Evaluating the Clinical Utility of Robotic Systems in Plastic and Reconstructive Surgery: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Risk of Bias
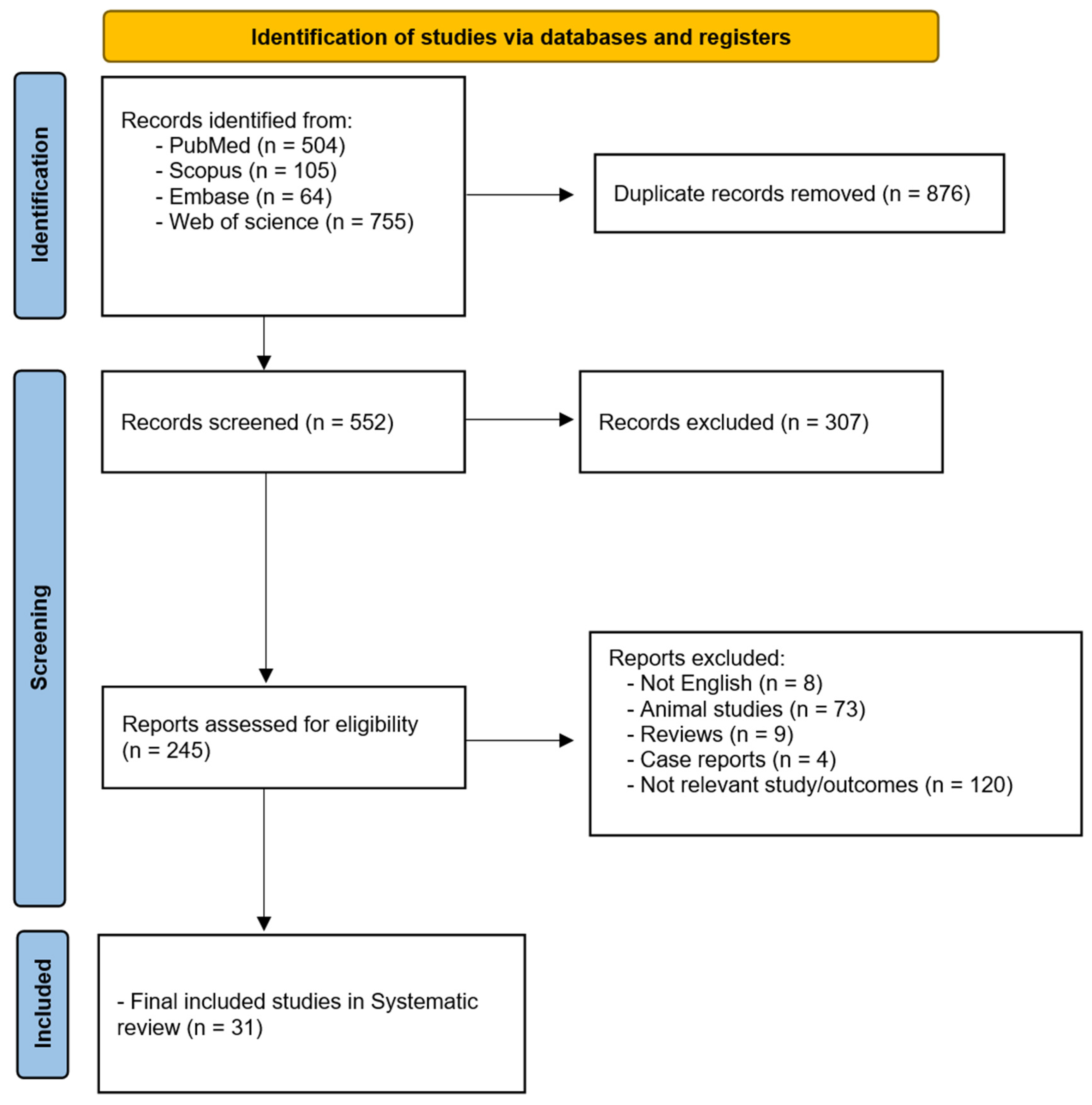
3. Results
Literature Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandra, R.; Agarwal, R.; Agarwal, D. Redefining Plastic Surgery. Plast. Reconstr. Surg. Glob. Open 2016, 4, e706. [Google Scholar] [CrossRef] [PubMed]
- Gorgy, A.; Xu, H.H.; Hawary, H.E.; Nepon, H.; Lee, J.; Vorstenbosch, J. Integrating AI into Breast Reconstruction Surgery: Exploring Opportunities, Applications, and Challenges. Plast. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Graziano, F.D.; Lu, J.; Sbitany, H.; Levin, L.S.; Taylor, G.I.; Taub, P.J.; Henderson, P.W. Fifty years of free tissue transfer: The past, present and future of microsurgical reconstruction. Anz. J. Surg. 2025, 95, E123–E130. [Google Scholar] [CrossRef]
- Rusch, M.; Hoffmann, G.; Wieker, H.; Bürger, M.; Kapahnke, S.; Berndt, R.; Rusch, R. Evaluation of the MMI Symani® robotic microsurgical system for coronary-bypass anastomoses in a cadaveric porcine model. J. Robot. Surg. 2024, 18, 168. [Google Scholar] [CrossRef]
- Menichini, G.; Malzone, G.; Tamburello, S.; Andreoli, A.L.; Mori, F.; Ballestín, A.; Shiraki, T. Safety and efficacy of Symani robotic-assisted microsurgery: Assessment of vascular anastomosis patency, thrombus, and stenosis in a randomized preclinical study. J. Plast. Reconstr. Aesthet. Surg. 2024, 96, 1–10. [Google Scholar] [CrossRef]
- Cho, J.; Kim, D.; Kim, T.; Pak, C.J.; Suh, H.P.; Hong, J.P. Further validating the robotic microsurgery platform through preclinical studies on rat femoral artery and vein. J. Reconstr. Microsurg. 2024. [Google Scholar] [CrossRef]
- Wessel, K.J.; Dahmann, S.; Kueckelhaus, M. Expanding Applications and Future of Robotic Microsurgery. J. Craniofac. Surg. 2025, 36, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, R.B.A.; Chakari, W.; Thomsen, J.B.; Sørensen, J.A. Evaluation of microsurgical free flap procedures utilizing the Symani Surgical System: A preliminary analysis. J. Plast. Reconstr. Aesthet. Surg. 2025, 102, 66–70. [Google Scholar] [CrossRef]
- Lin, L.; Xu, C.; Shi, Y.; Zhou, C.; Zhu, M.; Chai, G.; Xie, L. Preliminary clinical experience of robot-assisted surgery in treatment with genioplasty. Sci. Rep. 2021, 11, 6365. [Google Scholar] [CrossRef]
- Chung, J.H.; You, H.J.; Kim, H.S.; Lee, B.I.; Park, S.H.; Yoon, E.S. A novel technique for robot assisted latissimus dorsi flap harvest. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 966–972. [Google Scholar] [CrossRef]
- Nadjmi, N. Transoral robotic cleft palate surgery. Cleft Palate Craniofac. J. 2016, 53, 326–331. [Google Scholar] [CrossRef]
- Arora, R.; Verma, V.K.; Mishra, K.S.; Bhoye, H.; Kapoor, R. Reconstruction with free flaps in robotic head-and-neck onco-surgeries. Indian J. Plast. Surg. 2018, 51, 283–289. [Google Scholar] [CrossRef]
- Lai, H.W.; Chen, S.T.; Lin, S.L.; Chen, C.J.; Lin, Y.L.; Pai, S.H.; Chen, D.R.; Kuo, S.J. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: Technique, preliminary results and patient-reported cosmetic outcome. Ann. Surg. Oncol. 2019, 26, 42–52. [Google Scholar] [CrossRef]
- Ahn, S.J.; Song, S.Y.; Park, H.S.; Park, S.H.; Lew, D.H.; Roh, T.S.; Lee, D.W. Early experiences with robot-assisted prosthetic breast reconstruction. Arch. Plast. Surg. 2019, 46, 79–83. [Google Scholar] [CrossRef]
- Moon, K.C.; Yeo, H.D.; Yoon, E.S.; Lee, B.I.; Park, S.H.; Chung, J.H.; Lee, H.C. Robotic-assisted latissimus dorsi muscle flap for autologous chest reconstruction in Poland syndrome. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1506–1513. [Google Scholar] [CrossRef]
