Gamma-Ray Analysis of Reed Samples from the Danube Delta
Abstract
1. Introduction
2. Materials and Methods
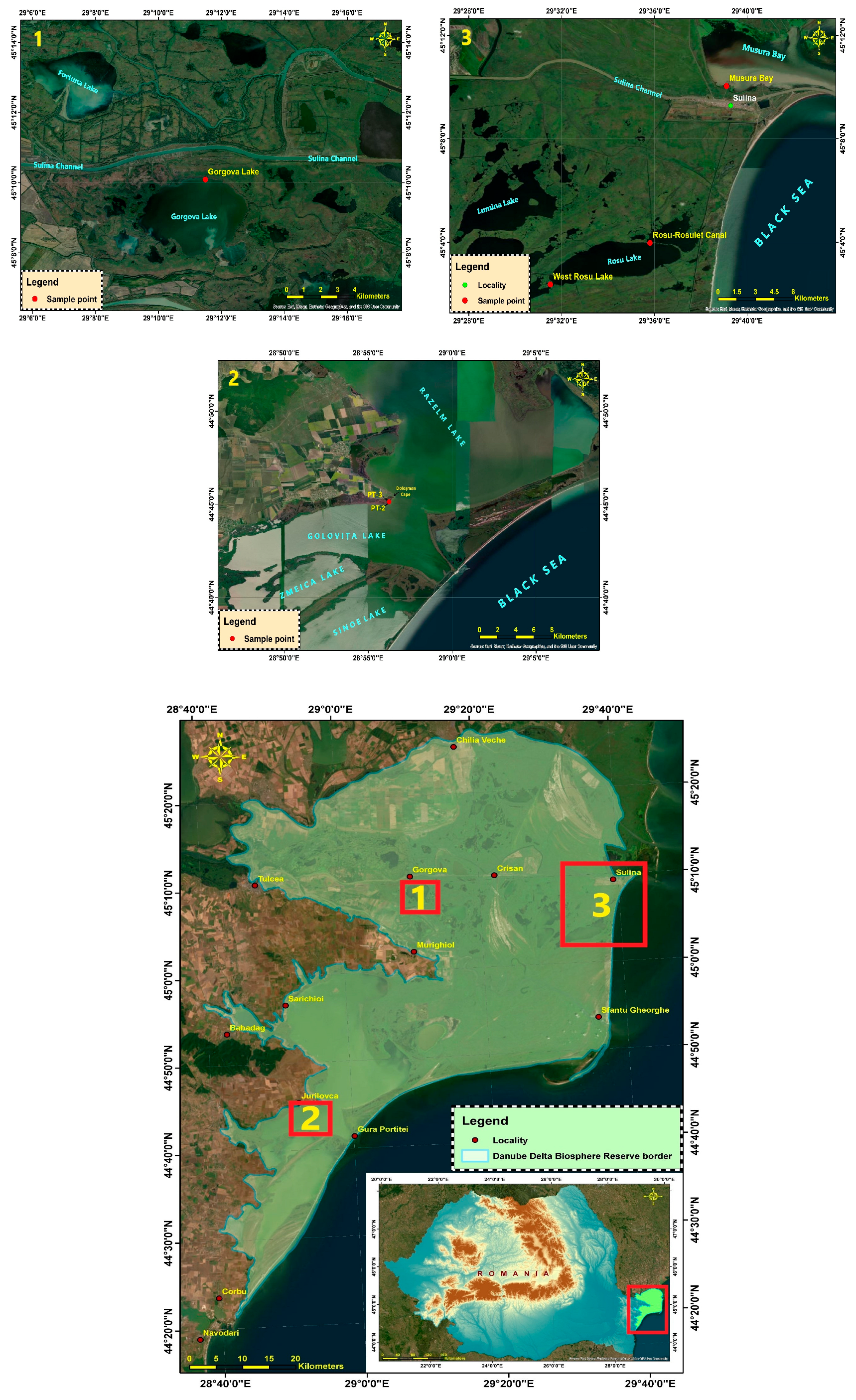
2.1. Study Area
2.2. Sample Processing and Analysis
3. Results
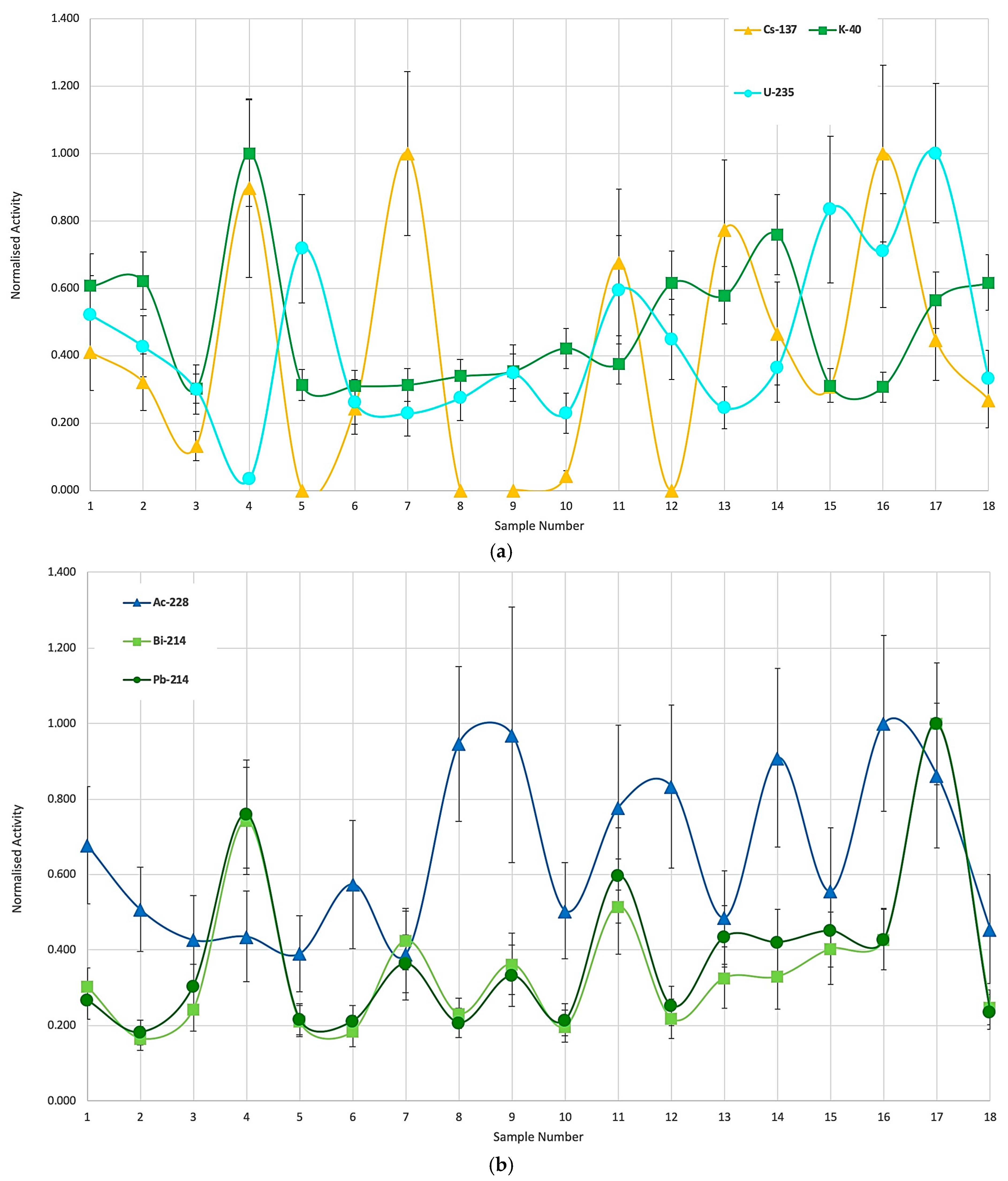
3.1. Specific Activities and Normalized Values
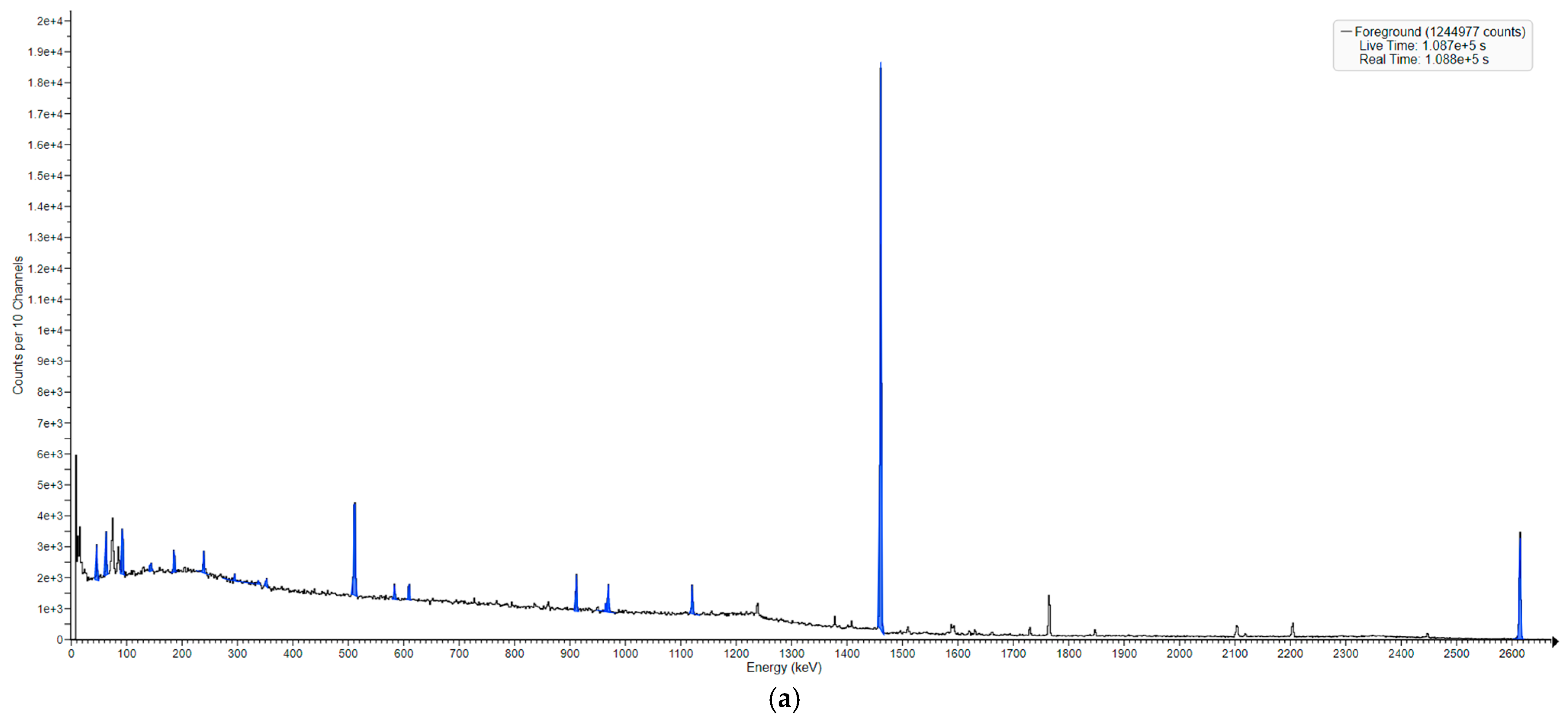
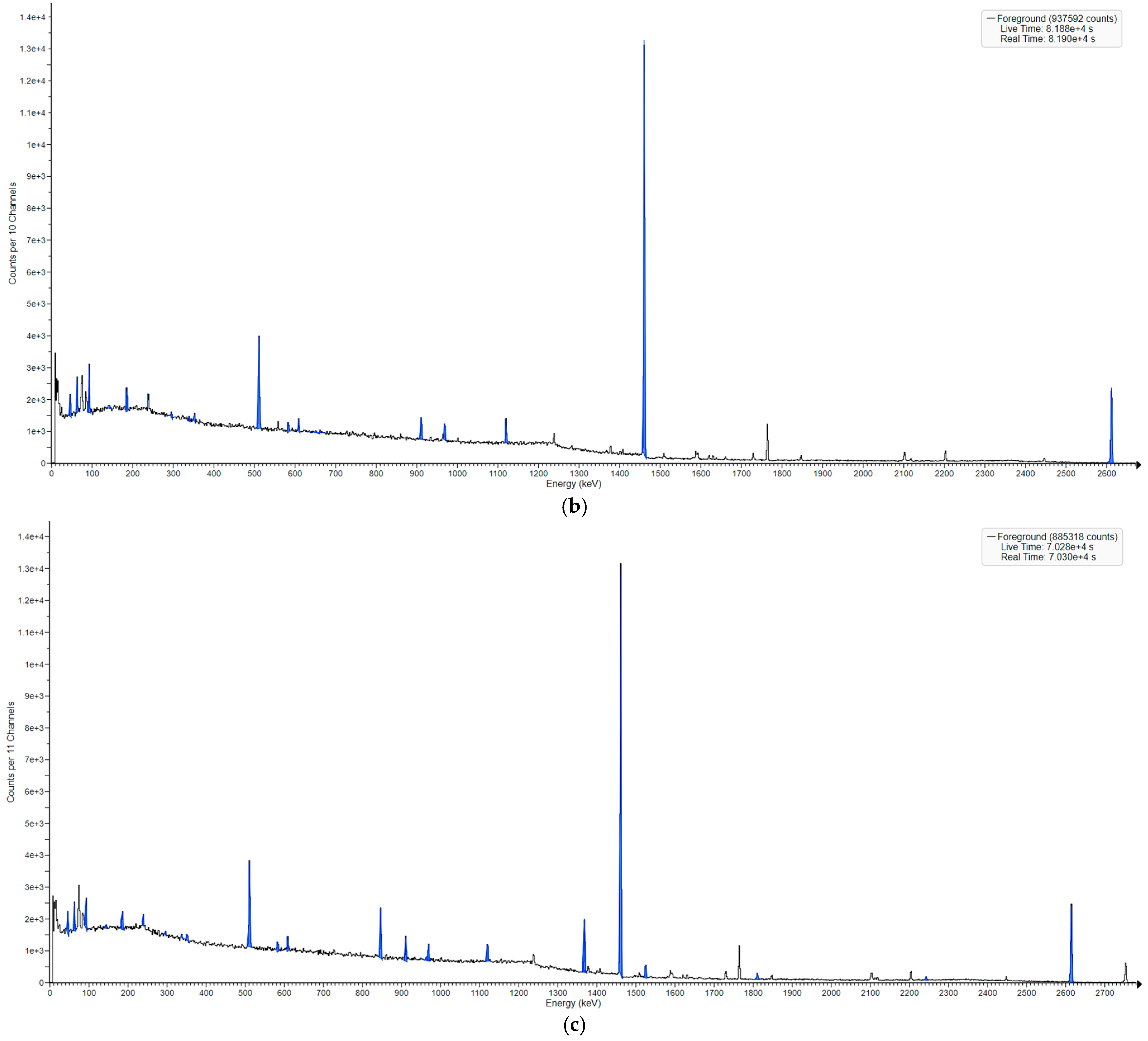
3.2. Neutron-Induced Emissions
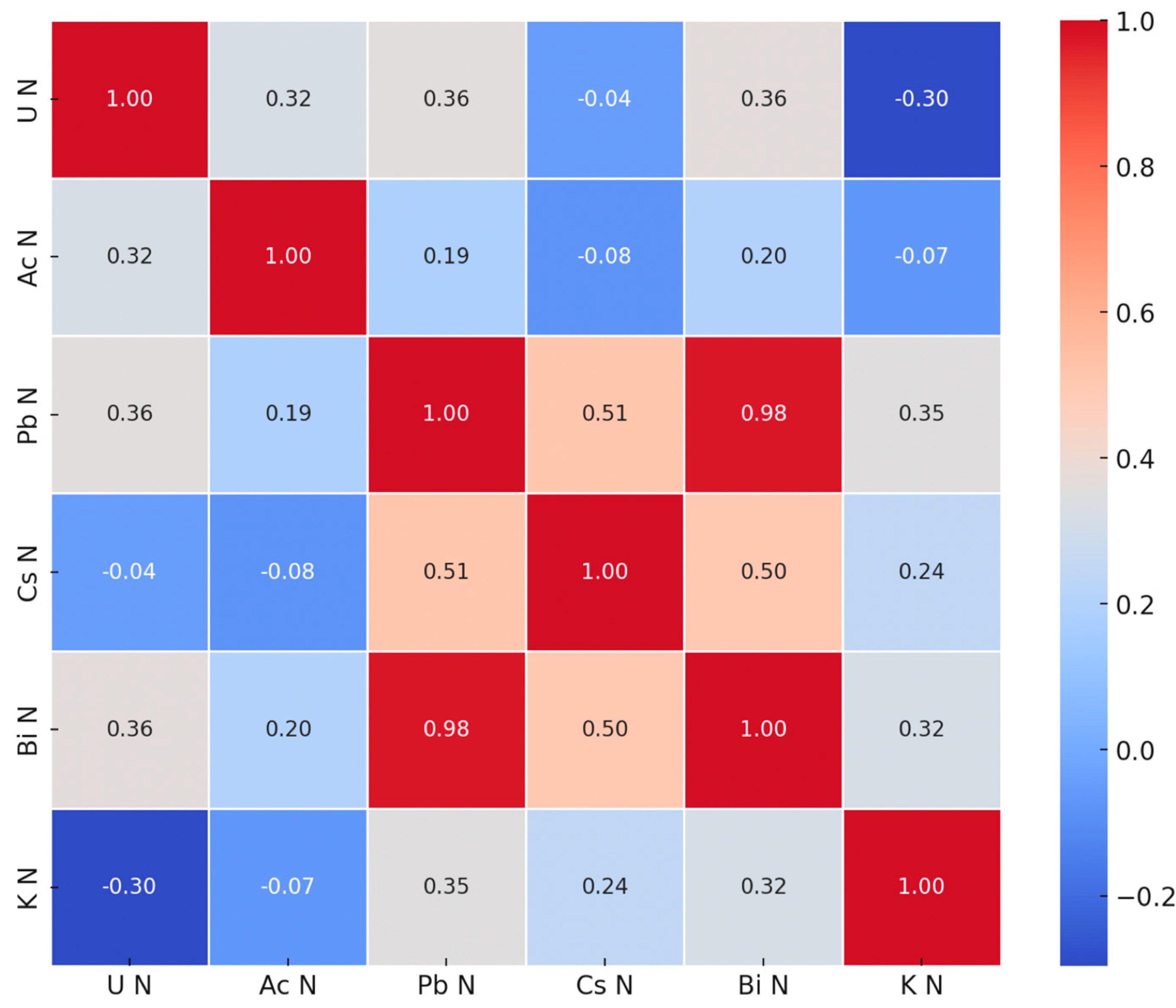
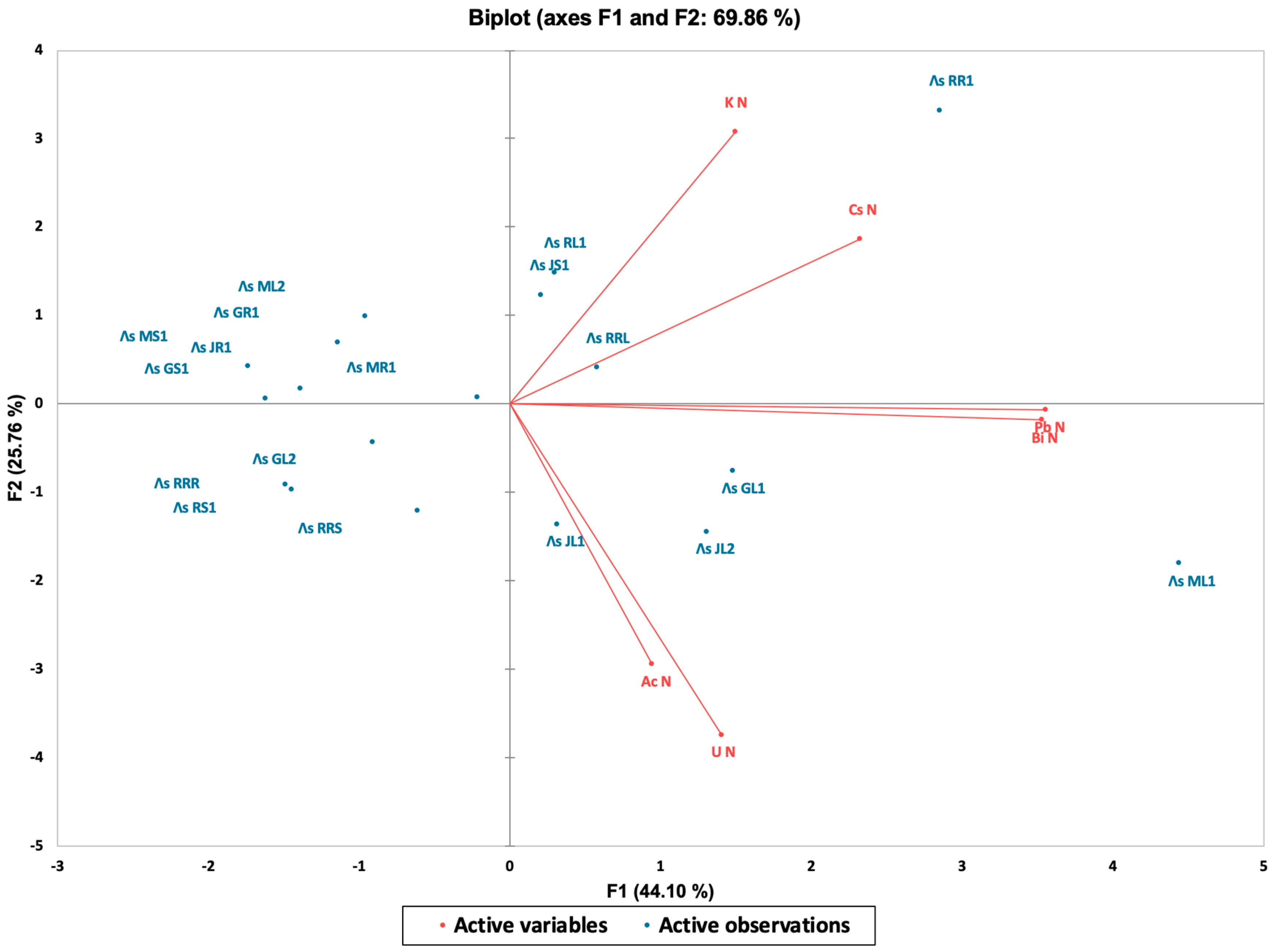
3.3. Covariance and Clustering
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, M.; Eguchi, T.; Azuma, K.; Nakao, A.; Kubo, K.; Fujimura, S.; Syaifudin, M.; Maruyama, H.; Watanabe, T.; Shinano, T. The ratio of plant 137Cs to exchangeable 137Cs in soil is a crucial factor in explaining the variation in 137Cs transferability from soil to plant. Sci. Total Environ. 2023, 857, 159208. