A Zinc Oxide Interconnected Hydroxypropyl-Beta-Cyclodextrin/rGO Nanocomposite as an Electrocatalyst for Melatonin Detection: An Ultra-Sensitive Electrochemical Sensor
Abstract
1. Introduction
2. Chemicals and Techniques
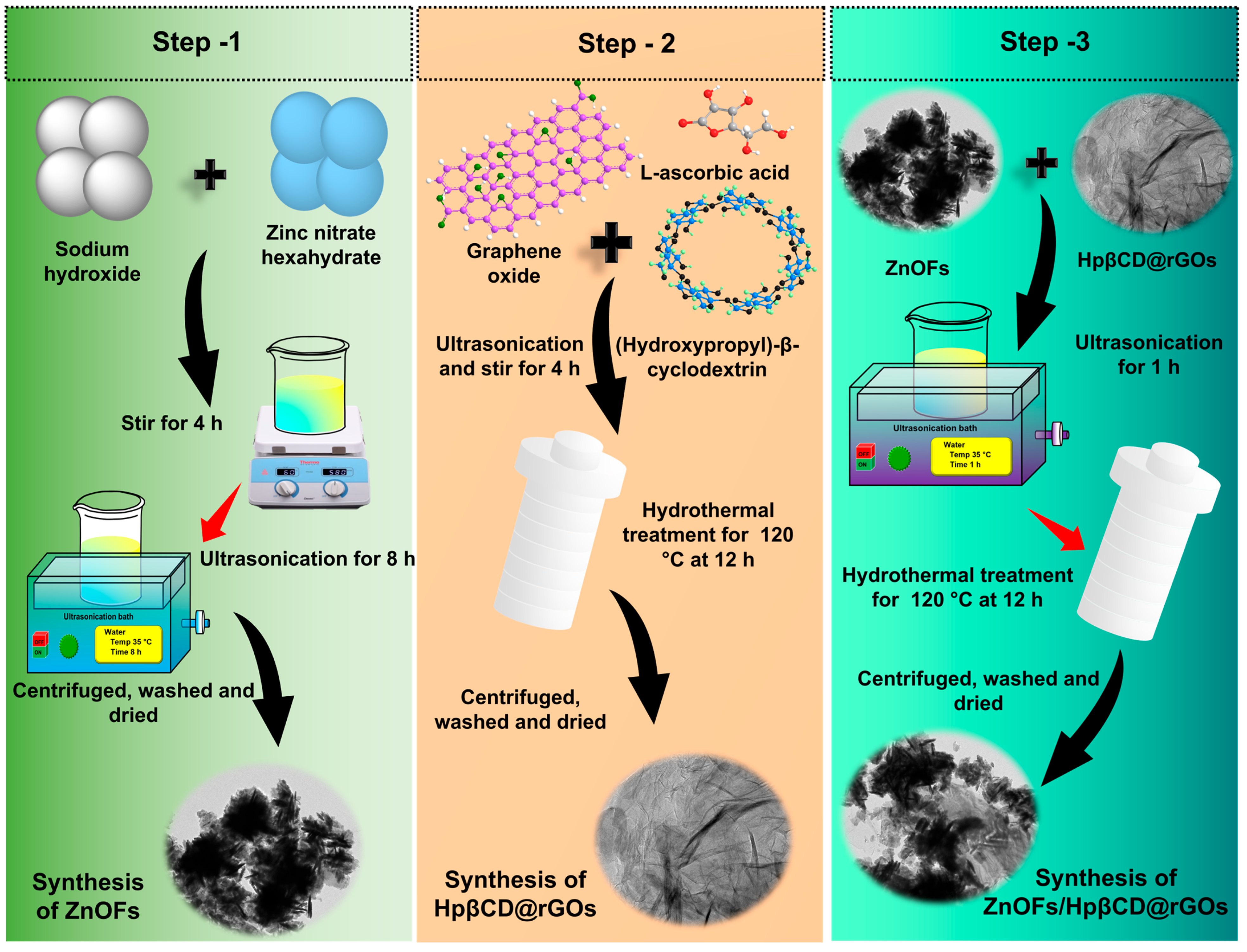
2.1. Preparation of ZnOFs, HpβCD@rGOs, and ZnOFs/HpβCD@rGOs
2.2. Fabrication of ZnOFs/HpβCD@rGOs/GCE
2.3. Choice of Materials
3. Results and Discussion
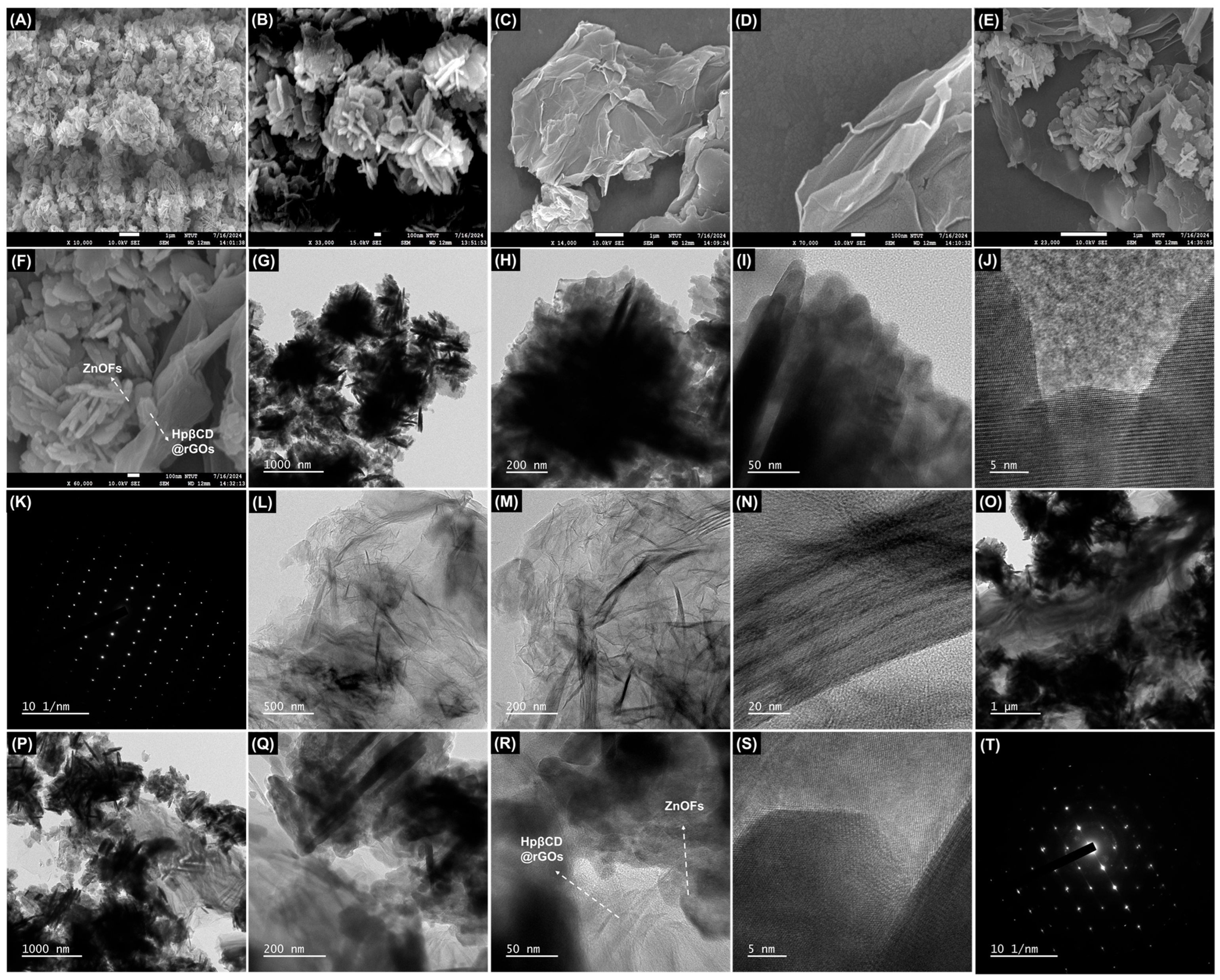
3.1. Morphological Studies
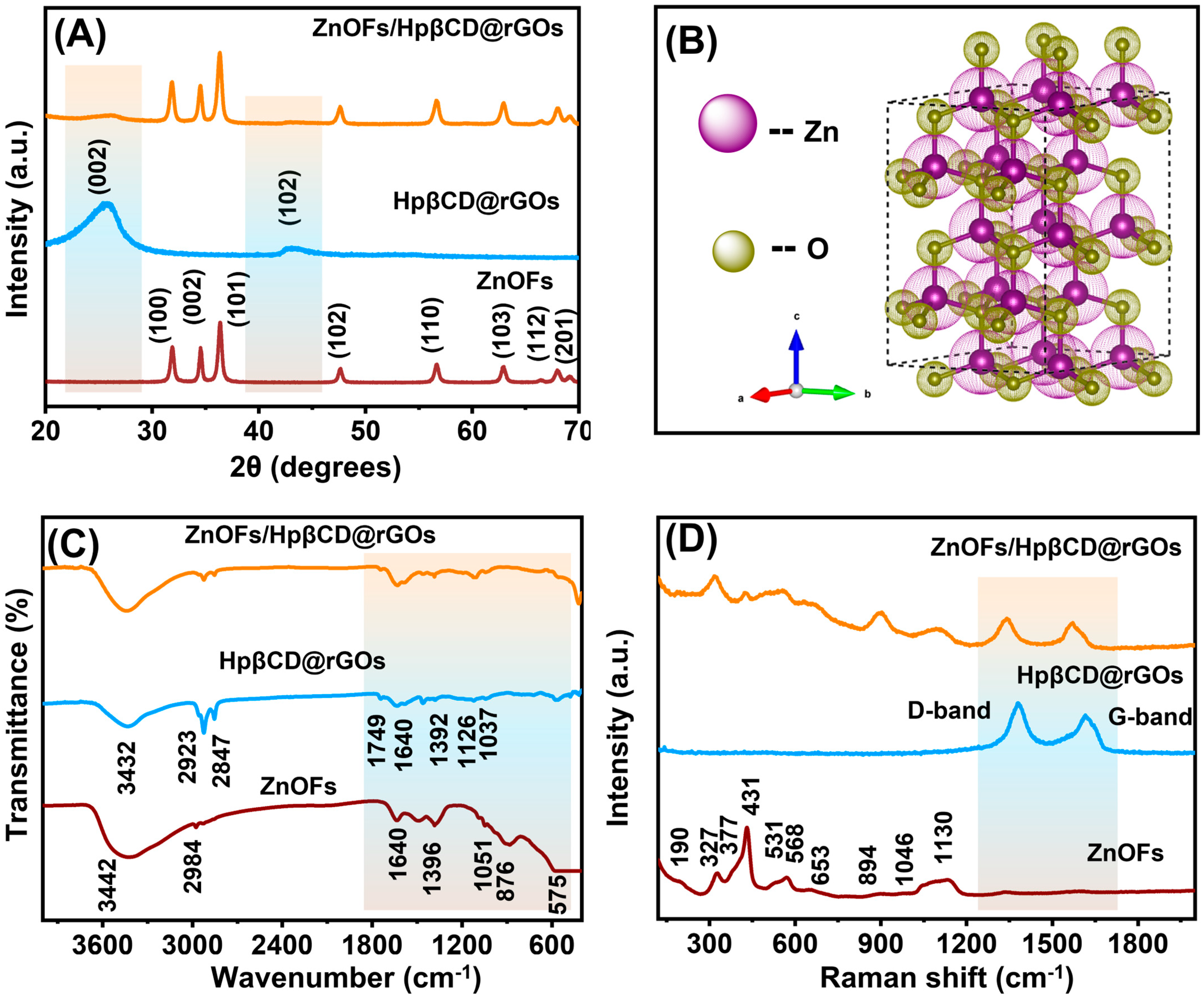
3.2. XRD and XPS Analysis
3.3. FTIR
3.4. Raman
4. Electrochemical Studies
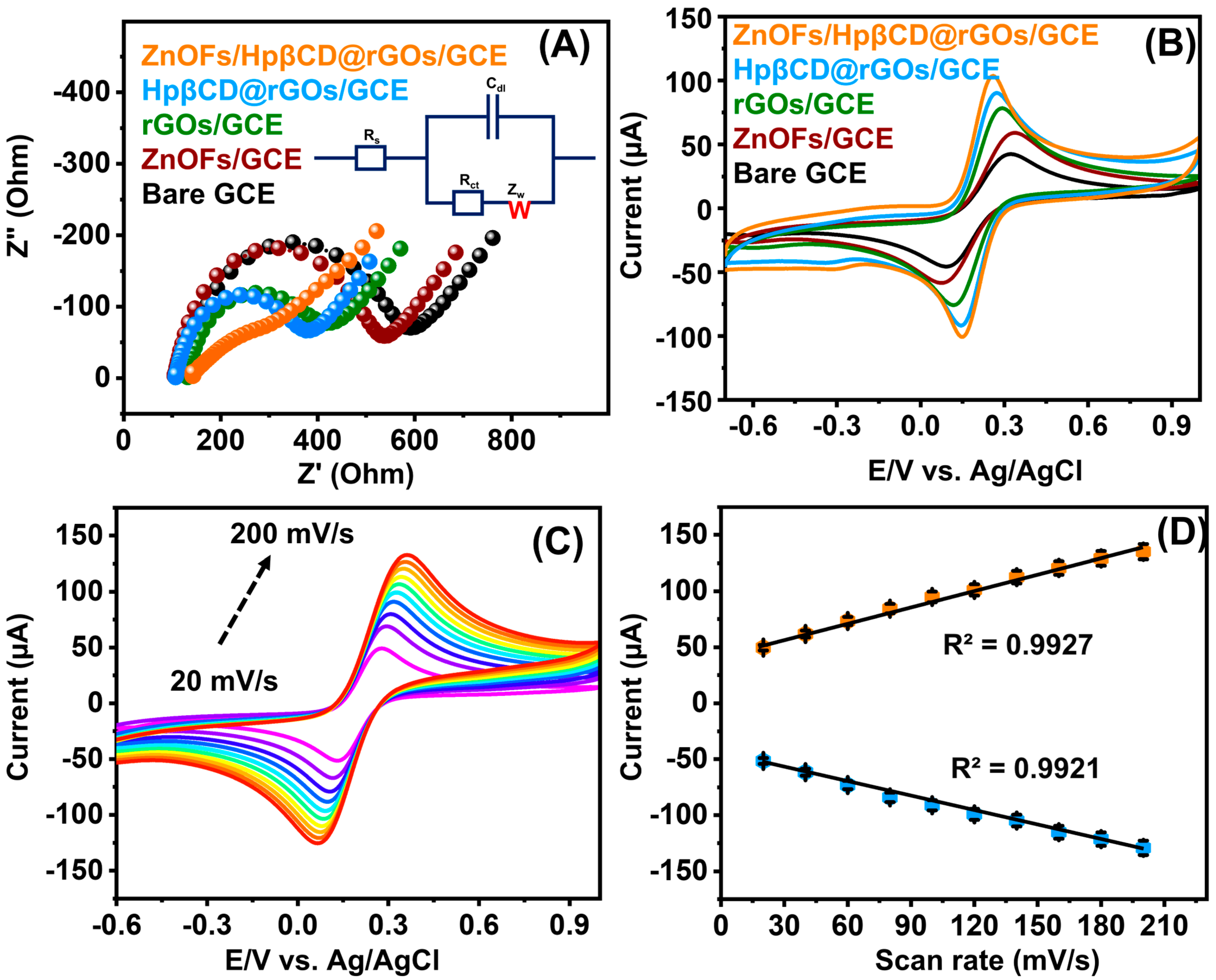
4.1. Electrochemical Impedance Spectroscopy
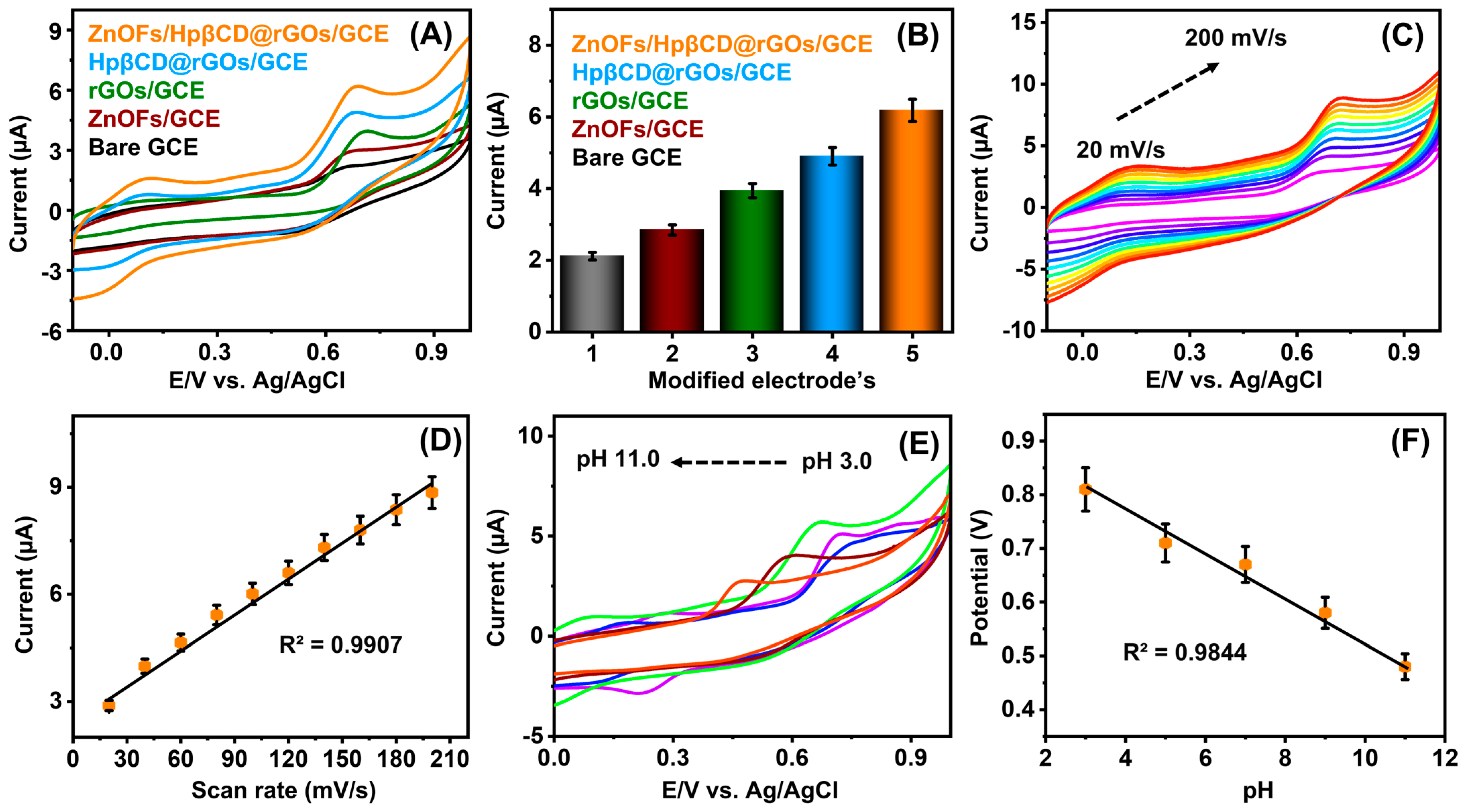
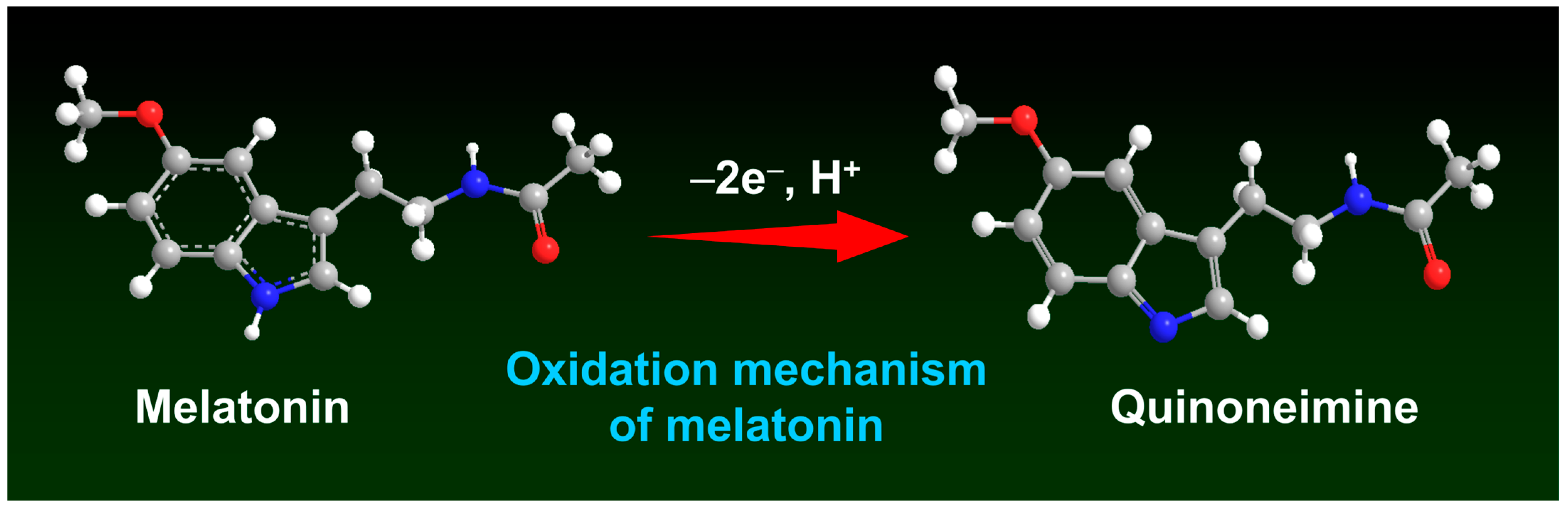
4.2. Electrochemical Detection of MTN with Modified Electrodes, Scan Rate, and pH Performance
4.3. MTN Concentration Variation in CV and DPV
4.4. Selectivity, Repeatability, and Reproducibility Study of ZnOFs/HpβCD@rGOs/GCE by the DPV Method
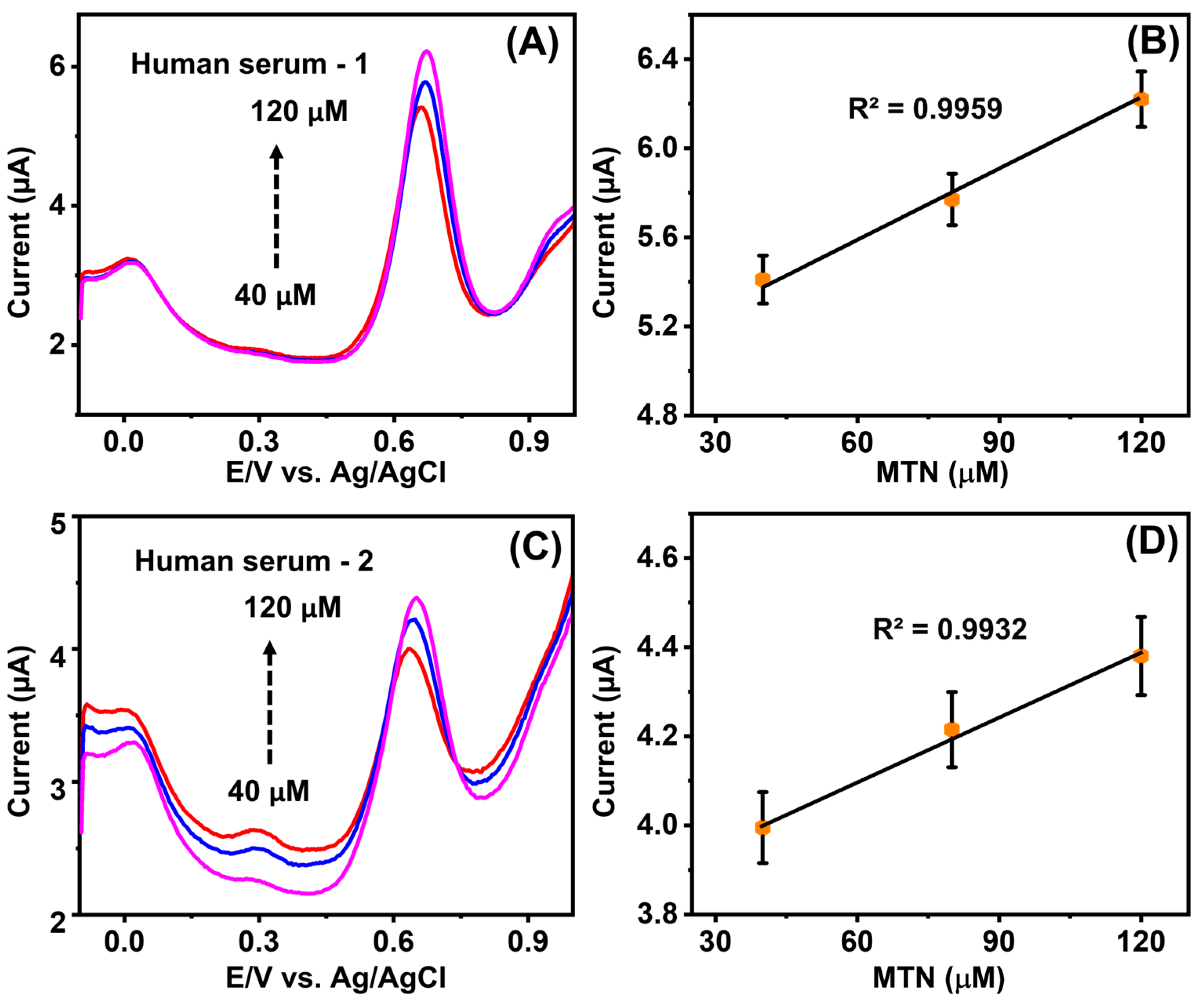
4.5. Real Sample Investigation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, Z.A.; Hong, P.J.S.; Lee, C.H.; Hong, Y. Recent Advances in Electrochemical and Optical Sensors for Detecting Tryptophan and Melatonin. Int. J. Nanomed. 2021, 16, 6861–6888. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Ding, Y.; Li, L.; Ma, G. Rapid Quantitative Detection of Melatonin by Electrochemical Sensor Based on Carbon Nanofibers Embedded with FeCo Alloy Nanoparticles. J. Electroanal. Chem. 2020, 873, 114422. [Google Scholar] [CrossRef]
- Johns, N.P.; Johns, J.; Porasuphatana, S.; Plaimee, P.; Sae-Teaw, M. Dietary Intake of Melatonin from Tropical Fruit Altered Urinary Excretion of 6-Sulfatoxymelatonin in Healthy Volunteers. J. Agric. Food Chem. 2013, 61, 913–919. [Google Scholar] [CrossRef]
- Diss, L.B.; Robinson, S.D.; Wu, Y.; Fidalgo, S.; Yeoman, M.S.; Patel, B.A. Age-Related Changes in Melatonin Release in the Murine Distal Colon. ACS Chem. Neurosci. 2013, 4, 879–887. [Google Scholar] [CrossRef]