- Jeon, D.N.; Kim, J.; Ko, B.S.; Lee, S.B.; Kim, E.K.; Eom, J.S.; Han, H.H. Robot-assisted breast reconstruction using the prepectoral anterior tenting method. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2906–2915. [Google Scholar] [CrossRef]
- Novo, J.; Seth, I.; Mon, Y.; Soni, A.; Elkington, O.; Marcaccini, G.; Rozen, W.M. Use of Robotic Surgery in Plastic and Reconstructive Surgery: A Narrative Review. Biomimetics 2025, 10, 97. [Google Scholar] [CrossRef]
- Kanayama, K.; Kato, H.; Mori, M.; Sakae, Y.; Okazaki, M. Robotically assisted recipient site preparation in hair restoration surgery: Surgical safety and clinical outcomes in 31 consecutive patients. Dermatol. Surg. 2021, 47, 1365–1370. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Chung, J.H.; Lee, H.C.; Park, S.H.; Yoon, E.S. Single-port transaxillary robot-assisted latissimus dorsi muscle flap reconstruction for Poland syndrome: Concomitant application of robotic system to contralateral augmentation mammoplasty. Arch. Plast. Surg. 2022, 49, 373–377. [Google Scholar] [CrossRef]
- Wittesaele, W.; Vandevoort, M. Implementing the robotic deep inferior epigastric perforator flap in daily practice: A series of 10 cases. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 2577–2583. [Google Scholar] [CrossRef]
- Weinzierl, A.; Barbon, C.; Gousopoulos, E.; von Reibnitz, D.; Giovanoli, P.; Grünherz, L.; Lindenblatt, N. Benefits of robotic-assisted lymphatic microsurgery in deep anatomical planes. JPRAS Open 2023, 37, 145–154. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Kim, B.-S.; Kuo, W.-L.; Liu, K.-H.; Chang, T.N.-J.; Cheong, D.C.-F.; Huang, J.-J. Novel port placement in robot-assisted DIEP flap harvest improves visibility and bilateral DIEP access: Early controlled cohort study. Plast. Reconst. Surg. 2023, 152, 590e–595e. [Google Scholar] [CrossRef]
- Aman, M.; Struebing, F.; Weigel, J.; Bigdeli, A.K.; Gazyakan, E.; Kneser, U.; Harhaus, L.; Boecker, A.H. Technical strategies and learning curve in robotic-assisted peripheral nerve surgery. Plast. Reconstr. Surg. Glob. Open 2024, 12, e6221. [Google Scholar] [CrossRef]
- Dastagir, N.; Obed, D.; Tamulevicius, M.; Dastagir, K.; Vogt, P.M. The use of the Symani Surgical System® in emergency hand trauma care. Surg. Innov. 2024, 31, 460–465. [Google Scholar] [CrossRef]
- Tolksdorf, K.; Hohberger, F.S.; Ernst, C.; Tietz, S.; Schultze-Mosgau, S.; Tautenhahn, F. First experience using a novel microsurgical robotic device for free flap surgery in cranio- and maxillofacial surgery. J. Cranio-Maxillofac. Surg. 2024, 52, 704–706. [Google Scholar] [CrossRef]
- Gorji, S.; Wessel, K.; Dermietzel, A.; Aitzetmueller, M.; Wendenburg, I.; Varnava, C.; Klietz, M.L.; Wiebringhaus, P.; Hirsch, T.; Kueckelhaus, M. Fully telemetric robotic microsurgery: Clinical experience wth 23 cases. Microsurgery 2024, 44, e31227. [Google Scholar] [CrossRef]
- Könneker, S.; Watson, J.A.; Weinzierl, A.; von Reibniz, D.; Besmens, I.; Kim, B.S.; Giovanoli, P.; Lindenblatt, N. Advances in reconstructive robotic microsurgery in the extremity. J. Craniofac. Surg. 2024, 36, 354–357. [Google Scholar] [CrossRef]
- Farr, D.E.; Haddock, N.T.; Tellez, J.; Radi, I.; Alterio, R.; Sayers, B.; Zeh, H., 3rd. Safety and feasibility of single-port robotic-assisted nipple-sparing mastectomy. JAMA Surg. 2024, 159, 269–276. [Google Scholar] [CrossRef]
- Oh, S.M.; Han, W.Y.; Eom, J.S.; Kim, E.K.; Han, H.H. Robot-assisted capsulectomy with immediate reimplantation in breast reconstruction. Plast. Reconstr. Surg. 2024, 153, 523e–526e. [Google Scholar] [CrossRef]
