Fourier Transform Infrared (FT-IR) Spectroscopy and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Cotton Fiber Maturity and Crystallinity for Plant Mapping
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Agronomic Fiber Property Measurements with Individual Bolls
2.3. ATR FT-IR Spectral Collection and Data Analysis with the Lint Harvested from Individual Bolls
2.4. Conventional Fiber Property Measurements
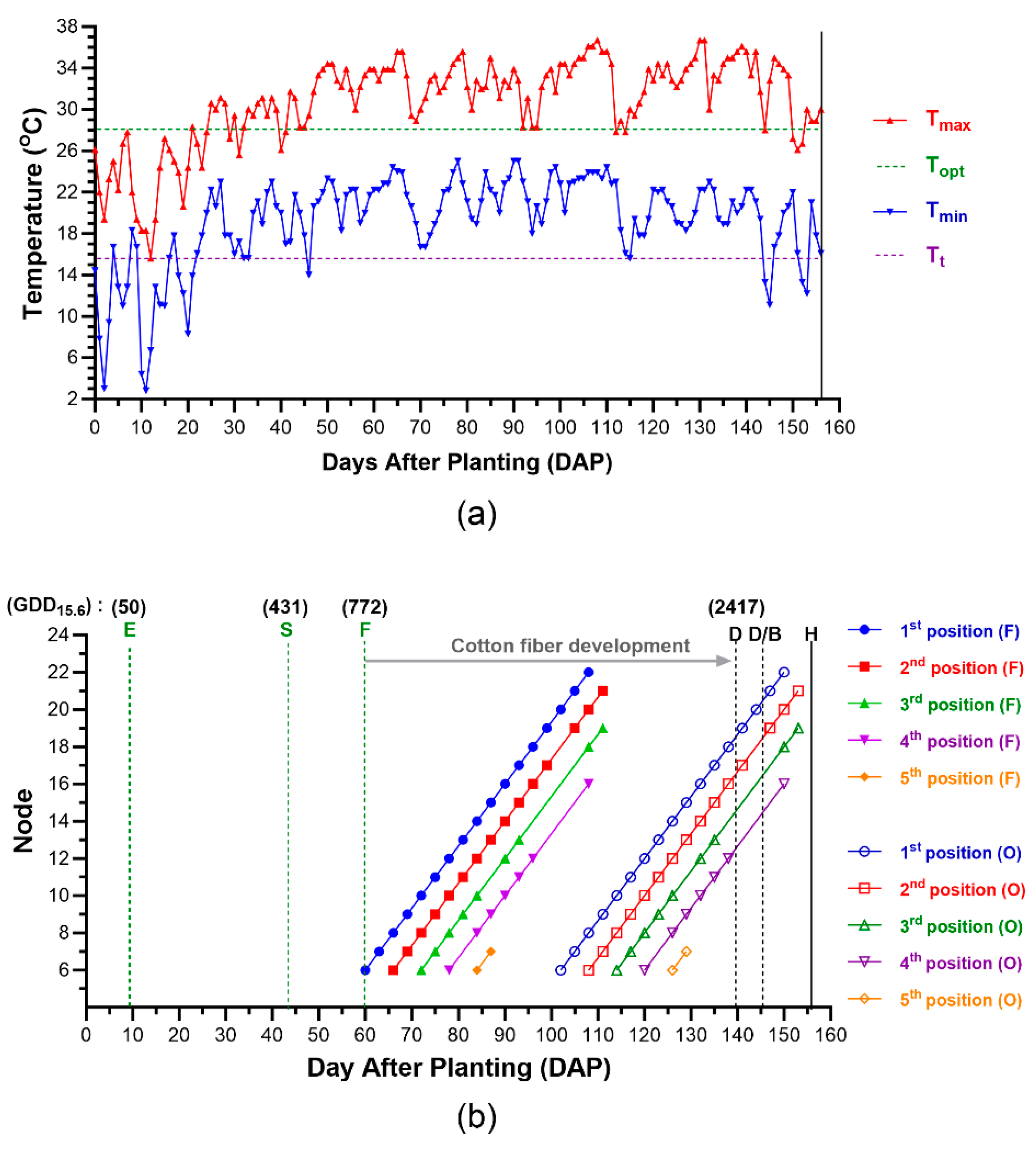
2.5. Calculation of Growing Degree Days (GDDs)
2.6. Statistical Analyses
3. Results and Discussion
3.1. Cotton Boll Distribution and Within-Plant Variability of Cotton Yield and Fiber Quality
3.2. Environmental and Managemental Effects on Cotton Fiber Maturation of Each Cotton Boll
3.3. Determination of Fiber Maturity and Crystallinity from a Single Boll Using FT-IR Spectroscopy
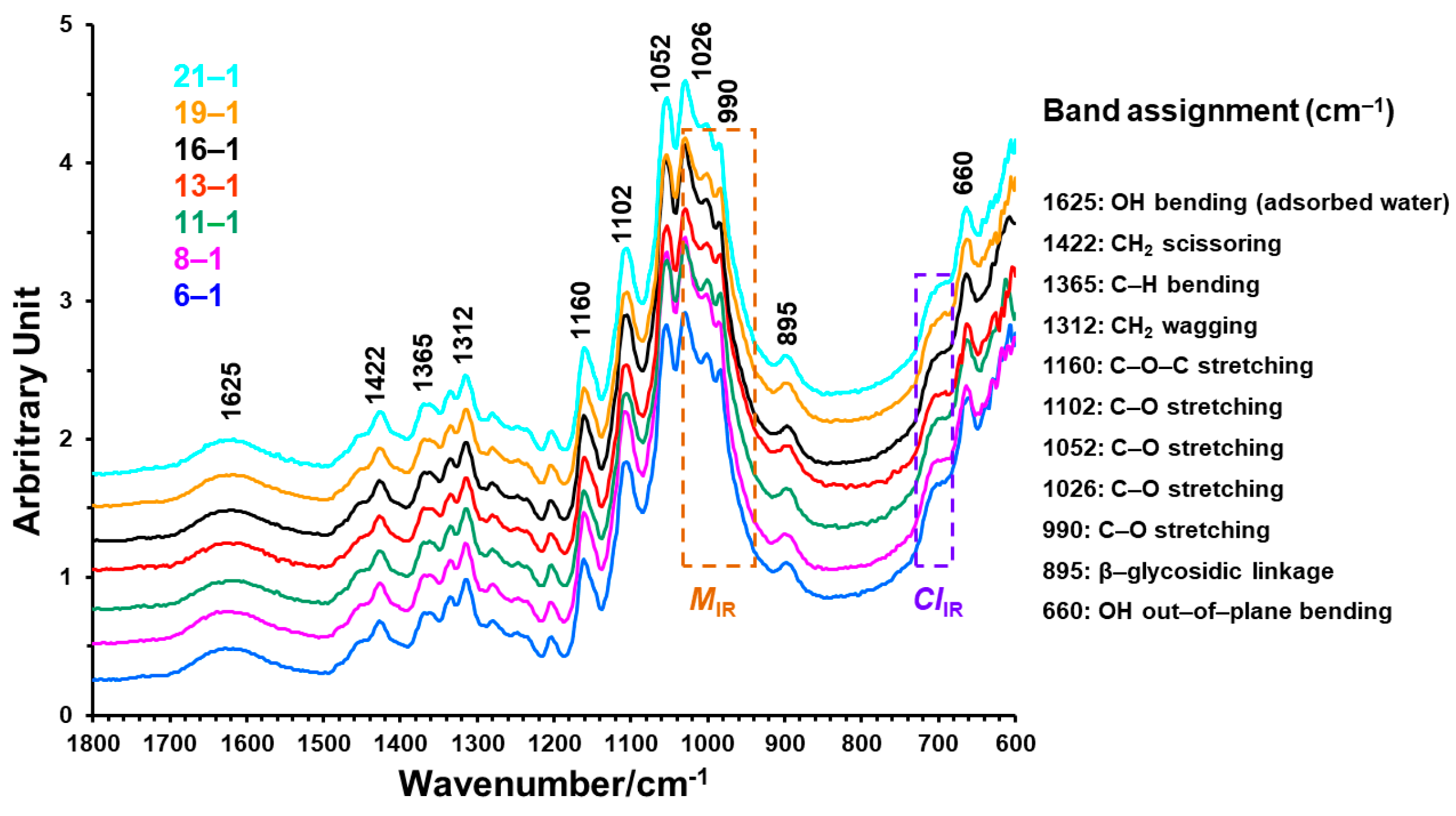
3.3.1. ATR FT-IR Spectral Characteristics of Cotton Fibers Harvested from a Single Boll at Various Nodes and Positions within a Plant