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, M.; Yabe, T.; Furukawa, J.; Watanabe, M.; Ikeda, K.; Yasutani, I.; Nishizawa, T. Comparison of Potentials of Higher Plants for Phytoremediation of Radioactive Cesium from Contaminated Soil. Environ. Control Biol. 2016, 54, 65–69. [Google Scholar] [CrossRef]
- Cinelli, G.; De Cort, M.; Tollefsen, T. (Eds.) European Atlas of Natural Radiation; Publication Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Sources and Effects of Ionizing Radiation; Report to the General Assembly, with Scientific Annexes; United Nations Scientific Committee on the Effects of Atomic Radiation: New York, NY, USA, 2000; ISBN 92-1-142238-8.
- El-Reefy, H.I.; Sharshar, T.; Zaghloul, R.; Badran, H.M. Distribution of gamma-ray emitting radionuclides in the environment of Burullus Lake: I. Soils and vegetations. J. Environ. Radioact. 2006, 87, 148–169. [Google Scholar] [CrossRef]
- Djelic, G.; Krstic, D.; Stajic, J.M.; Milenkovic, B.; Topuzovic, M.; Nikezic, D.; Vucic, D.; Zeremski, T.; Stankovic, M.; Kostic, D. Transfer factors of natural radionuclides and 137Cs from soil to plants used in traditional medicine in central Serbia. J. Environ. Radioact. 2016, 158, 81–88. [Google Scholar] [CrossRef]
- Galhardi, J.A.; García-Tenorio, R.; Bonotto, D.M.; Francés, I.D.; Motta, J.G. Natural radionuclides in plants, soils and sediments affected by U-rich coal mining activities in Brazil. J. Environ. Radioact. 2017, 177, 37–47. [Google Scholar] [CrossRef]
- Saenboonruang, K.; Phonchanthuek, E.; Prasandee, K. Soil-to-plant transfer factors of natural radionuclides (226Ra and 40K) in selected Thai medicinal plants. J. Environ. Radioact. 