- Kim, H.B.; Min, J.C.; Lee, S.B.; Kim, J.; Ko, B.S.; Kim, H.J.; Son, B.H.; Han, H.H.; Eom, J.S. Conventional versus robot-assisted immediate breast reconstruction: Reconstructive outcome and patient-reported outcome measures. Plast. Reconstr. Surg. 2024, 154, 3S–12S. [Google Scholar] [CrossRef]
- Wong, A.W.; Kuo, W.L.; Cheong, D.C.; Tsai, H.P.; Kao, S.W.; Chen, C.F.; Huang, J.J. Six steps for a successful aesthetic free flap reconstruction after minimally invasive mastectomy: A retrospective case-control study. Int. J. Surg. 2024, 110, 645–653. [Google Scholar] [CrossRef]
- von Reibnitz, D.; Weinzierl, A.; Barbon, C.; Gutschow, C.A.; Giovanoli, P.; Grünherz, L.; Lindenblatt, N. 100 anastomoses: A two-year single-center experience with robotic-assisted micro- and supermicrosurgery for lymphatic reconstruction. J. Robot. Surg. 2024, 18, 164. [Google Scholar] [CrossRef]
- Jung, S.M.; Kim, Y.J.; Lee, K.T.; Jeon, B.J.; Mun, G.H.; Pyon, J.K.; Ryu, J.M. Learning curve for robot-assisted nipple-sparing mastectomy: A single institution experience. Eur. J. Surg. Oncol. 2024, 50, 108602. [Google Scholar] [CrossRef]
- Lindenblatt, N.; Grunherz, L.; Wang, A.; Gousopoulos, E.; Barbon, C.; Uyulmaz, S.; Giovanoli, P. Early Experience Using a New Robotic Microsurgical System for Lymphatic Surgery. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4013. [Google Scholar] [CrossRef]
- Beier, J.P.; Hackenberg, S.; Boos, A.M.; Modabber, A.; Duong Dinh, T.A.; Holzle, F. First Series of Free Flap Reconstruction Using a Dedicated Robotic System in a Multidisciplinary Microsurgical Center. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5240. [Google Scholar] [CrossRef]
- Besmens, I.S.; Politikou, O.; Giovanoli, P.; Calcagni, M.; Lindenblatt, N. Robotic Microsurgery in Extremity Reconstruction—Experience With a Novel Robotic System. Surg. Innov. 2024, 31, 42–47. [Google Scholar] [CrossRef]
- Struebing, F.; Bigdeli, A.; Weigel, J.; Gazyakan, E.; Vollbach, F.; Panayi, A.C.; Vogelpohl, J.; Boecker, A.; Kneser, U. Robot-assisted Microsurgery: Lessons Learned from 50 Consecutive Cases. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5685. [Google Scholar] [CrossRef]
- Grunherz, L.; Weinzierl, A.; Gutschow, C.A.; Puippe, G.D.; Gnannt, R.; von Reibnitz, D.; Gousopoulos, E.; Barbon, C.; Giovanoli, P.; Pieper, C.C.; et al. Robotic-assisted Lymphovenous Anastomosis of the Central Lymphatic System. Plast. Reconstr. Surg. Glob. Open 2024, 12, e6164. [Google Scholar] [CrossRef]
- Barbon, C.; Grünherz, L.; Uyulmaz, S.; Giovanoli, P.; Lindenblatt, N. Exploring the learning curve of a new robotic microsurgical system for microsurgery. JPRAS Open 2022, 34, 126–133. [Google Scholar] [CrossRef]
- Savastano, A.; Rizzo, S. A Novel Microsurgical Robot: Preliminary Feasibility Test in Ophthalmic Field. Transl. Vis. Sci. Technol. 2022, 11, 13. [Google Scholar] [CrossRef]
- Frost, S.J.; Mawad, D.; Higgins, M.J.; Ruprai, H.; Kuchel, R.; Tilley, R.D.; Myers, S.; Hook, J.M.; Lauto, A. Gecko-inspired chitosan adhesive for tissue repair. NPG Asia Mater. 2016, 8, e280. [Google Scholar] [CrossRef]
- Seth, I.; Lim, B.; Xie, Y.; Cevik, J.; Rozen, W.M.; Ross, R.J.; Lee, M. Comparing the efficacy of large language models ChatGPT, BARD, and Bing AI in providing information on rhinoplasty: An observational study. Aesthetic Surg. J. Open Forum 2023, 5, ojad084. [Google Scholar] [CrossRef]
- Marcaccini, G.; Seth, I.; Cuomo, R. Letter on: “Artificial Intelligence: Enhancing Scientific Presentations in Aesthetic Surgery”. Aesthetic Plast Surg. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Seth, I.; Bulloch, G.; Joseph, K.; Hunter-Smith, D.J.; Rozen, W.M. Use of artificial intelligence in the advancement of breast surgery and implications for breast reconstruction: A narrative review. J. Clin. Med. 2023, 12, 5143. [Google Scholar] [CrossRef] [PubMed]