- Freitas, R.C.; Orzari, L.O.; Ferreira, L.M.C.; Paixão, T.R.L.C.; Coltro, W.K.T.; Vicentini, F.C.; Janegitz, B.C. Electrochemical Determination of Melatonin Using Disposable Self-Adhesive Inked Paper Electrode. J. Electroanal. Chem. 2021, 897, 115550. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, X.; Sailjoi, A.; Zou, Y.; Lin, X.; Yan, F.; Su, B.; Liu, J. Vertical Silica Nanochannels Supported by Nanocarbon Composite for Simultaneous Detection of Serotonin and Melatonin in Biological Fluids. Sens. Actuators B Chem. 2022, 353, 131101. [Google Scholar] [CrossRef]
- Santhan, A.; Hwa, K.Y. Rational Design of Nanostructured Copper Phosphate Nanoflakes Supported Niobium Carbide for the Selective Electrochemical Detection of Melatonin. ACS Appl. Nano Mater. 2022, 5, 18256–18269. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Li, H.; Wang, G.; Long, Y.; Li, A.; Yang, B. In Situ Detection of Melatonin and Pyridoxine in Plants Using a CuO-Poly(l -Lysine)/Graphene-Based Electrochemical Sensor. ACS Sustain. Chem. Eng. 2019, 7, 19537–19545. [Google Scholar] [CrossRef]
- Rahmati, R.; Hemmati, A.; Mohammadi, R.; Hatamie, A.; Tamjid, E.; Simchi, A. Sensitive Voltammetric Detection of Melatonin in Pharmaceutical Products by Highly Conductive Porous Graphene-Gold Composites. ACS Sustain. Chem. Eng. 2020, 8, 18224–18236. [Google Scholar] [CrossRef]
- Castagnola, E.; Woeppel, K.; Golabchi, A.; McGuier, M.; Chodapaneedi, N.; Metro, J.; Taylor, I.M.; Cui, X.T. Electrochemical Detection of Exogenously Administered Melatonin in the Brain. Analyst 2020, 145, 2612–2620. [Google Scholar] [CrossRef]
- Blum, B.; Kaushal, S.; Khan, S.; Kim, J.H.; Alvarez Villalba, C.L. Melatonin in Traumatic Brain Injury and Cognition. Cureus 2021, 13, e17776. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, E.A.; Di Mascio, P.; Harumi, T.; Spence, D.W.; Moscovitch, A.; Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Measurement of Melatonin in Body Fluids: Standards, Protocols and Procedures. Child’s Nerv. Syst. 2011, 27, 879–891. [Google Scholar] [CrossRef]
- Chen, G.; Ding, X.; Cao, Z.; Ye, J. Determination of Melatonin and Pyridoxine in Pharmaceutical Preparations for Health-Caring Purposes by Capillary Electrophoresis with Electrochemical Detection. Anal. Chim. Acta 2000, 408, 249–256. [Google Scholar] [CrossRef]
- Shahsavari, M.; Tajik, S.; Sheikhshoaie, I.; Garkani Nejad, F.; Beitollahi, H. Synthesis of Fe3O4@copper(II) Imidazolate Nanoparticles: Catalytic Activity of Modified Graphite Screen Printed Electrode for the Determination of Levodopa in Presence of Melatonin. Microchem. J. 2021, 170, 106637. [Google Scholar] [CrossRef]