2018, 184, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tsvetnova, O.G.; Shcheglov, A.; Klyashtorin, A. 137Cs and K annual fluxes in a cropland and forest ecosystems twenty-four years after the Chernobyl accident. J. Environ. Radioact. 2018, 195, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Charro, E.; Moyano, A. Soil and vegetation influence in plants natural radionuclides uptake at a uranium mining site. Radiat. Phys. Chem. 2017, 141, 200–206. [Google Scholar] [CrossRef]
- Khan, H.M.; Ismail, M.; Khan, K.; Akhter, P. Measurement of radionuclides and gamma-ray dose rate in soil and transfer of radionuclides from soil to vegetation, vegetable of some Northern area of Pakistan using γ-ray spectrometry. Water Air Soil Pollut. 2011, 219, 129–142. [Google Scholar] [CrossRef]
- Tsabaris, C.; Androulakaki, E.G.; Ballas, D.; Alexakis, S.; Perivoliotis, L.; Iona, A. Radioactivity Monitoring at North Aegean Sea Integrating In-Situ Sensor in an Ocean Observing Platform. J. Mar. Sci. Eng. 2021, 9, 77. [Google Scholar] [CrossRef]
- Olacel, A.; Ujeniuc, S.; Suvaila, R.; Alexandrescu, B.; Pojar, I. Isotopic patterns via neutron irradiation and gamma spectrometry of environmental samples. Chem. Phys. Impact 2022, 4, 100065. [Google Scholar] [CrossRef]
- Driga, B. Delta Dunării, Sistemul de Circulație Apei; Editura Casa Cărții De Știință: Cluj Napoca, Romania, 2004; p. 256. [Google Scholar]
- Botnariuc, N. Fluxul de Energie din Ghiolurile Puiu, Roşu, Porcu şi Potenţialul lor Bioproductiv; St. com. ecol. Delta Dunării: Tulcea, Romania, 1985; Volume 1, pp. 9–14. [Google Scholar]
- Coteț, P. Evoluția Morfohidrografică Deltei Dunării—O Sinteză A Studiilor Existente Și O Nouă Interpretare. Probl. Geogr. 1960, 7, 53–81. [Google Scholar]
- SR EN ISO 5661–1; Water Quality. Part 1, General Guidelines for Establishing Sampling Programs and Techniques. ISS: Belgrade, Serbia, 2008.