- Seth, I.; Lim, B.; Lu, P.Y.; Xie, Y.; Cuomo, R.; Ng, S.K.; Rozen, W.M.; Sofiadellis, F. Digital twins use in plastic surgery: A systematic review. J. Clin. Med. 2024, 13, 7861. [Google Scholar] [CrossRef]
- Seth, I.; Gibson, D.; Bulloch, G.; Joseph, K.; Cevik, J.; Qin, K.R.; Shahbaz, S.; Rozen, W.M. Vasovasostomy: A systematic review and meta-analysis comparing macroscopic, microsurgical, and robot-assisted microsurgical techniques. Andrology 2024, 12, 740–767. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Study Design | Type of Plastic Surgery | Robotic System Used | Advantages | Surgical Outcomes | Limitations and Challenges |
|---|---|---|---|---|---|---|
| Lai et al., 2019 [9] | Case series | Head and neck: free flap reconstruction for oropharyngeal cancer | da Vinci Surgical System | High-definition 3D visualization; tremor elimination | 100% flap survival rate; precise suturing of vessels as small as 2.1 mm; no major complications reported | Lack of tactile feedback; long setup and operating times; high costs |
| Chung et al., 2015 [10] | Non-randomized experimental study | Breast reconstruction: RA latissimus dorsi muscle flap | da Vinci Surgical System | Flexible joints; motion scaling; tremor control | High patient satisfaction rates for aesthetics (mean scores: 9.2 to 9.9/10) and no donor site complications; improved aesthetics with hidden scars; docking time and operating time improved with experience | Difficulty dissecting anterior border due to simultaneous robot arm overlap and confined working space; large workspace required; increased time due to retractor adjustments; steep learning curve |
| Nadjmi 2016 [11] | Case series | Head and neck: cleft palate reconstruction | da Vinci Surgical System | 3D endoscopic vision; tremor filtration; EndoWrist simulating hand-like movements | Improved dexterity and precision in intraoral suturing; shortened hospital stays (1 vs. 2.4 ± 1.3 days); normal swallowing restored on surgery day | Lack of tactile feedback; longer operative times (122 ± 8 min vs. 87 ± 6 min); high costs; steep learning curve |
| Arora et al., 2018 [12] | Case series | Head and neck: robotic resection of the tumor with free flap reconstruction | da Vinci Si Surgical System | Flexible robotic arms; high-definition 3D binocular vision; tremor elimination | 100% flap survival; satisfactory functional and aesthetic outcomes; no postoperative complications; reduced surgical morbidity | Difficulty reconstructing flaps in narrow areas; limited access to recipient vessels; steep learning curve; longer docking/setup times |
| Lai et al., 2019 [13] | Case series | Breast reconstruction: RANSM with breast reconstruction | da Vinci Si Surgical Robot | 3D imaging providing enhanced spatial precision; flexible robotic arms for fine dissection | 87% of patients were graded as “excellent” for cosmetic outcomes; no total nipple-areolar complex necrosis was observed | Longer operative times; high costs |
| Ahn et al., 2019 [14] | Case series | Breast reconstruction: RANSM with immediate breast reconstruction | da Vinci Xi Surgical System | Flexible robotic arms; 3D magnified imaging | High patient satisfaction scores (BREAST-Q); invisible scars in most cases; no significant complications reported | High costs and initial long operative times; securing workspace was challenging |
| Moon et al., 2020 [15] | Case series | Chest reconstruction: RA latissimus dorsi muscle flap surgery for Poland Syndrome | da Vinci Surgical System | 3D camera for a magnified view; robotic arms mimicking hand movements | High patient satisfaction for aesthetics and chest symmetry; inconspicuous scars achieved; no serious complications or flap loss reported | Lack of control group; high cost associated with robotic system purchase and maintenance; insufficient follow-up; prolonged operative time; potential bias in scar assessment |
