Augmented Feedback in Post-Stroke Gait Rehabilitation Derived from Sensor-Based Gait Reports—A Longitudinal Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Description
2.2. Outcome Measures
2.3. Intervention
2.4. Augmented Feedback
2.5. Data Analysis
3. Results
3.1. Outcome Measures of the 10MWT Comfortable Speed
3.2. Outcome Measures of the 10MWT Fast Speed
3.3. Outcome Measures of the 6MWT
3.4. Patient-Reported Questionnaire
4. Discussion
Methodological Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Dalgas, U.; Severinsen, K.; Overgaard, K. Relations between 6 minute walking distance and 10 meter walking speed in patients with multiple sclerosis and stroke. Arch. Phys. Med. Rehabil. 2012, 93, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Ada, L.; Dean, C.M.; Lindley, R.; Lloyd, G. Improving community ambulation after stroke: The AMBULATE Trial. BMC Neurol. 2009, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, H.L.; Brown, S.J.; Price, M.; Butterworth, C.; Groenevelt, R.; Jackson, K.; Walker, L.; Rees, N.; Clayton, A.; Reeves, N.D. Return to Employment After Stroke in Young Adults: How Important Is the Speed and Energy Cost of Walking? Stroke 2019, 50, 3198–3204. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Cirstea, C.M.; Ptito, A.; Levin, M.F. Feedback and cognition in arm motor skill reacquisition after stroke. Stroke 2006, 37, 1237–1242. [Google Scholar] [CrossRef]
- van Dijk, H.; Jannink, M.J.; Hermens, H.J. Effect of augmented feedback on motor function of the affected upper extremity in rehabilitation patients: A systematic review of randomized controlled trials. J. Rehabil. Med. 2005, 37, 202–211. [Google Scholar] [CrossRef]
- Molier, B.I.; Van Asseldonk, E.H.; Hermens, H.J.; Jannink, M.J. Nature, timing, frequency and type of augmented feedback; does it influence motor relearning of the hemiparetic arm after stroke? A systematic review. Disabil. Rehabil. 2010, 32, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.A.; Chevidikunnan, M.F.; Khan, F.R.; Gaowgzeh, R.A. Effectiveness of knowledge of result and knowledge of performance in the learning of a skilled motor activity by healthy young adults. J. Phys. Ther. Sci. 2016, 28, 1482–1486. [Google Scholar] [CrossRef]