- Hernandez, G.; Abreu, P.; Alonso, R.; Calzadilla, C.H. Determination of Pineal Melatonin by High-Performance Liquid Chromatography with Electrochemical Detection: Application for Rhythm Studies and Tissue Explants. J. Proteome Res. 1990, 8, 11–19. [Google Scholar] [CrossRef]
- Madhavan, A.S.; Kunjappan, L.M.; Rajith, L. Simultaneous Electrochemical Determination of L-Dopa and Melatonin at Reduced Graphene Oxide-Cu0.5Co0.5Fe2O4 Modified Platinum Electrode. J. Electrochem. Soc. 2021, 168, 057533. [Google Scholar] [CrossRef]
- Selvam, S.P.; Hansa, M.; Yun, K. Simultaneous Differential Pulse Voltammetric Detection of Uric Acid and Melatonin Based on a Self-Assembled Au Nanoparticle–MoS2 Nanoflake Sensing Platform. Sens. Actuators B Chem. 2020, 307, 127683. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Q.; Zhong, Y.; Tahini, H.A.; Shao, Z.; Wang, H. Metal Oxide-Based Materials as an Emerging Family of Hydrogen Evolution Electrocatalysts. Energy Environ. Sci. 2020, 13, 3361–3392. [Google Scholar] [CrossRef]
- Venkatesh, N.; Aravindan, S.; Ramki, K.; Murugadoss, G.; Thangamuthu, R.; Sakthivel, P. Sunlight-Driven Enhanced Photocatalytic Activity of Bandgap Narrowing Sn-Doped ZnO Nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 16792–16803. [Google Scholar] [CrossRef]
- Murugesan, C.; Pasupathi, A.; Paul Blessington Selvadurai, A.; Palanisamy, B.; Subramaniam, Y. Effect of Feedstock Solution Concentration on the Spectroscopic and Electrical Transport Properties of Thermal Plasma Synthesized ZnO Nanoparticles. Ceram. Int. 2023, 49, 22837–22845. [Google Scholar] [CrossRef]
- Hwa, K.Y.; Subramani, B. Synthesis of Zinc Oxide Nanoparticles on Graphene-Carbon Nanotube Hybrid for Glucose Biosensor Applications. Biosens. Bioelectron. 2014, 62, 127–133. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Petru, M. Synthesis and Applications of ZnO Nanostructures (ZONSs): A Review. Crit. Rev. Solid State Mater. Sci. 2022, 47, 99–141. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Garkani Nejad, F.; Safaei, M. Recent Advances in ZnO Nanostructure-Based Electrochemical Sensors and Biosensors. J. Mater. Chem. B 2020, 8, 5826–5844. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-Based Nanostructured Electrodes for Electrochemical Sensors and Biosensors in Biomedical Applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef]
