Synthesis and Sensing Response of Magnesium Antimoniate Oxide (MgSb2O6) in the Presence of Propane Atmospheres at Different Operating Voltages
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Physical Characterization
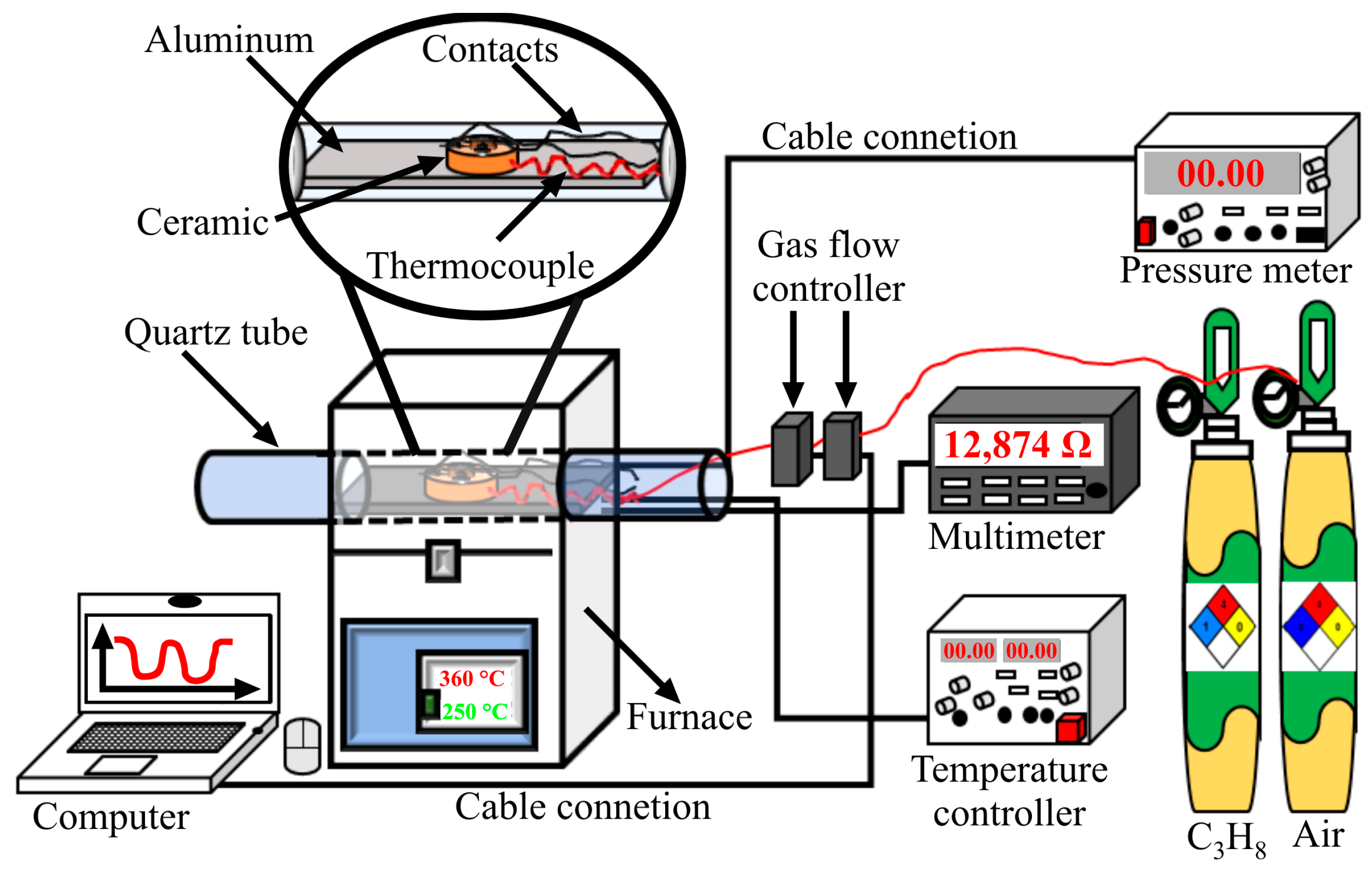
2.3. Dynamic Tests in C3H8 Atmospheres
3. Results
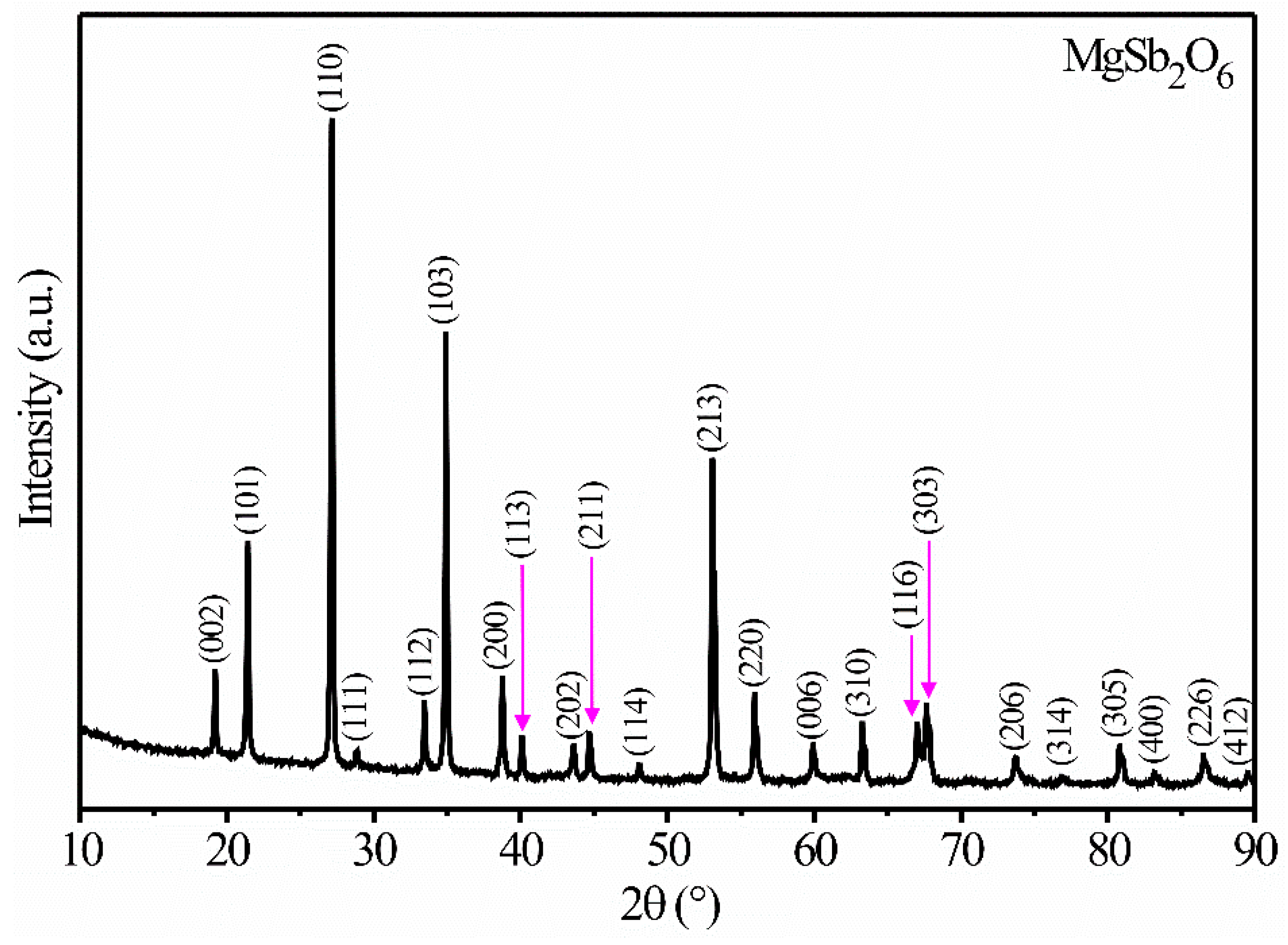
3.1. XRD Analysis
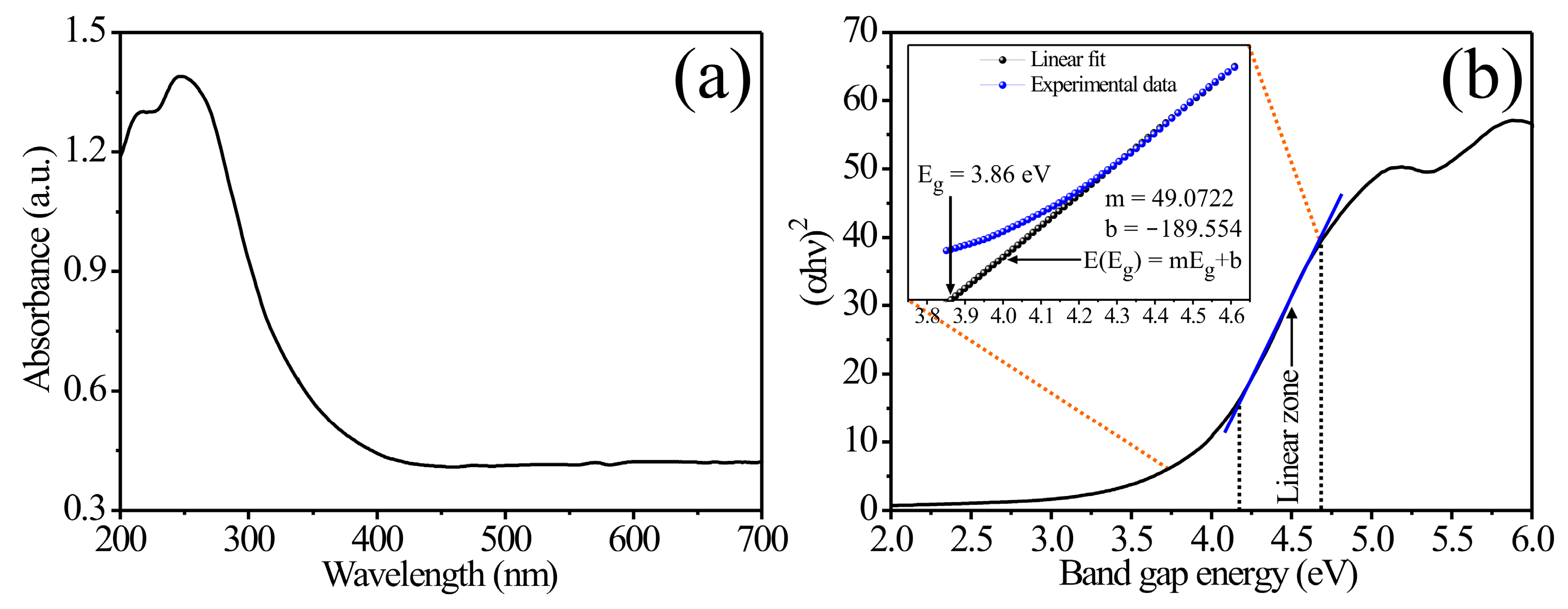
3.2. UV-Vis Analysis
3.3. SEM Analysis
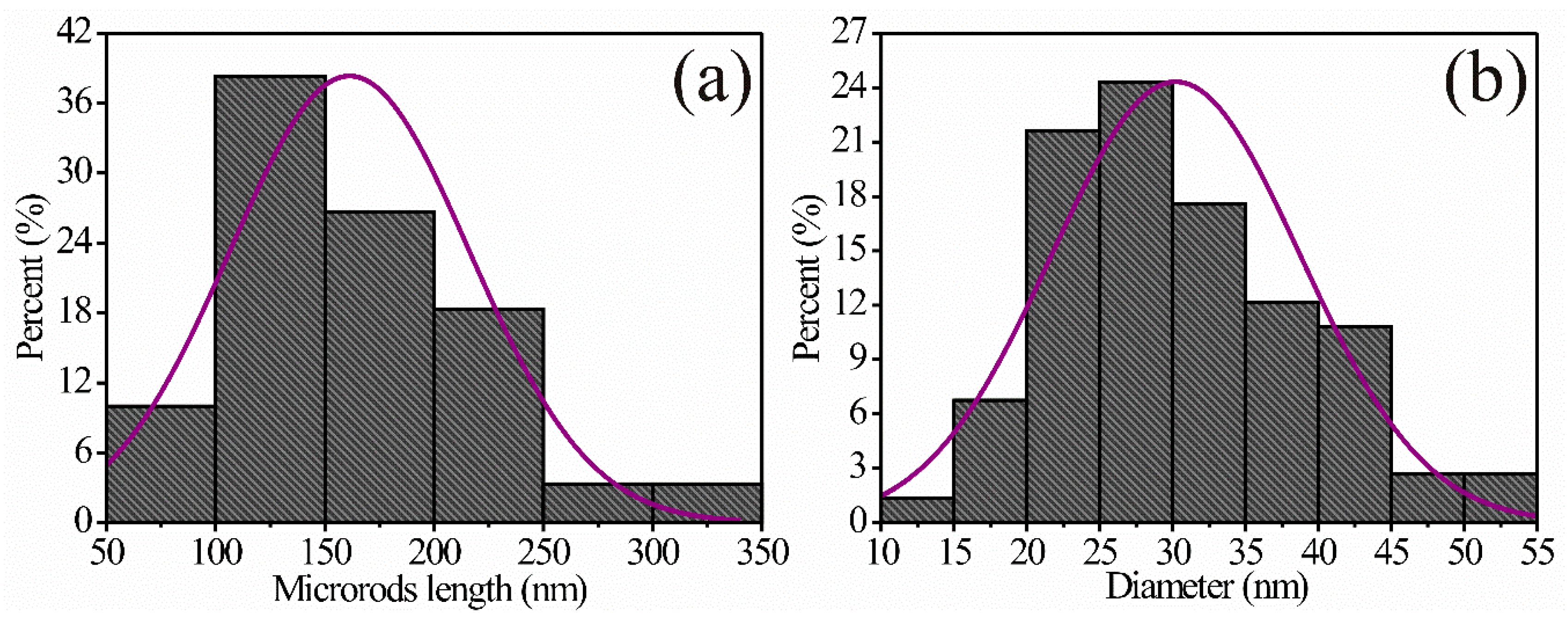
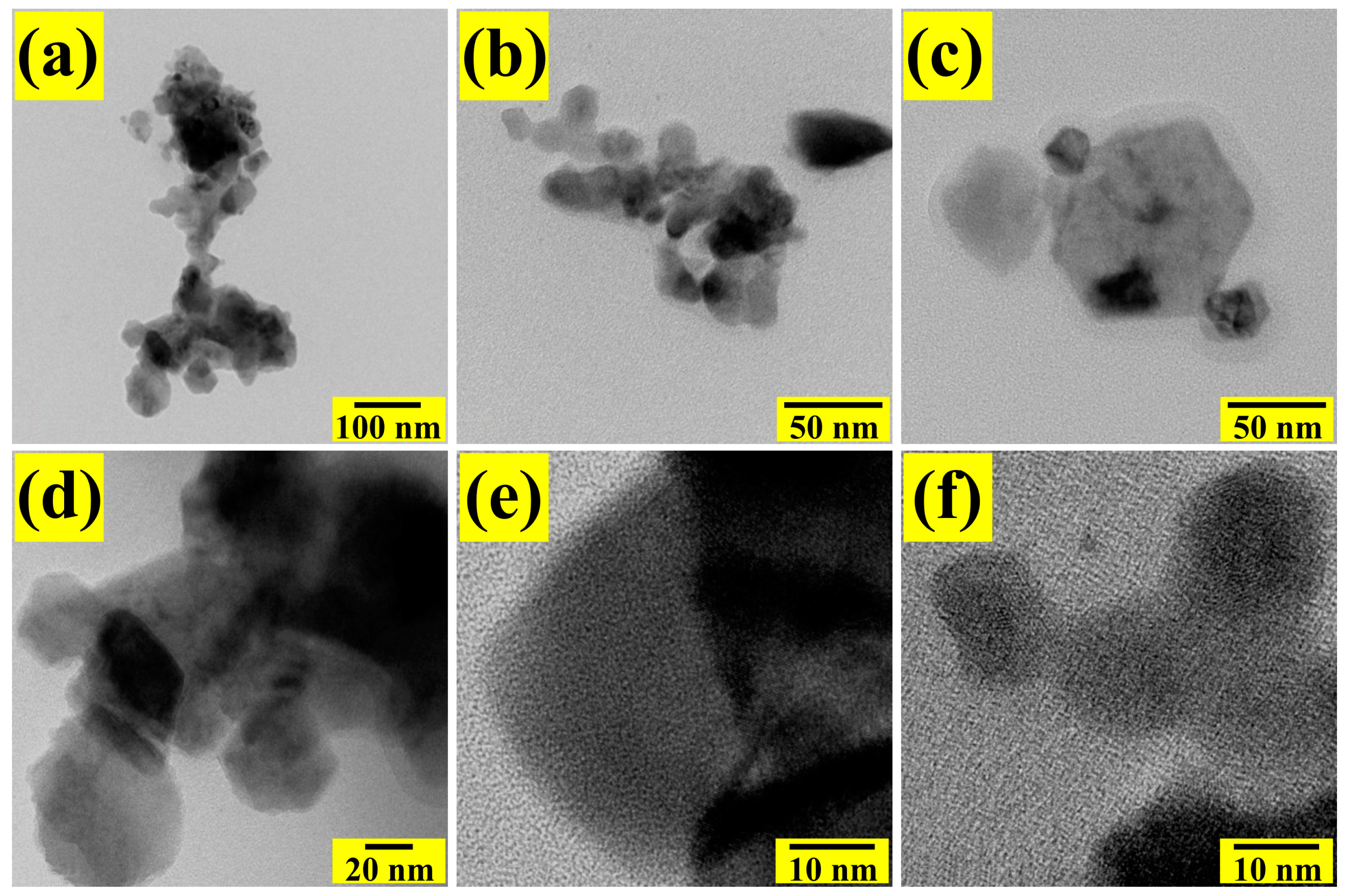
3.4. TEM Analysis
3.5. Gas-Sensing Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayesh, A.I. Metal/metal-Oxide nanoclusters for gas sensor applications. J. Nanomater. 2016, 2016, 2359019. [Google Scholar] [CrossRef]
- Fazio, E.; Spadaro, S.; Corsaro, C.; Neri, G.; Gianluca Leonardi, S.; Neri, F.; Lavanya, N.; Sekar, C.; Donato, N.; Neri, G. Metal-Oxide based nanomaterials: Synthesis, characterization and their applications in electrical and electrochemical sensors. Sensors 2021, 21, 2494. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Concina, I.; Faglia, G.; Falasconi, M.; Ferroni, M.; Galstyan, V.; Gobbi, E.; Ponzoni, A.; Vomiero, A. Metal oxide nanoscience and nanotechnology for chemical sensors. Sens. Actuators B Chem. 2013, 179, 3–20. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Munasinghe Arachchige, H.M.M.; Sisman, O.; Comini, E. “Metal oxide -based heterostructures for gas sensors”—A review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef]
- Shingange, K.; Swart, H.C.; Mhlongo, G.H. Design of porous p-type LaCoO3 nanofibers with remarkable response and selectivity to ethanol at low operating temperature. Sens. Actuators B Chem. 2020, 308, 127670. [Google Scholar] [CrossRef]
- Guillen Bonilla, J.T.; Guillen Bonilla, H.; Rodríguez-Betancourtt, V.M.; Guillen Bonilla, A.; Casillas Zamora, A.; Blanco Alonso, O.; Ramírez Ortega, J.A. A gas sensor for application as a propane leak detector. J. Sens. 2021, 2021, 8871166. [Google Scholar] [CrossRef]
- Vesna Nikolic, M.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Hua, Z.; Tian, C.; Huang, D.; Yuan, W.; Zhang, C.; Tian, X.; Wang, M.; Li, E. Power-law response of metal oxide semiconductor gas sensors to oxygen in presence of reducing gases. Sens. Actuators B Chem. 2018, 267, 510–518. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuator 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Jerome McAleer, F.; Patrick Moseley, T.; Norris John, O.W.; Williams, D.E. Tin dioxide gas sensors. J. Chem. Soc. Faraday Trans. I 1987, 83, 1323–1346. [Google Scholar]
- Huízar-Padilla, E.; Guillén-Bonilla, H.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.M.; Sánchez-Martínez, A.; Guillen-Bonilla, J.T.; Gildo-Ortiz, L.; Reyes-Gómez, J. Synthesis of ZnAl2O4 and Evaluation of the Response in Propane Atmospheres of Pellets and Thick Films Manufactured with Powders of the Oxide. Sensors 2021, 21, 2362. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, T. An overview: Facet-dependent metal oxide semiconductor gas sensors. Sens. Actuators B Chem. 2018, 277, 604–633. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Yu, Q.; Zhao, L.; Sun, P.; Wang, T.; Liu, F.; Yan, X.; Gao, Y.; Liang, X.; et al. One step synthesis of branched SnO2/ZnO heterostructures and their enhanced gas-sensing properties. Sens. Actuators B Chem. 2018, 2019, 415–423. [Google Scholar] [CrossRef]
- Saruhan, B.; Lontio Fomekong, R.; Nahirniak, S. Review: Influences of semiconductor metal oxide properties on gas sensing characteristics. Front. Sens. 2021, 2, 1–24. [Google Scholar]
- Qin, W.; Yuan, Z.; Gao, H.; Zhang, R.; Meng, F. Perovskite-structured LaCoO3 modified ZnO gas sensor and investigation on its gas sensing mechanism by first principle. Sens. Actuators B Chem. 2021, 341, 130015. [Google Scholar] [CrossRef]
- Ateia, E.E.; Arman, M.M.; Morsy, M. Synthesis, characterization of NdCoO3 perovskite and its uses as humidity sensor. Appl. Phys. 2019, 125, 883. [Google Scholar] [CrossRef]
- Guillén-Lopez, E.S.; Lopez-Urías, F.; Munoz-Sandoval, E.; Courel-Piedrahita, M.; Sanchez-Tizapa, M.; Guillén-Bonilla, H.; Rodríguez-Betancourtt, V.M.; Blanco-Alonso, O.; Guillén-Bonilla, A.; Moran-Lázaro, J.P. High performance isopropanol sensor based on spinel ZnMn2O4 nanoparticles. Mater. Today Commun. 2021, 26, 102138. [Google Scholar] [CrossRef]