- Levin, M.F.; Demers, M. Motor learning in neurological rehabilitation. Disabil. Rehabil. 2021, 43, 3445–3453. [Google Scholar] [CrossRef]
- Subramanian, S.K.; Massie, C.L.; Malcolm, M.P.; Levin, M.F. Does provision of extrinsic feedback result in improved motor learning in the upper limb poststroke? A systematic review of the evidence. Neurorehabil. Neural Repair 2010, 24, 113–124. [Google Scholar] [CrossRef]
- Liu, J.; Drutz, C.; Kumar, R.; McVicar, L.; Weinberger, R.; Brooks, D.; Salbach, N.M. Use of the six-minute walk test poststroke: Is there a practice effect? Arch. Phys. Med. Rehabil. 2008, 89, 1686–1692. [Google Scholar] [CrossRef]
- van Bloemendaal, M.; van de Water, A.T.; van de Port, I.G. Walking tests for stroke survivors: A systematic review of their measurement properties. Disabil. Rehabil. 2012, 34, 2207–2221. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.; Truijen, S.; Umar, N.A.; Useh, U.; Egwuonwu, V.A.; Van Criekinge, T.; Saeys, W. Effects of Lower Limb Constraint Induced Movement Therapy in People With Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 638904. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F.; Kleim, J.A.; Wolf, S.L. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil. Neural Repair 2009, 23, 313–319. [Google Scholar] [CrossRef]
- Nedergård, H.; Schelin, L.; Liebermann, D.G.; Johansson, G.M.; Häger, C.K. Core Sets of Kinematic Variables to Consider for Evaluation of Gait Post-stroke. Front. Hum. Neurosci. 2021, 15, 820104. [Google Scholar] [CrossRef]
- Donno, L.; Monoli, C.; Frigo, C.A.; Galli, M. Forward and Backward Walking: Multifactorial Characterization of Gait Parameters. Sensors 2023, 23, 4671. [Google Scholar] [CrossRef]
- Muro-de-la-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait analysis methods: An overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef] [PubMed]
- Johansson, D.; Malmgren, K.; Alt Murphy, M. Wearable sensors for clinical applications in epilepsy, Parkinson’s disease, and stroke: A mixed-methods systematic review. J. Neurol. 2018, 265, 1740–1752. [Google Scholar] [CrossRef]
- Stock, R.; Mork, P.J. The effect of an intensive exercise programme on leg function in chronic stroke patients: A pilot study with one-year follow-up. Clin. Rehabil. 2009, 23, 790–799. [Google Scholar] [CrossRef]