- Hwa, K.Y.; Santhan, A.; Tata, S.K.S. Fabrication of Sn-Doped ZnO Hexagonal Micro Discs Anchored on RGO for Electrochemical Detection of Anti-Androgen Drug Flutamide in Water and Biological Samples. Microchem. J. 2021, 160, 105689. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon Based Materials: A Review of Adsorbents for Inorganic and Organic Compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Iqbal, S.; Khatoon, H.; Hussain Pandit, A.; Ahmad, S. Recent Development of Carbon Based Materials for Energy Storage Devices. Mater. Sci. Energy Technol. 2019, 2, 417–428. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Vo, D.V.N.; Gnana Prakash, D.; Adithya Joseph, A.; Viswanathan, S.; Arun, J. Environmental Applications of Carbon-Based Materials: A Review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Ahmed, A.; Singh, A.; Young, S.J.; Gupta, V.; Singh, M.; Arya, S. Synthesis Techniques and Advances in Sensing Applications of Reduced Graphene Oxide (RGO) Composites: A Review. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107373. [Google Scholar] [CrossRef]
- Agarwal, V.; Zetterlund, P.B. Strategies for Reduction of Graphene Oxide—A Comprehensive Review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Tamang, S.; Rai, S.; Bhujel, R.; Bhattacharyya, N.K.; Swain, B.P.; Biswas, J. A Concise Review on GO, RGO and Metal Oxide/RGO Composites: Fabrication and Their Supercapacitor and Catalytic Applications. J. Alloys Compd. 2023, 947, 169588. [Google Scholar] [CrossRef]
- Mondal, A.; Prabhakaran, A.; Gupta, S.; Subramanian, V.R. Boosting Photocatalytic Activity Using Reduced Graphene Oxide (RGO)/Semiconductor Nanocomposites: Issues and Future Scope. ACS Omega 2021, 6, 8734–8743. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Q.; Zhang, H.; Passerini, S.; Qian, X. Two-Dimensional Titanium Carbide/RGO Composite for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 15661–15667. [Google Scholar] [CrossRef]
- Nataraj, N.; Chen, S.-M. Interfacial Influence of Strontium Niobium Engulfed Reduced Graphene Oxide Composite for Sulfamethazine Detection: Employing an Electrochemical Route in Real Samples. J. Electrochem. Soc. 2021, 168, 057512. [Google Scholar] [CrossRef]