| Jeon et al., 2021 [16] | Cohort study | Breast reconstruction: RA immediate prosthetic breast reconstruction | da Vinci Xi Surgical System | High-definition 3D imaging; articulated and flexible arms with precision motion; tremor reduction | Mean operative time: 194.7 min (oncology team), 80.8 min (plastic surgery team); minor complications (6% seroma, 6% superficial skin necrosis); small incisions (4.5 cm); enhanced visibility; precise tissue handling with minimal scarring | High costs; small patient cohort; short follow-up period; limited long-term outcomes or comparison with conventional techniques |
| Lin et al., 2021 [17] | Scientific report | Head and neck: genioplasty | Craniofacial-Plastic Surgical Robot (CPSR-I) system | Force feedback mechanism with automated drilling | Accurate osteotomy lines achieved high patient satisfaction without the need for additional surgeries | Heavy mechanical structure; insufficient navigation system for depth perception |
| Kanayama et al., 2021 [18] | Case series | Aesthetic: robotic recipient site preparation in hair transplantation | ARTAS Robotic System | Intelligent algorithms for hair identification; stereoscopic imaging | Efficient site creation with minimal complications; high patient and surgeon satisfaction ratings | Limited to the frontal scalp and requires preoperative hair trimming; familiarity affects speed initially |
| Hwang et al., 2022 [19] | Case series | Chest reconstruction: RA latissimus dorsi muscle flap surgery for Poland Syndrome | da Vinci SP Surgical System | Flexible robotic arms; 3D imaging; gas insufflation | Mean operative time: 449 min; no perioperative complications; superior scar aesthetics; enhanced operator ergonomics | Longer setup time for robotic docking compared to manual methods; high cost of robotic equipment; steep learning curve |
| Wittesaele et al., 2022 [20] | Case series | Breast reconstruction: RA DIEP flap | da Vinci Xi Surgical System (Multiport) | Tremor elimination, motion scaling; 3D imaging | No flap losses or conversions; reduced fascial disruption; successful flap survival with minimal complications | Longer operative times due to the learning curve, limited patient selection, high equipment costs |
| Weinzierl et al., 2023 [21] | Case series | Microsurgery: lymphatic microsurgery, including VLNT and LVA | Symani Surgical System with conventional or 3D exoscope | Motion scaling; tremor elimination; 3D depth perception | Precise anastomoses (mean time: 22.6 min); 25.2% limb volume reduction at 3 months; no complications | High cost; steep learning curve; fixed robotic angles required longer incisions in deep spaces |
| Tsai et al., 2023 [22] | Cohort study | Breast reconstruction: RA DIEP flap harvest | da Vinci Surgical System | Articulated robotic arms; high-definition 3D imaging | Reduced anterior rectus sheath incision length (RA 2.67 ± 1.13 cm vs. conventional 8.14 ± 1.69 cm); no flap loss; comparable pain scores; bilateral DIEP access achieved without robotic repositioning | RA procedure requires extra time (~100 min); costly disposables of approximately USD 3500; initial adaptation to post-placement technique |
| Aman et al., 2024 [23] | Cohort study | Hand surgery: RA peripheral nerve surgery | Symani Surgical System | Tremor reduction; precise nerve coaptations | Coaptation time averaged 23 min; 100% patency achieved; some complications like hematoma were reported | High cost; technical logistics; inferior grip strength of instruments; steep learning curve |