- Marklund, I.; Sefastsson, A.; Fure, B.; Klässbo, M.; Liv, P.; Stålnacke, B.M.; Hu, X. Lower-extremity constraint-induced movement therapy improved motor function, mobility, and walking after stroke. Eur. J. Phys. Rehabil. Med. 2023, 59, 136–144. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef]
- Nilsson, S.; Ertzgaard, P.; Lundgren, M.; Grip, H. Test-Retest Reliability of Kinematic and Temporal Outcome Measures for Clinical Gait and Stair Walking Tests, Based on Wearable Inertial Sensors. Sensors 2022, 22, 1171. [Google Scholar] [CrossRef] [PubMed]
- Marklund, I.; Klässbo, M. Effects of lower limb intensive mass practice in poststroke patients: Single-subject experimental design with long-term follow-up. Clin. Rehabil. 2006, 20, 568–576. [Google Scholar] [CrossRef]
- Tesio, L.; Rota, V.; Malloggi, C.; Brugliera, L.; Catino, L. Crouch gait can be an effective form of forced-use/no constraint exercise for the paretic lower limb in stroke. Int. J. Rehabil. Res. 2017, 40, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Supiot, A.; Pradon, D.; Do, M.C.; Zory, R.; Roche, N. Minimal detectable change of kinematic and spatiotemporal parameters in patients with chronic stroke across three sessions of gait analysis. Hum. Mov. Sci. 2019, 64, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Pollet, J.; Buraschi, R.; Villafañe, J.H.; Piovanelli, B.; Negrini, S. Gait parameters assessed with inertial measurement unit during 6-minute walk test in people after stroke. Int. J. Rehabil. Res. 2021, 44, 358–363. [Google Scholar] [CrossRef]
- Lewek, M.D.; Sykes, R., 3rd. Minimal Detectable Change for Gait Speed Depends on Baseline Speed in Individuals With Chronic Stroke. J. Neurol. Phys. Ther. 2019, 43, 122–127. [Google Scholar] [CrossRef]
- Flansbjer, U.B.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef]
- Wutzke, C.J.; Mercer, V.S.; Lewek, M.D. Influence of lower extremity sensory function on locomotor adaptation following stroke: A review. Top. Stroke Rehabil. 2013, 20, 233–240. [Google Scholar] [CrossRef]
- Awad, L.; Reisman, D.; Binder-Macleod, S. Distance-Induced Changes in Walking Speed After Stroke: Relationship to Community Walking Activity. J. Neurol. Phys. Ther. 2019, 43, 220–223. [Google Scholar] [CrossRef]