- Selvi, S.V.; Nataraj, N.; Chen, S.M. The Electro-Catalytic Activity of Nanosphere Strontium Doped Zinc Oxide with RGO Layers Screen-Printed Carbon Electrode for the Sensing of Chloramphenicol. Microchem. J. 2020, 159, 105580. [Google Scholar] [CrossRef]
- Santhan, A.; Hwa, K.Y.; Ganguly, A. Self-Assembled Nanorods with Reduced Graphene Oxide as Efficient Nano-Catalyst for Dual Modality Sensing of Hazardous Phenolic Compound. Chemosphere 2022, 307, 135715. [Google Scholar] [CrossRef]
- Nataraj, N.; Chen, T.W.; Chen, S.M.; Rwei, S.P. An Efficient Electrochemical Sensor Based on Zirconium Molybdate Decorated Reduced Graphene Oxide for the Detection of Hydroquinone. Int. J. Electrochem. Sci. 2020, 15, 8321–8335. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Wan, L.; Lin, J.; Wang, X. Microwave-Assisted Covalent Modification of Graphene Nanosheets with Hydroxypropyl-β-Cyclodextrin and Its Electrochemical Detection of Phenolic Organic Pollutants. J. Mater. Chem. 2011, 21, 10463–10471. [Google Scholar] [CrossRef]
- Chen, X.; Li, N.; Rong, Y.; Hou, Y.; Huang, Y.; Liang, W. β-Cyclodextrin Functionalized 3D Reduced Graphene Oxide Composite-Based Electrochemical Sensor for the Sensitive Detection of Dopamine. RSC Adv. 2021, 11, 28052–28060. [Google Scholar] [CrossRef]
- Santhan, A.; Hwa, K.Y.; Murugan, R. Facile Synthesis of Silver Selenide Anchored on β-Cd/Reduced Graphene Oxide Hybrid Composites for Electrochemical Sensing of Azithromycin in Biological and Environmental Samples. J. Taiwan Inst. Chem. Eng. 2024, 157, 105406. [Google Scholar] [CrossRef]
- Ganguly, A.; Hwa, K.Y.; Santhan, A.; Kanna Sharma, T.S. Strategic Orchestration of MoSe2 Microspheres on β-Cd Functionalized RGO: A Sustainable Electrocatalyst for Detection of Rifampicin in Real Samples. Chemosphere 2022, 307, 135373. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.B.; Badnore, A.U. Effect of PH on Sonication Assisted Synthesis of ZnO Nanostructures: Process Details. Chem. Eng. Process. Process Intensif. 2017, 122, 235–244. [Google Scholar] [CrossRef]
- Khorsand Zak, A.; Majid, W.H.A.; Wang, H.Z.; Yousefi, R.; Moradi Golsheikh, A.; Ren, Z.F. Sonochemical Synthesis of Hierarchical ZnO Nanostructures. Ultrason. Sonochem. 2013, 20, 395–400. [Google Scholar] [CrossRef]