- Covaliov, S.; Doroftei, M.; Dumitrița, S.; Hanganu, C.J. Reedbeds in the Danube Delta, National Institute for Research and Development of the Danube Delta; C.I.T.D.D.—Danube Delta Technological Information Center: Tulcea, Romania, 2023; 186p. [Google Scholar]
- Suvaila, R.; Sima, O.; Osvath, I. Improved method for the assessment of Co-60 and Cs-134 point sources in samples with non-homogeneous matrix. Appl. Radiat. Isot. 2014, 87, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Suvaila, R.; Osvath, I.; Sima, O. Improving the assessment of activity in samples with non-uniform distribution using the sum peak count rate. Appl. Radiat. Isot. 2013, 81, 76–80. [Google Scholar] [CrossRef]
- Suvaila, R.; Stancu, E.; Sima, O. On within sample homogeneity testing using gamma-ray spectrometry. Appl. Radiat. Isot. 2012, 70, 2144–2148. [Google Scholar] [CrossRef]
- Rudescu, L.; Niculescu, C.; Chivu, I. Monography of the Danube Delta Reed; Academiei R.S.R: Cluj-Napoca, Romania, 1965; 542p. [Google Scholar]
- Currie, L.A. Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal. Chem. 1968, 40, 586. [Google Scholar] [CrossRef]
- De Geer, L.E. Currie detection limits in gamma-ray spectroscopy. Appl. Radiat. Isot. 2004, 61, 151–160. [Google Scholar] [CrossRef] [PubMed]
- ISO 11929. Available online: https://www.iso.org/standard/69579.html (accessed on 9 April 2025).
- Addinsoft. XLSTAT Statistical and Data Analysis Solution; Addinsoft: New York, NY, USA, 2020; Available online: https://www.xlstat.com (accessed on 10 January 2025).
- Rodríguez, P.B.; Tomé, F.V.; Lozano, J.C.; Fernández, M.P. Transfer of 238U, 230Th, 226Ra, and 210Pb from soils to tree and shrub species in a Mediterranean area. Appl. Radiat. Isot. 2010, 68, 1154–1159. [Google Scholar] [CrossRef]
- Vandenhove, H.; Olyslaegers, G.; Sanzharova, N.; Shubina, O.; Reed, E.; Shang, Z.; Velasco, H. Proposal for new best estimates of the soil-to-plant transfer factor of U, Th, Ra, Pb and Po. J. Environ. Radioact. 2009, 100, 721–732. [Google Scholar] [CrossRef]
- Alexandrescu, B.; Sima, O.; Ujeniuc, S.; Pojar, I.; Suvaila, R. Distribution of 222Rn decay products in a soil sample. Phys. Scr. 2023, 98, 035009. [Google Scholar] [CrossRef]
- Suvaila, R.; Ujeniuc, S.; Scrieciu, A.; Pojar, I.; Alexandrescu, B. Methodology for regularity studies by precision environmental gamma ray spectrometry. Int. J. Environ. Sci. Technol. 2024, 22, 6265–6276. [Google Scholar] [CrossRef]