- Sibley, K.M.; Tang, A.; Patterson, K.K.; Brooks, D.; McIlroy, W.E. Changes in spatiotemporal gait variables over time during a test of functional capacity after stroke. J. Neuroeng. Rehabil. 2009, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, S.M.; Morris, D.M.; Taub, E. Constraint-Induced Movement Therapy for Improving Motor Function of the Paretic Lower Extremity After Stroke. Am. J. Phys. Med. Rehabil. 2020, 99, e75–e78. [Google Scholar] [CrossRef]
- Dos Anjos, S.; Morris, D.; Taub, E. Constraint-Induced Movement Therapy for Lower Extremity Function: Describing the LE-CIMT Protocol. Phys. Ther. 2020, 100, 698–707. [Google Scholar] [CrossRef]
- Abdullahi, A.; Aliyu, N.U.; Useh, U.; Abba, M.A.; Akindele, M.O.; Truijen, S.; Saeys, W. Comparing Two Different Modes of Task Practice during Lower Limb Constraint-Induced Movement Therapy in People with Stroke: A Randomized Clinical Trial. Neural Plast. 2021, 2021, 6664058. [Google Scholar] [CrossRef]
- Kobari, T.; Murayama, T.; Matsuzawa, K.; Sakai, K. Effects of a treatment program based on constraint-induced movement therapy for the lower extremities on gait and balance in chronic stroke: A 6-month follow-up pilot study. Int. J. Rehabil. Res. 2023, 46, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Duarte Pereira, N.; Ilha, J.; Dos Anjos, S.M.; Morris, D. Constraint-induced movement therapy for lower extremity use in activities of daily living in people with chronic hemiparesis: Multiple case study. Int. J. Rehabil. Res. 2022, 45, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Kim, Y.; Cha, Y.; In, T.S.; Hur, Y.G.; Chung, Y. Effects of gait training with a cane and an augmented pressure sensor for enhancement of weight bearing over the affected lower limb in patients with stroke: A randomized controlled pilot study. Clin. Rehabil. 2015, 29, 135–142. [Google Scholar] [CrossRef]
- Aruin, A.S.; Rao, N.; Sharma, A.; Chaudhuri, G. Compelled body weight shift approach in rehabilitation of individuals with chronic stroke. Top. Stroke Rehabil. 2012, 19, 556–563. [Google Scholar] [CrossRef]