- Murugan, R.; Hwa, K.-Y.; Santhan, A.; Ibrahim, S. ZnO/B-g-C3N4 Nanoplatelet/Nanosheet Heterostructures for the Electrochemical Detection of Metol in Real Sample Analysis. ACS Appl. Nano Mater. 2024, 7, 2072–2085. [Google Scholar] [CrossRef]
- Xu, S.; Fu, L.; Pham, T.S.H.; Yu, A.; Han, F.; Chen, L. Preparation of ZnO Flower/Reduced Graphene Oxide Composite with Enhanced Photocatalytic Performance under Sunlight. Ceram. Int. 2015, 41, 4007–4013. [Google Scholar] [CrossRef]
- Paul, J.; Santhosh Kumar, M.C. Enhanced Photocatalytic Activity of Graphene Oxide Incorporated ZnO Nanorods Doped with Post-Transition Metals. Ceram. Int. 2024, 50, 9081–9088. [Google Scholar] [CrossRef]
- Hossain, M.M.; Ku, B.C.; Hahn, J.R. Synthesis of an Efficient White-Light Photocatalyst Composite of Graphene and ZnO Nanoparticles: Application to Methylene Blue Dye Decomposition. Appl. Surf. Sci. 2015, 354, 55–65. [Google Scholar] [CrossRef]
- Pruna, A.; Poliac, I.; Busquets Mataix, D.; Ruotolo, A. Synergistic Effects in ZnO Nanorod Films by Pulsed Electrodeposition on Graphene Oxide towards Enhanced Photocatalytic Degradation. Ceram. Int. 2024, 50, 4622–4631. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Kanan, V.; Joshi, S.; Visoly-Fisher, I. Role of Oxygen Functional Groups in Reduced Graphene Oxide for Lubrication. Sci. Rep. 2017, 7, 45030. [Google Scholar] [CrossRef]
- Hwa, K.Y.; Santhan, A.; Sharma, T.S.K. One-Dimensional Self-Assembled Co2SnO4 Nanosphere to Nanocubes Intertwined in Two-Dimensional Reduced Graphene Oxide: An Intriguing Electrocatalytic Sensor toward Mesalamine Detection. Mater. Today Chem. 2022, 23, 100739. [Google Scholar] [CrossRef]
- Santhan, A.; Hwa, K.Y. Zinc Phosphate-Incorporated Niobium Carbide as an Effective Electrocatalyst for Ultrasensitive and Selective Monitoring of Monoamine Neurotransmitter. ACS Sustain. Chem. Eng. 2023, 11, 4329–4341. [Google Scholar] [CrossRef]
- Liang, W.; Rong, Y.; Fan, L.; Zhang, C.; Dong, W.; Li, J.; Niu, J.; Yang, C.; Shuang, S.; Dong, C.; et al. Simultaneous Electrochemical Sensing of Serotonin, Dopamine and Ascorbic Acid by Using a Nanocomposite Prepared from Reduced Graphene Oxide, Fe3O4 and Hydroxypropyl-β-Cyclodextrin. Microchim. Acta 2019, 186, 751. [Google Scholar] [CrossRef]
- Shukla, S.K.; Agorku, E.S.; Mittal, H.; Mishra, A.K. Synthesis, Characterization and Photoluminescence Properties of Ce 3+-Doped ZnO-Nanophosphors. Chem. Pap. 2014, 68, 217–222. [Google Scholar] [CrossRef]