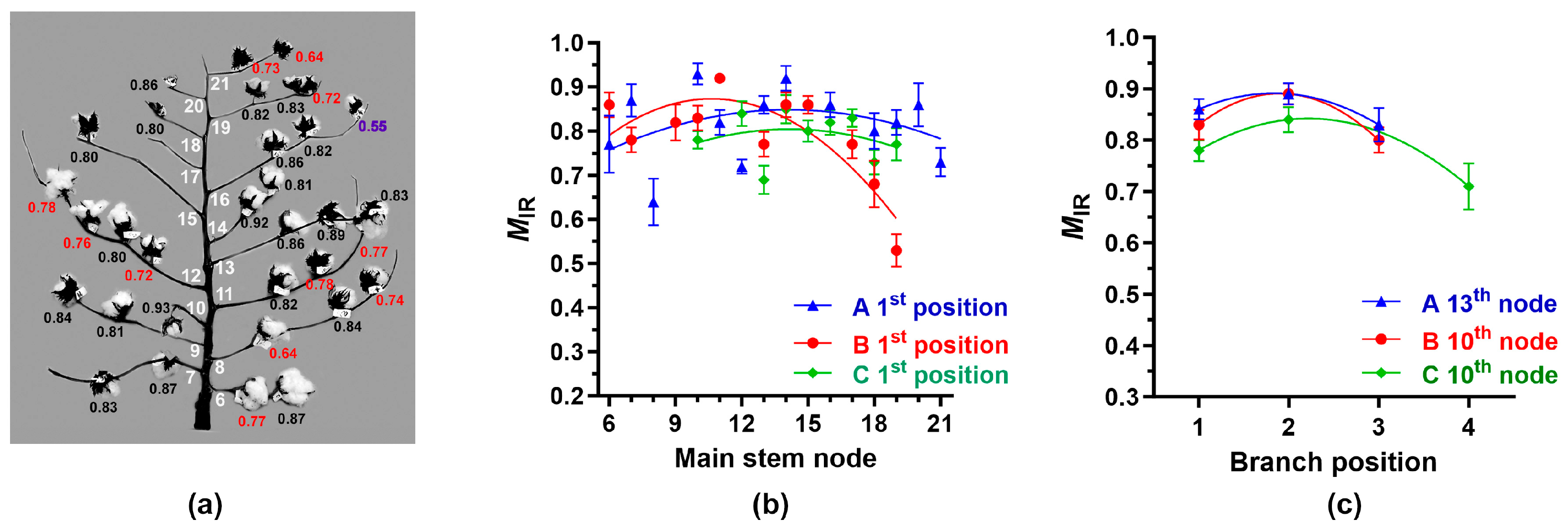
3.3.2. IR Maturity Variation among Individual Bolls within Cotton Plants
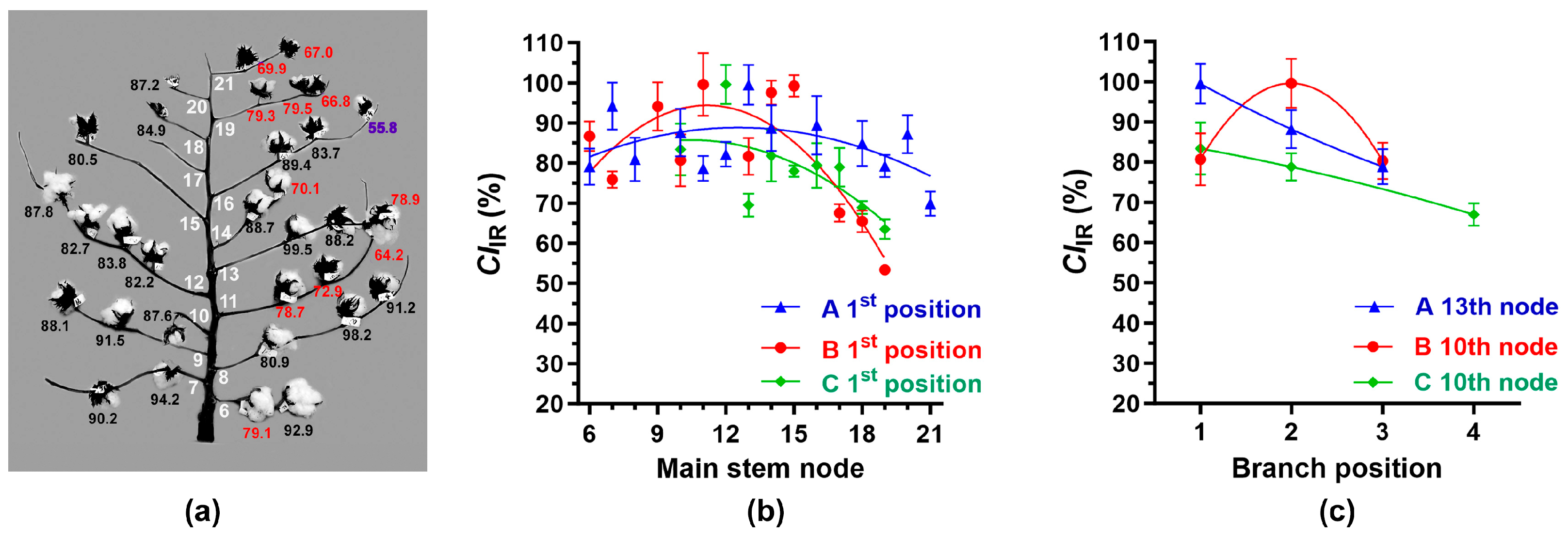
3.3.3. IR Crystallinity Variation among Individual Bolls within Cotton Plants
3.4. Comparisons of Fiber Properties Measured between FT-IR Spectroscopy and Conventional Methods with the Combined Samples at the Same Positions of the Three Plants
3.4.1. Determination of Fiber Properties via Conventional Methods
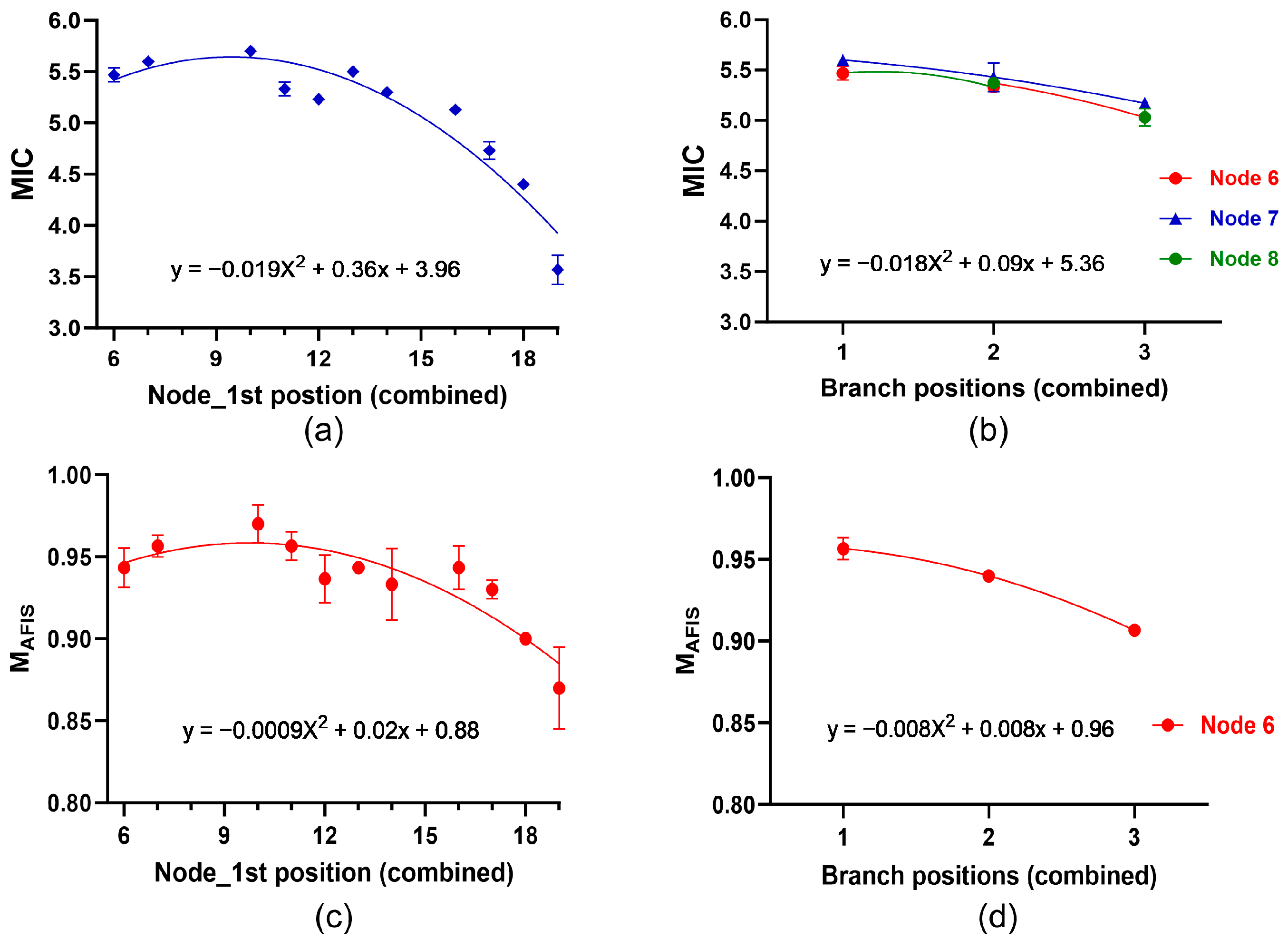
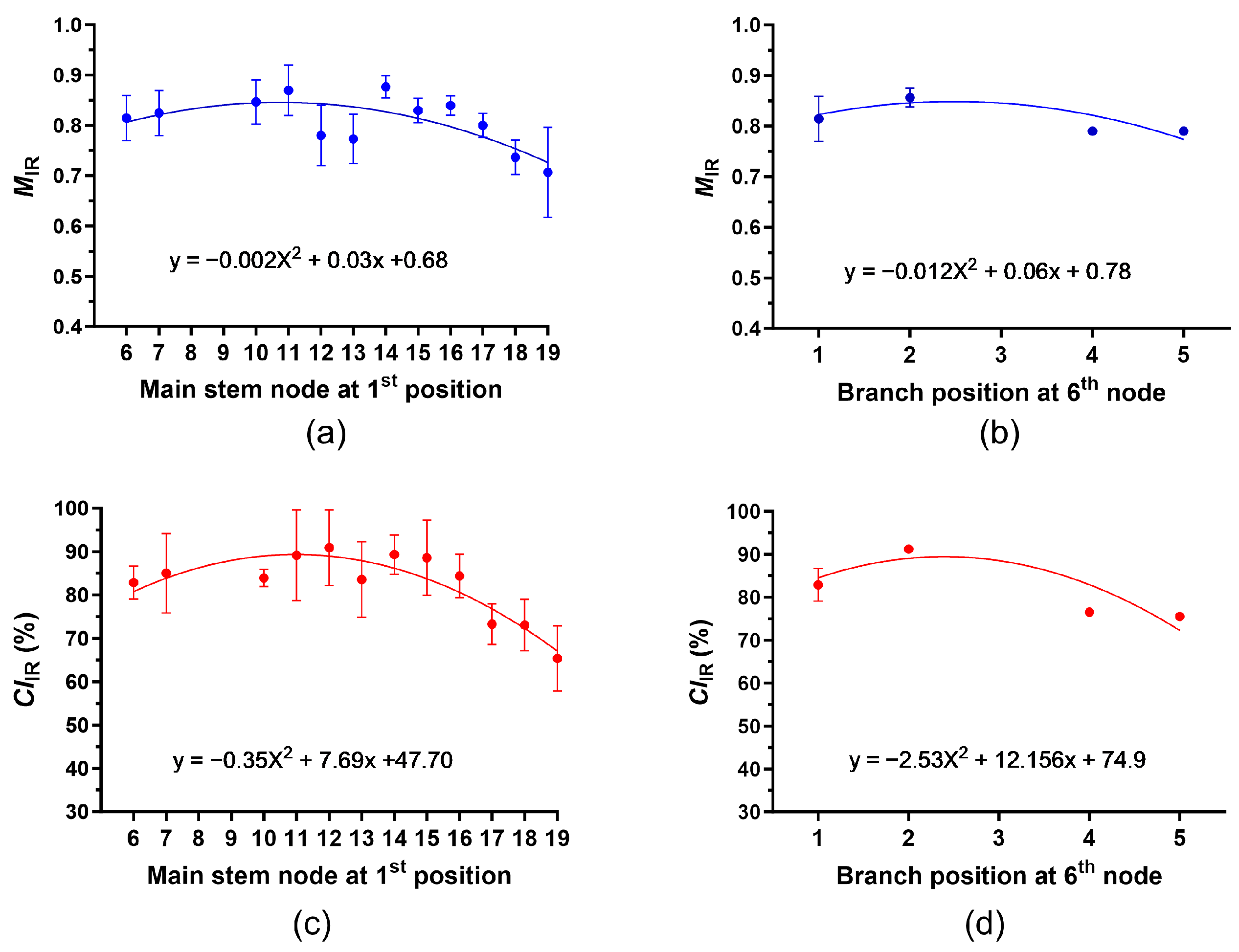
3.4.2. Determination of Average MIR and CIIR at Each Node and Position among the Plants
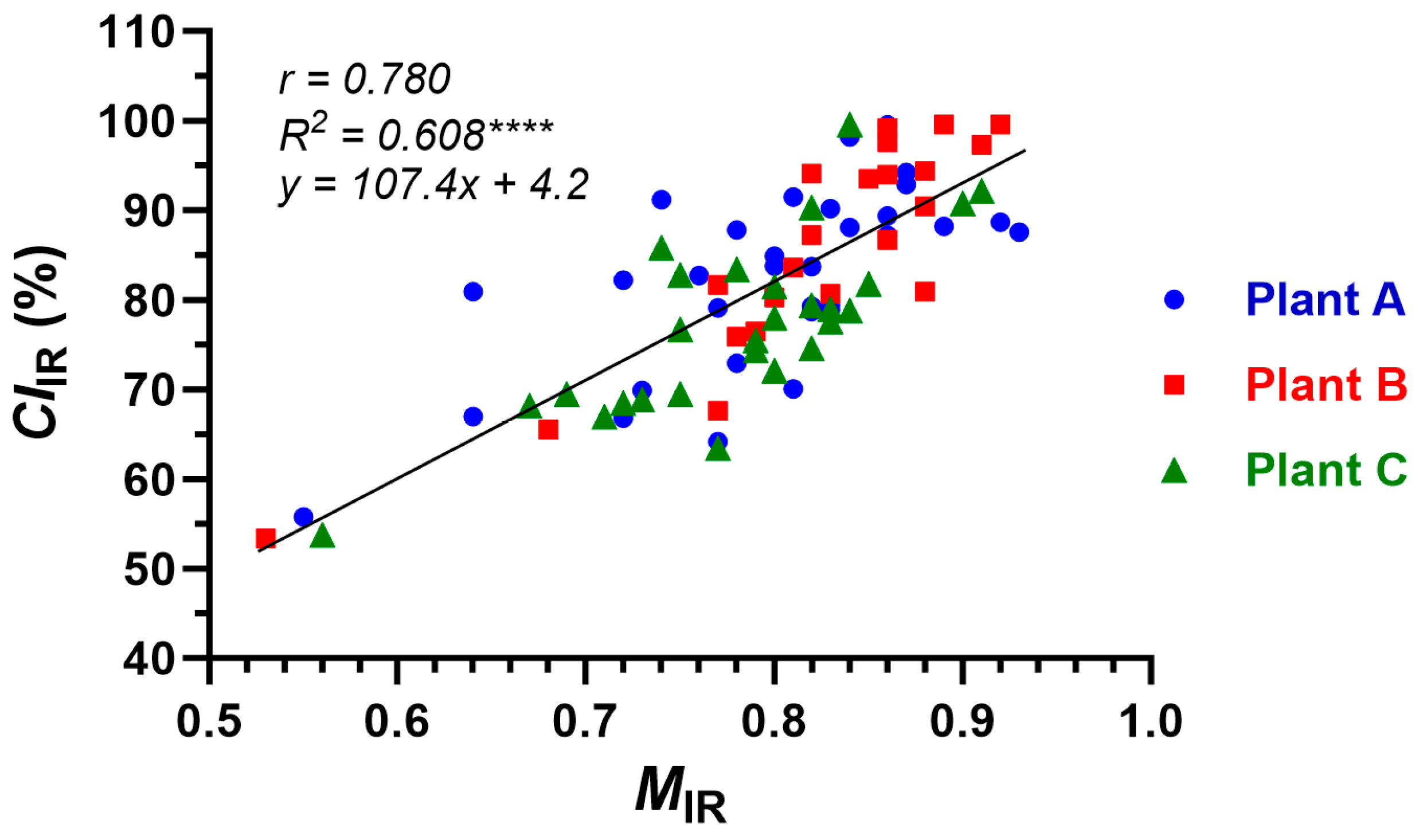
3.4.3. Relationships between IR Properties and Conventional Maturity Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, W.; Roberts, B. Integrated crop management for cotton production in the 21st century. In Cotton: Technology for the 21st Century; First International Cotton Advisory Committee: Washington, DC, USA, 2010; pp. 63–98. [Google Scholar]