- Mazzarella, J.; McNally, M.; Richie, D.; Chaudhari, A.M.W.; Buford, J.A.; Pan, X.; Heathcock, J.C. 3D Motion Capture May Detect Spatiotemporal Changes in Pre-Reaching Upper Extremity Movements with and without a Real-Time Constraint Condition in Infants with Perinatal Stroke and Cerebral Palsy: A Longitudinal Case Series. Sensors 2020, 20, 7312. [Google Scholar] [CrossRef]
- Demers, M.; Cain, A.; Bishop, L.; Gunby, T.; Rowe, J.B.; Zondervan, D.K.; Winstein, C.J. Understanding stroke survivors’ preferences regarding wearable sensor feedback on functional movement: A mixed-methods study. J. Neuroeng. Rehabil. 2023, 20, 146. [Google Scholar] [CrossRef]
- Sun, Y.; Song, Z.; Mo, L.; Li, B.; Liang, F.; Yin, M.; Wang, D. IMU-Based quantitative assessment of stroke from gait. Sci. Rep. 2025, 15, 9541. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.M.; O’Brien, E.S.; Kamrud, K.E.; Roberts, S.M.; Rooney, T.A.; Thibodeau, K.P.; Balakrishnan, S.; Gell, N.; Mohapatra, S. Utilization of wearable technology to assess gait and mobility post-stroke: A systematic review. J. Neuroeng. Rehabil. 2021, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.S.D.; Silva, S.T.D.; Cardoso, D.C.R.; Quirino, M.A.F.; Silva, M.H.A.; Gomes, L.A.; Fernandes, J.D.; Oliveira, R.; Fernandes, A.; Ribeiro, T.S. Psychometric properties of wearable technologies to assess post-stroke gait parameters: A systematic review. Gait Posture 2024, 113, 543–552. [Google Scholar] [CrossRef] [PubMed]
- ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef]
- Felius, R.A.W.; Geerars, M.; Bruijn, S.M.; van Dieën, J.H.; Wouda, N.C.; Punt, M. Reliability of IMU-Based Gait Assessment in Clinical Stroke Rehabilitation. Sensors 2022, 22, 908. [Google Scholar] [CrossRef]
- Felius, R.A.W.; Punt, M.; Wouda, N.C.; Geerars, M.; Bruijn, S.M.; Wittink, H.; van Dieën, J.H. Mapping Trajectories of Gait Recovery in Clinical Stroke Rehabilitation. Neurorehabil. Neural Repair 2025, 39, 274–285. [Google Scholar] [CrossRef]
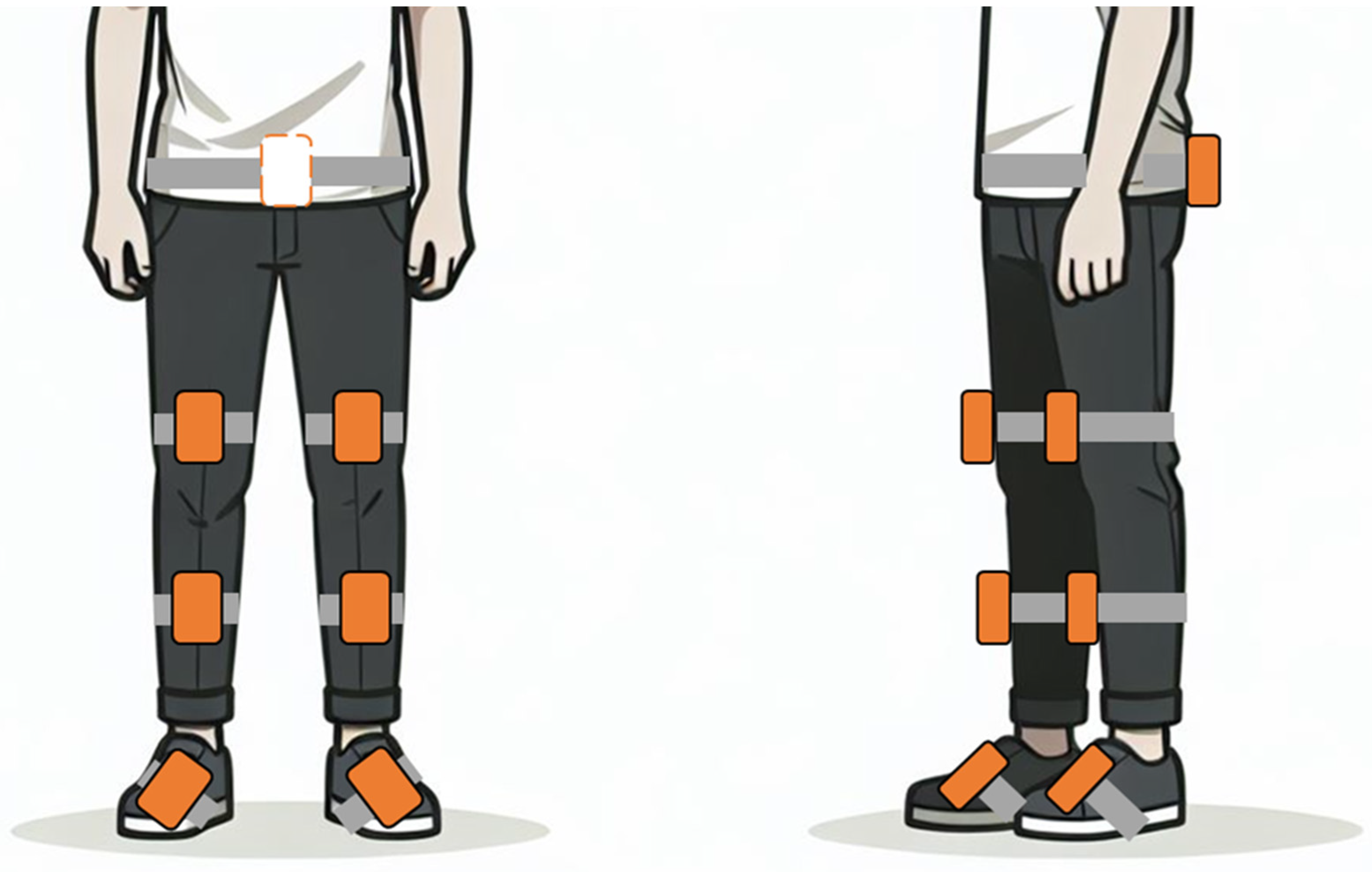
| A | B | C | D | |
|---|---|---|---|---|
| Age (years) | 25 | 57 | 55 | 56 |
| Sex (female/male) | F | F | M | M |
| Affected side (right/left) | L | R | R | R |
| Time since stroke (months) | 14 | 10 | 15 | 12 |
| Type of stroke (ischemic/haemorrhagic) | H | H | I | H |
| Body Mass Index (kg/m2) | 19.6 | 23.7 | 24.6 | 27.5 |
| FMA-LE (scores) | ||||