| Dastagir et al., 2024 [24] | Case series | Hand surgery: microsurgical anastomosis | Symani Surgical System | NanoWrist instruments; motion scaling; tremor reduction | Patency rate of 100%; significant ergonomic benefits; anastomosis time reduced by 30% with experience | Steep learning curve; equipment positioning; high cost of devices and training |
| Tolksdorf et al., 2024 [25] | Case series | Craniofacial surgery: free flap surgery with robotic-assisted and conventional anastomosis | Symani Surgical System with Orbeye exoscope for 3D magnification | NanoWrists; 7 degrees of freedom; motion scaling; tremor filtering | Anastomosis times longer with robotic methods; flap survival comparable to manual methods; minor complications reported | Long learning curve; technical issues (arm collisions, software errors); inadequate grip strength for thin sutures |
| Gorji et al., 2024 [26] | Case series | Breast, trauma, and head and neck surgery: free flap reconstruction | Symani Surgical System and RoboticScope | Motion scaling, telemetric control, orbital view adjustment | 95.7% flap survival; ischemia time 100.6 min; end-to-side anastomoses performed efficiently | High costs; increased surgical time; intraoperative ventilation issues; robot setup complexity |
| Könnecker et al., 2024 [27] | Case series | Limb reconstruction: free tissue transfer with microsurgical anastomosis for extremity reconstruction | Symani Surgical System | Motion scaling, tremor filtering, NanoWrists, ergonomic telemanipulators | Successful flap survival; mean anastomosis time 33.2 min; no vascular complications | Small sample size; lack of comparative manual data; challenging grip strength for delicate movements |
| Farr et al., 2024 [28] | Case series | Breast reconstruction: RANSM | da Vinci SP Robotic Surgical System | Flexible 3D camera, robotic arms with improved motion range | Median operative time was 277 min; learning curve improvement was observed with time; minor complications observed | Limited to a single surgeon; insufficient generalizability; technical ease dependent on breast size |
| Oh et al., 2024 [29] | Case series | Breast surgery: RA capsulectomy | da Vinci SP Robotic Surgical System | Curved scissors; bipolar forceps; 3D magnified imaging | Enhanced precision in capsulectomy; clear visualization in confined spaces | Longer operative times than conventional methods; high costs associated with robotic setup |
| Kim et al., 2024 [30] | Cohort study | Breast reconstruction: immediate breast reconstruction following mastectomy (implant-based and autologous DIEP flap reconstruction) | da Vinci SP Robotic Surgical System | Articulated robotic arms; motion scaling; tremor reduction | Fewer complications (skin necrosis 2% robotic vs. 8% conventional); improved sexual well-being scores | Longer operative times for robotic procedures; high costs; steep learning curve for surgeons |
| Wong et al., 2024 [31] | Case-control study | Breast reconstruction: free flap reconstruction after endoscopic or robotic mastectomy | da Vinci Xi Surgical System | Flexible robotic arms mimicking human wrist movements; 3D imaging and tremor reduction | Scar placement was aesthetically superior in the robotic group; 70.7% of scars were hidden laterally compared to 70.7% in the conventional group | Aesthetic revision rates similar to conventional mastectomy; no direct aesthetic advantage with da Vinci Xi apart from scar position |
| von Reibnitz et al., 2024 [32] | Case series | Microsurgery: RA lymphatic reconstruction with LTT and/or LVA or LLA | Symani Surgical System | Motion scaling; tremor reduction; 7 degrees of freedom | Limb volume reduction of 1–10% (80 to 1250 mL); wound healing complications in 6.4% of cases; no need for surgical assistant | Lack of direct comparison with manual methods; inability to monitor long-term flap survival; single-center study |