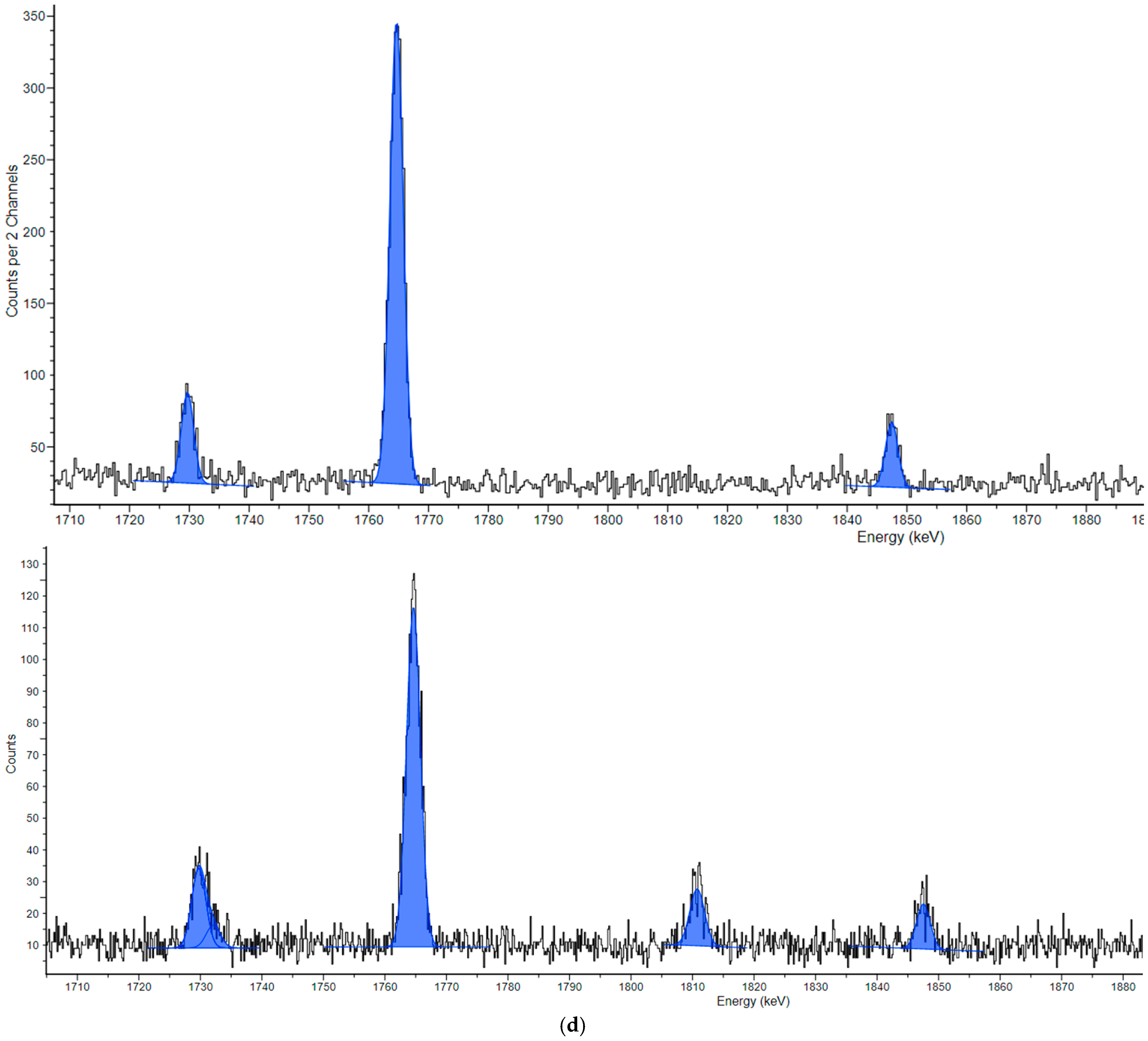
| Nuclide | 235U | 228Ac | 214Pb | 137Cs | 214Bi | 40K |
| Energy | 144 | 338 | 352 | 662 | 1120 | 1461 |
| D. L. (Bq·kg−1) | 1.8 | 3.4 | 3.5 | 4.8 | 5.2 | 5.1 |
| E (keV) | 144 | 338 | 352 | 662 | 1120 | 1461 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iγ (%) | 10.9 | U-235 | 11.27 | Ac-228 | 35.72 | Pb-214 | 85.1 | Cs-137 | 14.9 | Bi-214 | 10.77 | K-40 | ||||||||
| ε × 102 | 18.8 | 9.5 | 9.2 | 5.2 | 3.5 | 3.1 | ||||||||||||||
| No/Sample | Ls | u (U) | Norm | Ls | u (Ac) | Norm | Ls | u (Pb) | Norm | Ls | u (Cs) | Norm | Ls | u (Bi) | Norm | Ls | u (K) | Norm | Live (s) | Dry (g) |
| 1 Λs MR1 | 5.48 | 0.22 | 0.52 | 8.61 | 0.23 | 0.68 | 3.79 | 0.19 | 0.27 | 0.82 | 0.27 | 0.41 | 4.56 | 0.17 | 0.30 | 238 | 0.16 | 0.61 | 3 × 105 | 15.71 |
| 2 Λs GR1 | 4.49 | 0.21 | 0.43 | 6.45 | 0.22 | 0.51 | 2.58 | 0.18 | 0.18 | 0.65 | 0.26 | 0.32 | 2.50 | 0.16 | 0.17 | 244 | 0.14 | 0.62 | 3 × 105 | 18.13 |
| 3 Λs JR1 | 3.16 | 0.25 | 0.30 | 5.42 | 0.27 | 0.43 | 4.32 | 0.19 | 0.31 | 0.26 | 0.32 | 0.13 | 3.65 | 0.17 | 0.24 | 118 | 0.15 | 0.30 | 2 × 105 | 20.28 |
| 4 Λs RR1 | 0.35 | 0.29 | 0.03 | 5.53 | 0.28 | 0.44 | 10.78 | 0.19 | 0.76 | 1.80 | 0.30 | 0.90 | 11.16 | 0.17 | 0.74 | 392 | 0.16 | 1.00 | 2 × 105 | 7.20 |
| 5 Λs RRR | 7.54 | 0.22 | 0.72 | 4.96 | 0.26 | 0.39 | 3.07 | 0.19 | 0.22 | 0.00 | 0.26 | 0.00 | 3.18 | 0.17 | 0.21 | 123 | 0.15 | 0.31 | 2 × 105 | 18.42 |
| 6 Λs GS1 | 2.75 | 0.25 | 0.26 | 7.30 | 0.30 | 0.57 | 3.00 | 0.19 | 0.21 | 0.49 | 0.31 | 0.24 | 2.78 | 0.17 | 0.18 | 121 | 0.15 | 0.31 | 2 × 105 | 16.24 |
| 7 Λs JS1 | 2.41 | 0.29 | 0.23 | 4.95 | 0.31 | 0.39 | 5.16 | 0.21 | 0.36 | 2.01 | 0.24 | 1.00 | 6.40 | 0.19 | 0.43 | 123 | 0.16 | 0.31 | 96,600 | 16.07 |
| 8 Λs RS1 | 2.88 | 0.25 | 0.27 | 12.03 | 0.22 | 0.95 | 2.94 | 0.19 | 0.21 | 0.00 | LD | 0.00 | 3.48 | 0.17 | 0.23 | 133 | 0.15 | 0.34 | 2 × 105 | 20.43 |
| 9 Λs RRS | 3.65 | 0.24 | 0.35 | 12.32 | 0.35 | 0.97 | 4.71 | 0.25 | 0.33 | 0.00 | 0.19 | 0.00 | 5.46 | 0.20 | 0.36 | 139 | 0.15 | 0.35 | 1 × 105 | 14.17 |
| 10 Λs MS1 | 2.41 | 0.26 | 0.23 | 6.40 | 0.25 | 0.50 | 3.04 | 0.20 | 0.21 | 0.09 | 0.35 | 0.04 | 2.98 | 0.18 | 0.20 | 165 | 0.14 | 0.42 | 2 × 105 | 19.90 |
| 11 Λs GL1 | 6.25 | 0.27 | 0.59 | 9.88 | 0.28 | 0.78 | 8.47 | 0.21 | 0.60 | 1.36 | 0.32 | 0.68 | 7.76 | 0.20 | 0.52 | 147 | 0.16 | 0.38 | 80,200 | 17.15 |
| 12 Λs GL2 | 4.72 | 0.26 | 0.45 | 10.59 | 0.26 | 0.83 | 3.57 | 0.21 | 0.25 | 0.00 | LD | 0.00 | 3.28 | 0.19 | 0.22 | 241 | 0.15 | 0.61 | 1 × 105 | 19.48 |