| Total motor function (maximum 34) | 27 | 25 | 23 | 26 |
| Sensation (maximum 12) | 9 | 12 | 10 | 6 |
| Passive joint motion (maximum 20) | 20 | 20 | 20 | 20 |
| Pain (maximum 20) | 20 | 20 | 20 | 20 |
| Walking aids | No | No | No | Yes |
| 10MWT—Comfortable Speed | 10MWT—Fast Speed | ||||||
|---|---|---|---|---|---|---|---|
| Participants | Occasion | Time (s) | Cadence (steps/m) | Speed (m/s) | Time (s) | Cadence (steps/m) | Speed (m/s) |
| A | Pre | 14.05 | 94.05 | 0.71 | 10.04 | 118.23 | 1.00 |
| Post | 12.48 | 95.15 | 0.80 | 9.15 | 121.9 | 1.09 | |
| 3 months | 11.24 | 104.13 | 0.89 | 8.92 | 117.75 | 1.12 | |
| 6 months | 13.12 | 96.42 | 0.76 | 9.59 | 118.28 | 1.04 | |
| B | Pre | 13.18 | 103.40 | 0.76 | 8.94 | 134.25 | 1.12 |
| Post | 12.30 | 109.11 | 0.81 | 8.04 | 142.91 | 1.24 | |
| 3 months | 10.97 | 103.69 | 0.91 | 7.90 | 129.51 | 1.27 | |
| 6 months | 10.59 | 110.33 | 0.94 | 7.69 | 130.27 | 1.30 | |
| C | Pre | 13.82 | 95.67 | 0.72 | 9.10 | 108.38 | 1.10 |
| Post | 11.82 | 99.34 | 0.85 | 8.76 | 109.46 | 1.14 | |
| 3 months | 10.61 | 100.72 | 0.94 | 7.42 | 112.24 | 1.35 | |
| 6 months | 11.10 | 100.93 | 0.90 | 7.94 | 116.61 | 1.26 | |
| D | Pre | 22.04 | 67.21 | 0.45 | 10.18 | 106.52 | 0.98 |
| Post | 20.65 | 68.73 | 0.48 | 9.24 | 102.22 | 1.08 | |
| 3 months | 19.97 | 67.81 | 0.50 | 9.55 | 108.46 | 1.05 | |
| 6 months | 20.79 | 66.07 | 0.48 | 10.38 | 100.85 | 0.96 | |
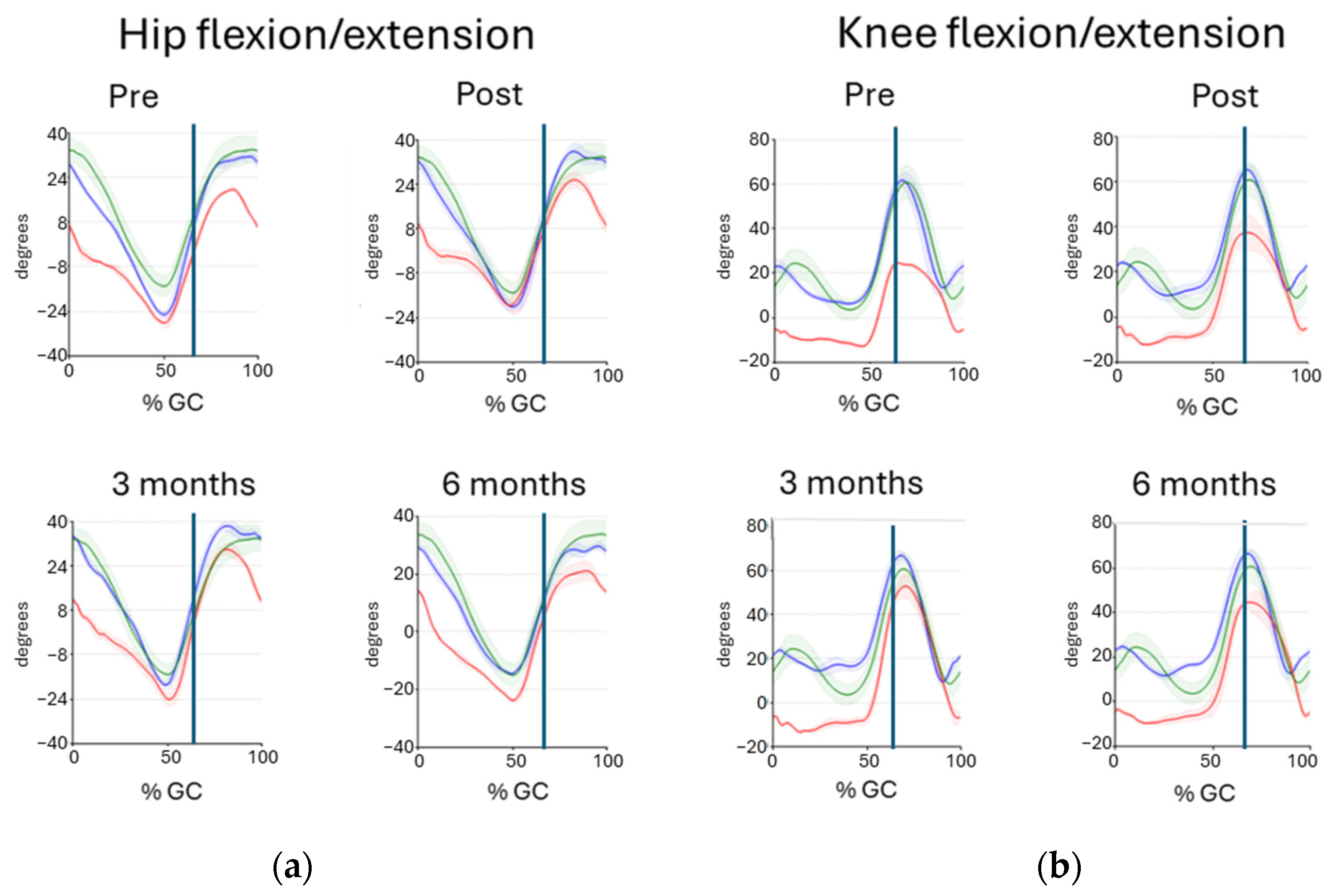
| Hip Joint | Knee Joint | ||||||
|---|---|---|---|---|---|---|---|
| Participants | Occasion | Max Angle ° | Min Angle ° | ROM ° | Max Angle ° | Min Angle ° | ROM ° |
| Reference data | 36.07 (4.46) | −15.35 (3.80) | 51.42 (4.69) | 61.55 (7.47) | 2.45 (4.06) | 59.09 (6.64) | |
| A | Pre | 19.94 | −28.40 | 48.34 | 25.09 | −12.85 | 37.94 |
| Post | 24.65 | −21.41 | 46.06 | 37.71 | −13.82 | 51.53 | |
| 3 months | 30.23 | −24.33 | 54.56 | 53.16 | −13.87 | 67.03 | |
| 6 months | 21.89 | −22.94 | 44.82 | 46.07 | −10.06 | 56.13 | |
| B | Pre | 34.62 | −13.79 | 48.40 | 39.74 | −9.79 | 49.52 |
| Post | 32.74 | −15.70 | 48.45 | 43.09 | −9.56 | 52.65 | |
| 3 months | 38.83 | −19.37 | 58.20 | 48.27 | −14.52 | 62.79 | |
| 6 months | 33.86 | −19.51 | 53.36 | 43.93 | −11.40 | 55.32 | |
| C | Pre | 27.75 | −7.61 | 35.36 | 41.78 | 7.48 | 34.31 |