- Manoharan, C.; Rajendran, V.; Sivaraj, R. Synthesis, Characterization and Applications of Zno/Tio2/Sio2 Nanocomposite. Orient. J. Chem. 2018, 34, 1333–1340. [Google Scholar] [CrossRef]
- N’Konou, K.; Haris, M.; Lare, Y.; Baneto, M.; Napo, K.; Torchio, P. Effect of Barium Doping on Structural and Optical Properties of Zinc Oxide Nanoparticles Synthesized by Microwave Hydrothermal Method. Phys. Status Solidi Basic Res. 2016, 253, 260–266. [Google Scholar] [CrossRef]
- Kumar, R.; Youssry, S.M.; Abdel-Galeil, M.M.; Matsuda, A. One-Pot Synthesis of Reduced Graphene Oxide Nanosheets Anchored ZnO Nanoparticles via Microwave Approach for Electrochemical Performance as Supercapacitor Electrode. J. Mater. Sci. Mater. Electron. 2020, 31, 15456–15465. [Google Scholar] [CrossRef]
- Silambarasan, M.; Saravanan, S.; Soga, T. Raman and Photoluminescence Studies of Ag and Fe-Doped ZnO Nanoparticles. Int. J. ChemTech Res. 2015, 7, 1644–1650. [Google Scholar]
- Morales-Mendoza, J.E.; Herrera-Pérez, G.; Fuentes-Cobas, L.; Hermida-Montero, L.A.; Pariona, N.; Paraguay-Delgado, F. Synthesis, Structural and Optical Properties of Cu Doped ZnO and CuO–ZnO Composite Nanoparticles. Nano-Struct. Nano-Objects 2023, 34, 100967. [Google Scholar] [CrossRef]
- Mrabet, S.; Ihzaz, N.; Bessadok, M.N.; Vázquez-Vázquez, C.; Alshammari, M.; El Mir, L. Microstructural, Raman, and Magnetic Investigations on Ca-Doped ZnO Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2024, 34, 2064–2073. [Google Scholar] [CrossRef]
- Santhan, A.; Hwa, K.Y.; Murugan, R. Rational Construction of 2D Heterostructures: Niobium Carbide Entangled RGO Aerogel for the Detection of Para Nitroaniline Isomer in Environmental Water Samples. Process Saf. Environ. Prot. 2023, 180, 907–922. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Voltammetric Determination of Melatonin Using a Graphene-Based Sensor in Pharmaceutical Products. Int. J. Nanomed. 2016, 11, 1859–1866. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Martín, A.; Silva, M.F.; Escarpa, A. Screen-Printed Electrodes Modified with Carbon Nanotubes or Graphene for Simultaneous Determination of Melatonin and Serotonin. Microchim. Acta 2015, 182, 1925–1931. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Gao, M.; Yu, H.; Fu, M.; Chen, W. Molecularly Imprinted Electrochemical Sensor Based on CuxO/MXene Nanocomposite for Sensing of Melatonin. ACS Appl. Nano Mater. 2024, 7, 22832–22842. [Google Scholar] [CrossRef]