- Liu, F.; Wang, B.; Yang, X.; Guan, Y.; Wang, Q.; Lianga, X.; Sun, P.; Wang, Y.; Lu, G. High-temperature NO2 gas sensor based on stabilized zirconia and CoTa2O6 sensing electrode. Sens. Actuators B Chem. 2017, 240, 148–157. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Singh, A.; Rathore, S.; Yadav, B.C.; Tandon, P. Nanostructured cobalt antimonate: A fast responsive and highly stable sensing material for liquefied petroleum gas detection at room temperature. RSC Adv. 2020, 10, 33770–33781. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.R.; Martínez, A.H.; Jiménez, S. Gas sensing response of nanostructured trirutile-type CoSb2O6 synthesized by solution-polymerization method. Sens. Actuators B Chem. 2008, 132, 45–51. [Google Scholar] [CrossRef]
- Jamal, A.; Rahman, M.M.; Khan, S.B.; Faisal, M.; Akhtar, K.; Rub, M.A.; Asiri, A.M.; Al-Youbi, A.O. Cobalt doped antimony oxide nano-particles based chemical sensor and photo-catalyst for environmental pollutants. Appl. Surf. Sci. 2012, 261, 52–58. [Google Scholar] [CrossRef]
- Casillas-Zamora, A.; Guillen-Bonilla, J.T.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, M.V.; Casallas-Moreno, Y.L.; Gildo-Ortiz, L.; Olvera-Amador, M.L.; Tomás, S.A.; Guillen-Bonilla, H. Synthesis of MnSb2O6 powders through a simple low-temperature method and their test as a gas sensor. J. Mater. Sci. Mater. Electron. 2020, 31, 7359–7372. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Singh, A.; Tandon, P. A stable and highly sensitive room-temperature liquefied petroleum gas sensor based on nanocubes/cuboids of zinc antimonate. RSC Adv. 2020, 10, 20349–20357. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Guillén-Bonilla, H.; Flores-Martínez, M.; Rodríguez-Betancourtt, V.M.; Guillen-Bonilla, A.; Reyes-Gómez, J.; Gildo-Ortiz, L.; Olvera Amador, M.L.; Santoyo-Salazar, J. A Novel Gas Sensor Based on MgSb2O6 Nanorods to Indicate Variations in Carbon Monoxide and Propane Concentrations. Sensors 2016, 16, 177. [Google Scholar] [CrossRef]
- Roper, A.; Leverett, P.; Murphy, T.; Williams, P. Stabilities of byströmite, MgSb2O6, ordoñezite, ZnSb2O6 and rosiaite, PbSb2O6, and their possible roles in limiting antimony mobility in the supergene zone. Mineral. Mag. 2015, 79, 537–544. [Google Scholar] [CrossRef]