| Post | 31.88 | −2.93 | 34.81 | 59.43 | 24.54 | 34.89 | |
| 3 months | 38.61 | −9.89 | 48.51 | 45.94 | 17.35 | 28.59 | |
| 6 months | 26.79 | −10.47 | 37.26 | 39.99 | 10.81 | 29.18 | |
| D | Pre | 22.33 | −18.44 | 40.76 | 12.96 | −0.77 | 13.73 |
| Post | 19.51 | −19.86 | 39.37 | 4.71 | −7.59 | 12.30 | |
| 3 months | 28.90 | −12.66 | 41.56 | 14.82 | 0.89 | 13.93 | |
| 6 months | 19.09 | −23.26 | 42.35 | 10.85 | −6.14 | 16.98 | |
| Hip Joint | Knee Joint | ||||||
|---|---|---|---|---|---|---|---|
| Participants | Occasion | Max Angle ° | Min Angle ° | ROM ° | Max Angle ° | Min Angle ° | ROM ° |
| Reference data | 42.94 (5.86) | −17.93 (4.41) | 60.87 (5.41) | 63.99 (8.73) | 0.65 (5.11) | 63.34 (8.18) | |
| A | Pre | 24.41 | −27.12 | 51.53 | 38.42 | −10.26 | 48.68 |
| Post | 26.44 | −21.94 | 48.38 | 41.87 | −17.47 | 59.34 | |
| 3 months | 35.17 | −18.75 | 53.92 | 52.98 | −10.78 | 63.76 | |
| 6 months | 23.48 | −27.07 | 50.55 | 57.94 | −13.73 | 71.67 | |
| B | Pre | 45.20 | −6.30 | 51.49 | 45.85 | −0.24 | 46.09 |
| Post | 54.25 | −0.47 | 54.72 | 50.36 | −2.29 | 52.65 | |
| 3 months | 50.25 | −11.14 | 61.40 | 54.03 | −5.10 | 59.13 | |
| 6 months | 36.68 | −17.89 | 54.57 | 48.09 | −8.75 | 56.83 | |
| C | Pre | 27.36 | −21.50 | 48.86 | 28.05 | −1.97 | 30.02 |
| Post | 35.05 | −8.43 | 43.48 | 44.74 | 15.81 | 28.93 | |
| 3 months | 43.87 | −13.60 | 57.47 | 34.42 | 3.50 | 30.92 | |
| 6 months | 38.25 | −10.51 | 48.76 | 45.83 | 18.99 | 26.84 | |
| D | Pre | 37.52 | −16.28 | 53.80 | 28.05 | 4.98 | 23.07 |
| Post | 29.18 | −26.32 | 55.50 | 17.25 | −4.02 | 21.27 | |
| 3 months | 14.82 | 0.89 | 13.93 | 23.52 | 5.33 | 18.20 | |
| 6 months | 10.85 | −6.14 | 16.98 | 21.62 | −1.01 | 22.62 | |
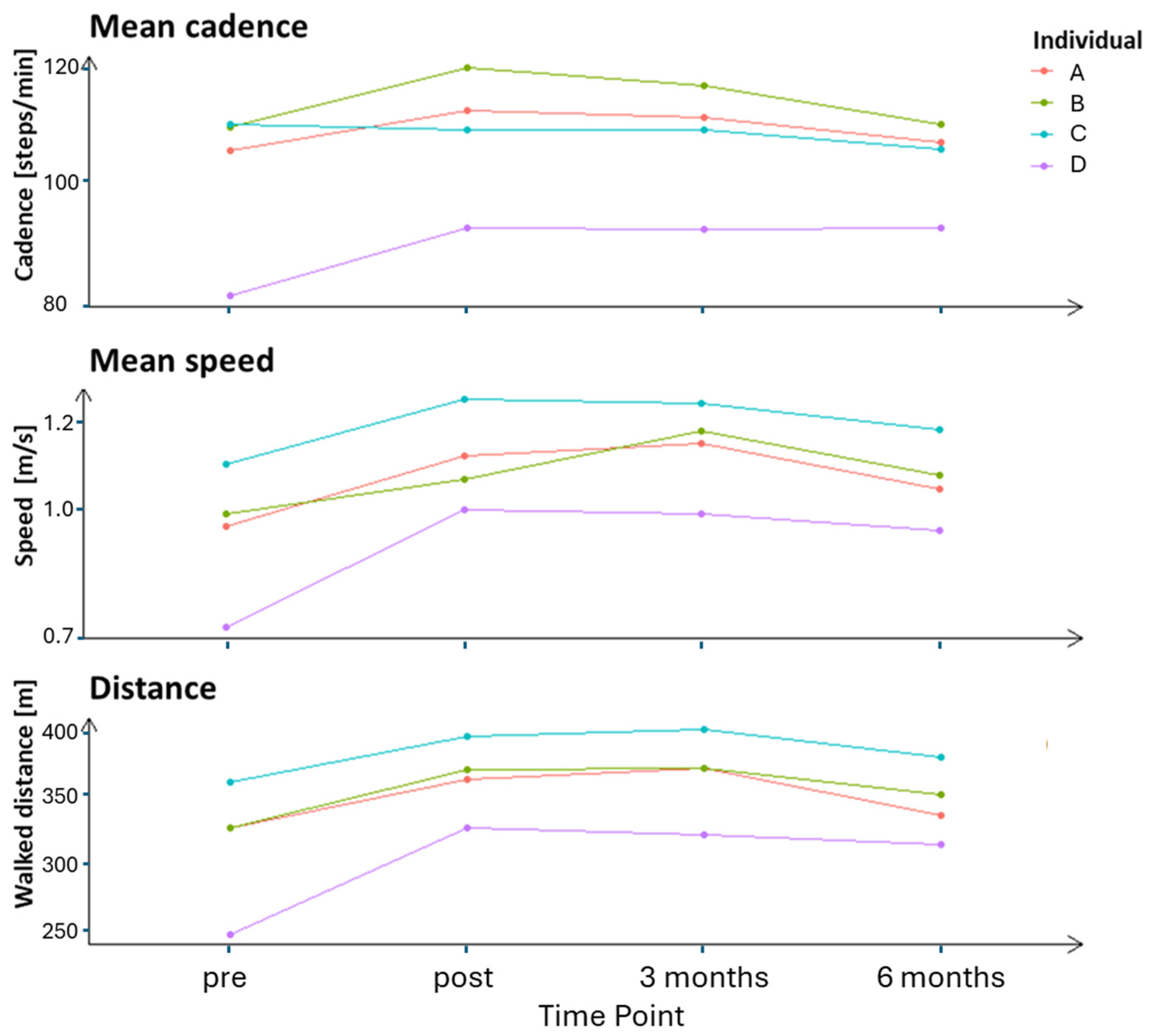
| Participant | Variable | Occasion | T0 | T1 | T2 |
|---|---|---|---|---|---|
| A | Cadence | Pre | 108.55 | 104.93 | 102.41 |
| (steps/min) | Post | 114.26 | 113.40 | 108.67 | |
| 3 months | 112.05 | 110.13 | 108.67 | ||
| 6 months | 109.74 | 105.95 | 104.25 | ||
| Speed | Pre | 0.94 | 0.97 | 0.95 | |
| (m/s) | Post | 1.10 | 1.19 | 1.06 | |
| 3 months | 1.17 | 1.19 | 1.08 | ||
| 6 months | 1.08 | 1.04 | 1.00 | ||
| B | Cadence | Pre | 109.8 | 109.59 | 108.67 |
| (steps/min) | Post | 125.28 | 115.87 | 116.41 | |