- Bahend, K.; Bakirhan, N.K.; El Fazdoune, M.; El-Asri, A.; Bazzaoui, E.A.; Bazzaoui, M. Nanostructured Electrochemical Platform for Sensitive Detection of Melatonin in Human Serum and Tablets. Synth. Met. 2024, 309, 117750. [Google Scholar] [CrossRef]


| Electrode Modified Materials | MTN Sensing Techniques | MTN Wide Linear Range (µM) | MTN Limit of Detection (µM) | Ref. |
|---|---|---|---|---|
| 1 CDE | CV | 0.0025–1.1 (mmol/L) | 1.3 | [13] |
| 2 GPH-CSPE | Amperometric (IT) | 1–300 | 0.87 | [62] |
| 3 VMSF/HErGO-CNT/ITO | DPV | 20–100 | 0.014 | [6] |
| 4 CNTs and graphene-based CSPE | DPV | 0.005–3mM | 1.1 | [63] |
| 5 Fe3O4@Cu (Im)2/GSPE | DPV | 1.0–300.0 | 0.24 | [14] |
| 6 MIP/CuxO/MXene/SPCE | DPV | 5–700 | 0.029 | [64] |
| 7 PTBO/MWCNTs/GCE | DPV | 1–1000 | 0.027 | [65] |
| ZnOFs/HpβCD@rGOs/GCE | DPV | 0.014–0.149 and 1.149–643.341 µM | 0.0105 µM or 10.5 nM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwa, K.-Y.; Santhan, A.; Ou, C.-W.; Wang, C.-H. A Zinc Oxide Interconnected Hydroxypropyl-Beta-Cyclodextrin/rGO Nanocomposite as an Electrocatalyst for Melatonin Detection: An Ultra-Sensitive Electrochemical Sensor. Sensors 2025, 25, 3266. https://doi.org/10.3390/s25113266
Hwa K-Y, Santhan A, Ou C-W, Wang C-H. A Zinc Oxide Interconnected Hydroxypropyl-Beta-Cyclodextrin/rGO Nanocomposite as an Electrocatalyst for Melatonin Detection: An Ultra-Sensitive Electrochemical Sensor. Sensors. 2025; 25(11):3266. https://doi.org/10.3390/s25113266
Chicago/Turabian StyleHwa, Kuo-Yuan, Aravindan Santhan, Chun-Wei Ou, and Cheng-Han Wang. 2025. "A Zinc Oxide Interconnected Hydroxypropyl-Beta-Cyclodextrin/rGO Nanocomposite as an Electrocatalyst for Melatonin Detection: An Ultra-Sensitive Electrochemical Sensor" Sensors 25, no. 11: 3266. https://doi.org/10.3390/s25113266
APA StyleHwa, K.-Y., Santhan, A., Ou, C.-W., & Wang, C.-H. (2025). A Zinc Oxide Interconnected Hydroxypropyl-Beta-Cyclodextrin/rGO Nanocomposite as an Electrocatalyst for Melatonin Detection: An Ultra-Sensitive Electrochemical Sensor. Sensors, 25(11), 3266. https://doi.org/10.3390/s25113266