| Jung et al., 2024 [33] | Cohort study | Breast reconstruction: RANSM with immediate breast reconstruction | da Vinci Xi Surgical System | Not specified | Significant improvements in operation times after 21 cases; mean docking time dropped from 8.2 to 5 min; minimal complications | Single-surgeon study; technical difficulties with the multiport systems, such as arm collisions and camera blind spots; limited tactile sensation was reported compared to conventional NSM |
| Lindenblatt et al., 2022 [34] | Case series | Lymphatic reconstruction | Symani Surgical System | Flexible robotic arms mimicking human wrist movements | 100% patency; no complications | Small sample size; no follow up |
| Bier et al., 2023 [35] | Case series | Free flap reconstruction | Symani Surgical System | Motion scaling; tremor reduction; 7 degrees of freedom | Mean end-to-end arterial anastomosis time of 69 min; mean end-to-side arterial anastomosis time of 50 min; mean venous anastomosis time of 94 min; 4.3% flap loss rate; 21.7% revision rate. | Small sample size; no follow up |
| Besmens et al., 2023 [36] | Case series | Extremity (limb) free flap reconstruction | Symani Surgical System | Motion scaling; tremor reduction; 7 degrees of freedom | 41.7 min mean end-to-end arterial anastomosis time; 29 min end-to-side arterial anastomosis; 22 min mean epineural coaptation time; 100% flap survival; 100% patency. | Small sample size; no follow up |
| Struebing et al., 2024 [37] | Case series | Free flap reconstruction, nerve reconstruction, lymphatic reconstruction | Symani Surgical System | Motion scaling; tremor reduction; 7 degrees of freedom | No statistically significant correlation between anastomosis time and case number; 31.0 ± 20.7 min end-to-end arterial anastomosis time; 37.4 ± 13.9 min end-to-side arterial anastomosis time; 23.8 ± 9.2 min venous anastomosis time; 28 ± 9.2 min LVA time; 16.5 ± 9.5 min epineural coaptation time; 2% partial flap loss rate; 2% revision rate. | No follow up |
| Grunherz et al. 2024 [38] | Case series | Central lymphatic reconstruction | Symani Surgical System | Motion scaling; tremor reduction; 7 degrees of freedom | Three-quarters of patients required further surgery or experienced symptoms within 7 months;1 patient asymptomatic at 11-month review | Small sample size; no follow up |
| Konneker et al., 2024 [39] | Case series | Free flap reconstruction | Symani Surgical System | Motion scaling; tremor reduction; 7 degrees of freedom | 33.2 ± 5.8 min arterial anastomosis time; 100% flap survival; 12.5% revision rate | Small sample size; no follow up |
| Study | Focus | Study Design | Tool Used | Quality Assessment |
|---|---|---|---|---|
| Lai et al., 2019 [9] | Free flap for oropharyngeal cancer | Case series | CARE Checklist | Moderate quality |
| Chung et al., 2015 [10] | Breast reconstruction | Non-randomized experimental study | NOS | Moderate quality |
| Nadjmi 2016 [11] | Cleft palate reconstruction | Case series | CARE Checklist | Moderate quality |
| Arora et al., 2018 [12] | Tumor resection + free flap | Case series | CARE Checklist | Moderate quality |
| Lai et al., 2019 [13] | RANSM + breast reconstruction | Case series | CARE Checklist | Moderate quality |
| Ahn et al., 2019 [14] | RANSM + immediate breast reconstruction | Case series | CARE Checklist | Moderate quality |
| Moon et al., 2020 [15] | Chest reconstruction for Poland Syndrome | Case series | CARE Checklist | Moderate quality |