- Kim, H.J.; Triplett, B.A. Cotton fiber growth in planta and in vitro. Models for plant cell elongation and cell wall biogenesis. Plant Physiol. 2001, 127, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, H.-J. Fourier transform infrared spectroscopy (FT-IR) and simple algorithm analysis for rapid and non-destructive assessment of developmental cotton fibers. Sensors 2017, 17, 1469. [Google Scholar] [CrossRef]
- Ayele, A.G.; Kelly, B.R.; Hequet, E.F. Evaluating within-plant variability of cotton fiber length and maturity. Agron. J. 2018, 110, 47–55. [Google Scholar] [CrossRef]
- Bradow, J.M.; Bauer, P.J.; Hinojosa, O.; Sassenrath-Cole, G. Quantitation of cotton fibre-quality variations arising from boll and plant growth environments. Eur. J. Agron. 1997, 6, 191–204. [Google Scholar] [CrossRef]
- Pabuayon, I.L.B.; Kelly, B.R.; Mitchell-McCallister, D.; Coldren, C.L.; Ritchie, G.L. Cotton boll distribution: A review. Agron. J. 2021, 113, 956–970. [Google Scholar] [CrossRef]
- Kothari, N.; Hague, S.; Hinze, L.; Dever, J. Boll sampling protocols and their impact on measurements of cotton fiber quality. Ind. Crops Prod. 2017, 109, 248–254. [Google Scholar] [CrossRef]
- Kothari, N.; Abidi, N.; Hequet, E.; Wilkins, T. Fiber Quality Variability within a Plant. In Proceedings of the World Cotton Research Conference-4, Lubbock, TX, USA, 10–14 September 2007. [Google Scholar]
- Oosterhuis, D.M.; Jernstedt, J. Morphology and anatomy of the cotton plant. In Cotton: Origin, History, Technology, and Production; Smith, C.W., Cothren, J.T., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 1999; pp. 175–206. [Google Scholar]
- Liu, Y.; Delhom, C.D. Investigation of fiber maturity and crystallinity information in Upland seed cottons by Fourier transform infrared spectroscopy. Text. Res. J. 2023, 93, 2507–2519. [Google Scholar] [CrossRef]