| 13 Λs RL1 | 2.58 | 0.26 | 0.24 | 6.17 | 0.25 | 0.49 | 6.18 | 0.19 | 0.44 | 1.55 | 0.27 | 0.77 | 4.91 | 0.18 | 0.33 | 227 | 0.15 | 0.58 | 2 × 105 | 19.00 |
| 14 Λs RRL | 3.82 | 0.28 | 0.36 | 11.54 | 0.26 | 0.91 | 5.97 | 0.21 | 0.42 | 0.93 | 0.33 | 0.46 | 4.96 | 0.19 | 0.33 | 298 | 0.16 | 0.76 | 87,300 | 19.32 |
| 15 Λs JL1 | 8.77 | 0.26 | 0.83 | 7.06 | 0.30 | 0.56 | 6.41 | 0.21 | 0.45 | 0.62 | LD | 0.31 | 6.07 | 0.20 | 0.40 | 122 | 0.16 | 0.31 | 85,900 | 12.75 |
| 16 Λs JL2 | 7.48 | 0.24 | 0.71 | 12.70 | 0.23 | 1.00 | 6.06 | 0.19 | 0.43 | 2.01 | 0.26 | 1.00 | 6.44 | 0.17 | 0.43 | 120 | 0.15 | 0.31 | 2 × 105 | 10.94 |
| 17 Λs ML1 | 10.52 | 0.21 | 1.00 | 10.95 | 0.22 | 0.86 | 14.17 | 0.16 | 1.00 | 0.89 | 0.27 | 0.45 | 15.03 | 0.15 | 1.00 | 221 | 0.15 | 0.56 | 4 × 105 | 5.48 |
| 18 Λs ML2 | 3.50 | 0.25 | 0.33 | 5.78 | 0.32 | 0.46 | 3.33 | 0.20 | 0.24 | 0.54 | 0.31 | 0.27 | 3.72 | 0.18 | 0.25 | 242 | 0.13 | 0.62 | 2 × 105 | 18.64 |
| Isotope | Minimum | Maximum | Mean | Std. Dev |
|---|---|---|---|---|
| U-235 | 0.033 | 1.000 | 0.437 | 0.248 |
| Ac-228 | 0.390 | 1.000 | 0.650 | 0.221 |
| Pb-214 | 0.182 | 1.000 | 0.383 | 0.217 |
| Bi-214 | 0.166 | 1.000 | 0.363 | 0.214 |
| K-40 | 0.302 | 1.000 | 0.484 | 0.198 |
| Cs-137 | 0.000 | 1.000 | 0.388 | 0.350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavel, A.B.; Ujeniuc, S.; Iordache, G.; Catianis, I.; Gavrila, C.; Scrieciu, A.; Seremet, R.; Andreicovici, I.; Ise, S.; Suvaila, R. Gamma-Ray Analysis of Reed Samples from the Danube Delta. Sensors 2025, 25, 3257. https://doi.org/10.3390/s25113257
Pavel AB, Ujeniuc S, Iordache G, Catianis I, Gavrila C, Scrieciu A, Seremet R, Andreicovici I, Ise S, Suvaila R. Gamma-Ray Analysis of Reed Samples from the Danube Delta. Sensors. 2025; 25(11):3257. https://doi.org/10.3390/s25113257
Chicago/Turabian StylePavel, Ana Bianca, Sorin Ujeniuc, Gabriel Iordache, Irina Catianis, Catalina Gavrila, Albert Scrieciu, Radu Seremet, Iulian Andreicovici, Silvia Ise, and Rares Suvaila. 2025. "Gamma-Ray Analysis of Reed Samples from the Danube Delta" Sensors 25, no. 11: 3257. https://doi.org/10.3390/s25113257
APA StylePavel, A. B., Ujeniuc, S., Iordache, G., Catianis, I., Gavrila, C., Scrieciu, A., Seremet, R., Andreicovici, I., Ise, S., & Suvaila, R. (2025). Gamma-Ray Analysis of Reed Samples from the Danube Delta. Sensors, 25(11), 3257. https://doi.org/10.3390/s25113257