| Jeon et al., 2021 [16] | RA prosthetic breast reconstruction | Cohort study | NOS | Moderate quality |
| Lin et al., 2021 [17] | Genioplasty | Scientific report | Not applicable | Moderate quality |
| Kanayama et al., 2021 [18] | Hair transplantation | Case series | CARE Checklist | Moderate quality |
| Hwang et al., 2022 [19] | Chest reconstruction for Poland Syndrome | Case series | CARE Checklist | Moderate quality |
| Wittesaele et al., 2022 [20] | RA DIEP flap | Case series | CARE Checklist | Moderate quality |
| Weinzierl et al., 2023 [21] | Lymphatic microsurgery | Case series | CARE Checklist | Moderate quality |
| Tsai et al., 2023 [22] | RA DIEP flap harvest | Cohort study | NOS | Moderate quality |
| Aman et al., 2024 [23] | Peripheral nerve surgery | Cohort study | NOS | Moderate quality |
| Dastagir et al., 2024 [24] | Microsurgical anastomosis | Case series | CARE Checklist | Moderate quality |
| Tolksdorf et al., 2024 [25] | Free flap surgery (craniofacial) | Case series | CARE Checklist | Moderate quality |
| Gorji et al., 2024 [26] | Free flap reconstruction | Case series | CARE Checklist | Moderate quality |
| Könnecker et al., 2024 [27] | Limb reconstruction | Case series | CARE Checklist | Moderate quality |
| Farr et al., 2024 [28] | RANSM breast reconstruction | Case series | CARE Checklist | Moderate quality |
| Oh et al., 2024 [29] | Capsulectomy | Case series | CARE Checklist | Moderate quality |
| Kim et al., 2024 [30] | Immediate breast reconstruction | Cohort study | NOS | Moderate quality |
| Wong et al., 2024 [31] | Free flap after mastectomy | Case-control study | NOS | Moderate quality |
| von Reibnitz et al., 2024 [32] | Lymphatic reconstruction | Case series | CARE Checklist | Moderate quality |
| Jung et al., 2024 [33] | RANSM with breast reconstruction | Cohort study | NOS | Moderate quality |
| Lindenblatt et al., 2022 [34] | Lymphatic reconstruction | Case series | GRADE | Moderate quality |
| Bier et al., 2023 [35] | Free flap reconstruction | Case series | CARE Checklist | Moderate quality |
| Besmens et al., 2023 [36] | Extremity reconstruction | Case series | CARE Checklist | Moderate quality |
| Struebing et al., 2024 [37] | Flap, nerve, lymphatic recon. | Case series | CARE Checklist | Moderate quality |
| Grünherz et al., 2024 [38] | Central lymphatic reconstruction | Case series | CARE Checklist | Moderate quality |
| Konneker et al., 2024 [39] | Free flap reconstruction | Case series | CARE Checklist | Moderate quality |
| Robotic System | Advantages | Disadvantages |
|---|---|---|
| Da Vinci Surgical System |
|
|
| Symani Surgical System |
|
|
| ARTAS Robotic System |
|
|
| Craniofacial Plastic Surgical Robot (CPSR-I) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seth, I.; Lim, K.; Chang, E.; Rozen, W.M.; Ng, S.K.-H. Evaluating the Clinical Utility of Robotic Systems in Plastic and Reconstructive Surgery: A Systematic Review. Sensors 2025, 25, 3238. https://doi.org/10.3390/s25103238
Seth I, Lim K, Chang E, Rozen WM, Ng SK-H. Evaluating the Clinical Utility of Robotic Systems in Plastic and Reconstructive Surgery: A Systematic Review. Sensors. 2025; 25(10):3238. https://doi.org/10.3390/s25103238
Chicago/Turabian StyleSeth, Ishith, Kaiyang Lim, Edmond Chang, Warren M. Rozen, and Sally Kiu-Huen Ng. 2025. "Evaluating the Clinical Utility of Robotic Systems in Plastic and Reconstructive Surgery: A Systematic Review" Sensors 25, no. 10: 3238. https://doi.org/10.3390/s25103238
APA StyleSeth, I., Lim, K., Chang, E., Rozen, W. M., & Ng, S. K.-H. (2025). Evaluating the Clinical Utility of Robotic Systems in Plastic and Reconstructive Surgery: A Systematic Review. Sensors, 25(10), 3238. https://doi.org/10.3390/s25103238