- Kohel, R.J. Linkage tests in Upland cotton, Gossypium hirsutum L. II.1. Crop Sci. 1972, 12, 66–69. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Hequet, E. Fourier transform infrared spectroscopic approach to the study of the secondary cell wall development in cotton fibers. Cellulose 2010, 17, 309–320. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Abidi, N.; Manike, M. X-ray diffraction and FTIR investigations of cellulose deposition during cotton fiber development. Text. Res. J. 2018, 88, 719–730. [Google Scholar] [CrossRef]
- He, Z.; Nam, S.; Fang, D.D.; Cheng, H.N.; He, J. Surface and thermal characterization of cotton fibers of phenotypes differing in fiber length. Polymers 2021, 13, 994. [Google Scholar] [CrossRef]
- Santiago, C.M.; Hinchliffe, D.J. FT-IR examination of the development of secondary cell wall in cotton fibers. Fibers 2015, 3, 30–40. [Google Scholar] [CrossRef]
- Averett, L.A.; Griffiths, P.A.; Nishikida, K. Effective path length in attenuated total reflection spectroscopy. Anal. Chem. 2008, 80, 3045–3049. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011; pp. 27–54. [Google Scholar]
- Van der Sluijs, M.H.J. Cotton appearance. In Cotton Fibers: Characteristics, Uses and Performance; Gordon, S., Abidi, N., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 135–158. [Google Scholar]
- Singh, R.P.; Prasad, P.V.V.; Sunita, K.; Giri, S.N.; Reddy, K.R. Influence of high temperature and breeding for heat tolerance in cotton: A review. Adv. Agron. 2007, 93, 313–385. [Google Scholar] [CrossRef]
- Pearson, K. Contributions to the mathematical theory of evolution. III. Regression, Heredity, and Panmixia. Proc. R. Soc. Lond. 1895, 59, 69–71. [Google Scholar]
- Oosterhuis, D.M. Growth and development of a cotton plant. In Nitrogen Nutrition in Cotton: Practical Issues, Proceedings of the Southern Branch Workshop for Practicing Agronomists, North Little Rock, AR, USA, 7 February 1990; Publications of the American Society of Agronomy: Madison, WI, USA, 1990; pp. 1–24. [Google Scholar]