| 3 months | 122.89 | 113.89 | 110.13 | ||
| 6 months | 112.05 | 108.70 | 108.24 | ||
| Speed | Pre | 0.95 | 0.96 | 1.04 | |
| (m/s) | Post | 1.01 | 1.12 | 1.05 | |
| 3 months | 1.25 | 1.17 | 1.11 | ||
| 6 months | 1.05 | 1.06 | 1.11 | ||
| C | Cadence | Pre | 109.26 | 109.27 | 110.52 |
| (steps/min) | Post | 112.04 | 104.32 | 109.99 | |
| 3 months | 110.38 | 106.80 | 109.28 | ||
| 6 months | 106.23 | 106.22 | 104.63 | ||
| Speed | Pre | 1.14 | 1.08 | 1.08 | |
| (m/s) | Post | 1.27 | 1.24 | 1.25 | |
| 3 months | 1.22 | 1.20 | 1.30 | ||
| 6 months | 1.14 | 1.21 | 1.19 | ||
| D | Cadence | Pre | 79.29 | 81.55 | 82.32 |
| (steps/min) | Post | 97.61 | 88.24 | 112.40 | |
| 3 months | 98.63 | 91.97 | 90.82 | ||
| 6 months | 93.61 | 93.79 | 89.60 | ||
| Speed | Pre | 0.70 | 0.72 | 0.73 | |
| (m/s) | Post | 1.01 | 0.97 | 0.98 | |
| 3 months | 1.00 | 1.00 | 0.95 | ||
| 6 months | 0.94 | 0.94 | 0.95 |
| Perception Aspects | A | B | C | D |
|---|---|---|---|---|
| Understanding of gait curves a | 5 | 8 | 8 | 9 |
| Interest in gait curves b | 10 | 10 | 10 | 10 |
| Comparison of own results with reference group’s curves b | 10 | 10 | 10 | 8 |
| Following development over time b | 10 | 10 | 10 | 10 |
| Understanding of movement problems c | 10 | 10 | 10 | 9 |
| Understanding of compensatory movements c | 0 | 6 | 9 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johansson, G.M.; Öhberg, F. Augmented Feedback in Post-Stroke Gait Rehabilitation Derived from Sensor-Based Gait Reports—A Longitudinal Case Series. Sensors 2025, 25, 3109. https://doi.org/10.3390/s25103109
Johansson GM, Öhberg F. Augmented Feedback in Post-Stroke Gait Rehabilitation Derived from Sensor-Based Gait Reports—A Longitudinal Case Series. Sensors. 2025; 25(10):3109. https://doi.org/10.3390/s25103109
Chicago/Turabian StyleJohansson, Gudrun M., and Fredrik Öhberg. 2025. "Augmented Feedback in Post-Stroke Gait Rehabilitation Derived from Sensor-Based Gait Reports—A Longitudinal Case Series" Sensors 25, no. 10: 3109. https://doi.org/10.3390/s25103109
APA StyleJohansson, G. M., & Öhberg, F. (2025). Augmented Feedback in Post-Stroke Gait Rehabilitation Derived from Sensor-Based Gait Reports—A Longitudinal Case Series. Sensors, 25(10), 3109. https://doi.org/10.3390/s25103109