- Benedict, C.R.; Kohel, J.R.; Lewis, H.L. Cotton fiber quality. In Cotton: Origin, History, Technology, and Production; Smith, C.W., Cothren, J.T., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 1999; pp. 269–288. [Google Scholar]
- Delhom, C.D.; Kelly, B.; Martin, V. Physical properties of cotton fiber and their measurement. In Cotton Fiber: Physics, Chemistry and Biology; Fang, D.D., Ed.; Springer: Cham, Switzerland, 2018; pp. 41–73. [Google Scholar]
- Ratner, B. The correlation coefficient: Its values range between +1/−1, or do they? J. Target Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]


| Classification | Property | A Plant | B Plant | C Plant |
|---|---|---|---|---|
| Total | Boll number | 33 | 22 | 27 |
| Lint mass | 78.06 g | 47.99 g | 70.73 g | |
| Lint% | 34.69% | 36.97% | 36.22% | |
| Single boll | Mean lint mass (g) | 2.36 ± 0.67 g | 2.18 ± 0.43 g | 2.62 ± 0.67 g |
| Lint% | 34.45 ± 1.49% | 36.95 ± 1.37% | 35.97 ± 2.03% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Liu, Y.; Zeng, L. Fourier Transform Infrared (FT-IR) Spectroscopy and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Cotton Fiber Maturity and Crystallinity for Plant Mapping. Sensors 2024, 24, 2888. https://doi.org/10.3390/s24092888
Kim H-J, Liu Y, Zeng L. Fourier Transform Infrared (FT-IR) Spectroscopy and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Cotton Fiber Maturity and Crystallinity for Plant Mapping. Sensors. 2024; 24(9):2888. https://doi.org/10.3390/s24092888
Chicago/Turabian StyleKim, Hee-Jin, Yongliang Liu, and Linghe Zeng. 2024. "Fourier Transform Infrared (FT-IR) Spectroscopy and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Cotton Fiber Maturity and Crystallinity for Plant Mapping" Sensors 24, no. 9: 2888. https://doi.org/10.3390/s24092888
APA StyleKim, H.-J., Liu, Y., & Zeng, L. (2024). Fourier Transform Infrared (FT-IR) Spectroscopy and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Cotton Fiber Maturity and Crystallinity for Plant Mapping. Sensors, 24(9), 2888. https://doi.org/10.3390/s24092888