- Nagarajan, A.; Naraginti, S. Facile synthesis of N-MgSb2O6 trirutile antimonate and its enhanced photocatalytic performance. Int. J. Environ. Anal. Chem. 2020, 102, 7938–7952. [Google Scholar] [CrossRef]
- Arunkumar, N.; Naraginti, S. Facile synthesis of nanostructured trirutile antimonates M(II)Sb2O6 (M = Co, Cu, Ni, Fe) and its visible photocatalytic studies. Inorg. Nano-Met. Chem. 2022, 52, 151–160. [Google Scholar] [CrossRef]
- Singh, J.; Bhardwaj, N.; Uma, S. Single step hydrothermal based synthesis of M(II)Sb2O6 (M = Cd and Zn) type antimonates and their photocatalytic properties. Bull. Mater. Sci. 2013, 36, 287–291. [Google Scholar] [CrossRef]
- Litong, H.; Qiang, Z.; Fangfei, L.; Liang, L. Optical properties of trirutile structure MgTa2O6 single crystals grown by optical floating zone method. Mod. Phys. Lett. B 2020, 34, 2050281. [Google Scholar]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Voorhees, P.W. The theory of Ostwald ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef]
- Yu, H.; Regulacio, D.; Ye, M.D.; Han, M.Y. Chemical routes to top-down nanofabrication. Chem. Soc. Rev. 2013, 42, 6006–6018. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Kida, T.; Kuroiwa, T.; Yuasa, M.; Shimanoe, K.; Yamazoe, N. Study on the response and recovery properties of semiconductor gas sensors using a high-speed gas-switching system. Sens. Actuators B Chem. 2008, 134, 928–933. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Ramírez-Ortega, J.; Guillén-Bonilla, H.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.M.; Sánchez-Martínez, A.; Guillén-Bonilla, J.T.; Gildo-Ortiz, L.; Huizar, E.; Reyes-Gómez, J. Synthesis of the oxide NiSb2O6 and its electrical characterization in toxic atmospheres for its application as a gas sensor. J. Mater. Sci. Mater. Electron. 2022, 33, 18268–18283. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Pd-functionalized core-shell composite nanowires for self-heating, sensitive, and benzene-selective gas sensors. Sens. Actuator A Phys. 2020, 308, 112011. [Google Scholar] [CrossRef]
- Avila-García, A.; Chaudhary, A.; Rojas-Chávez, H. Iridium oxide films as propane sensors. Thin Solid Films 2021, 724, 138617. [Google Scholar] [CrossRef]


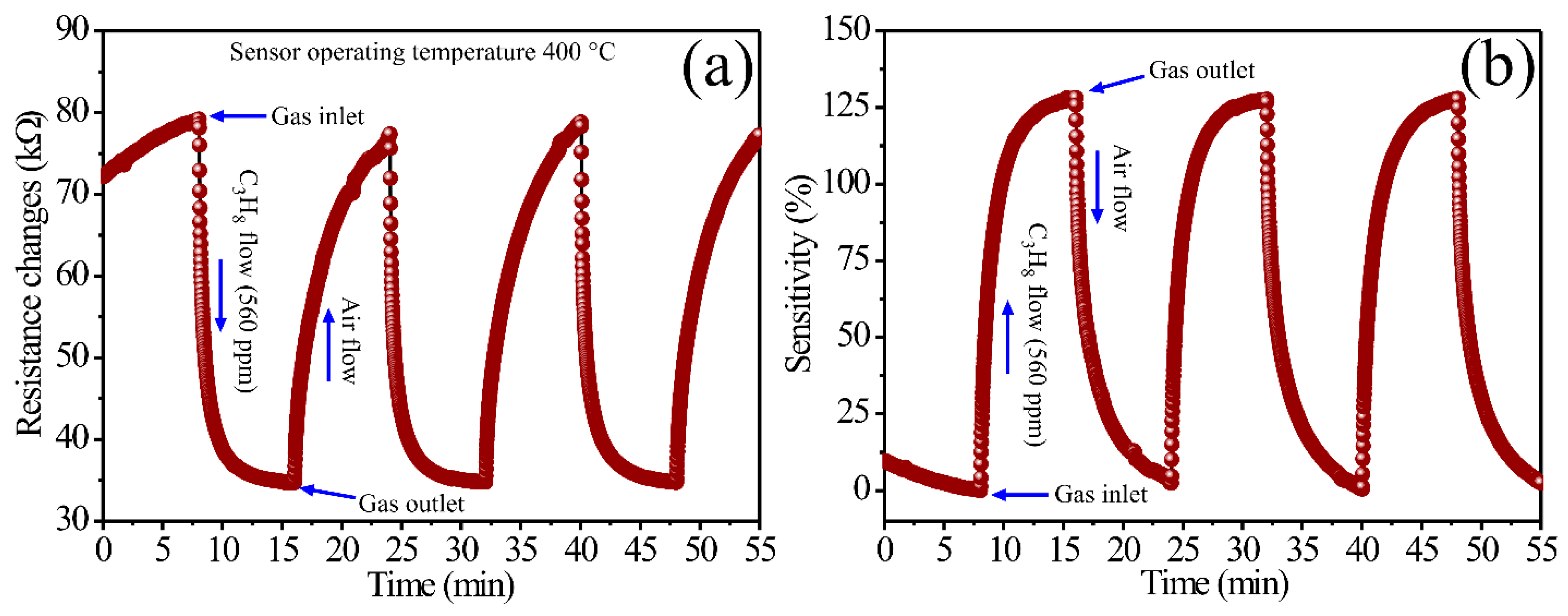

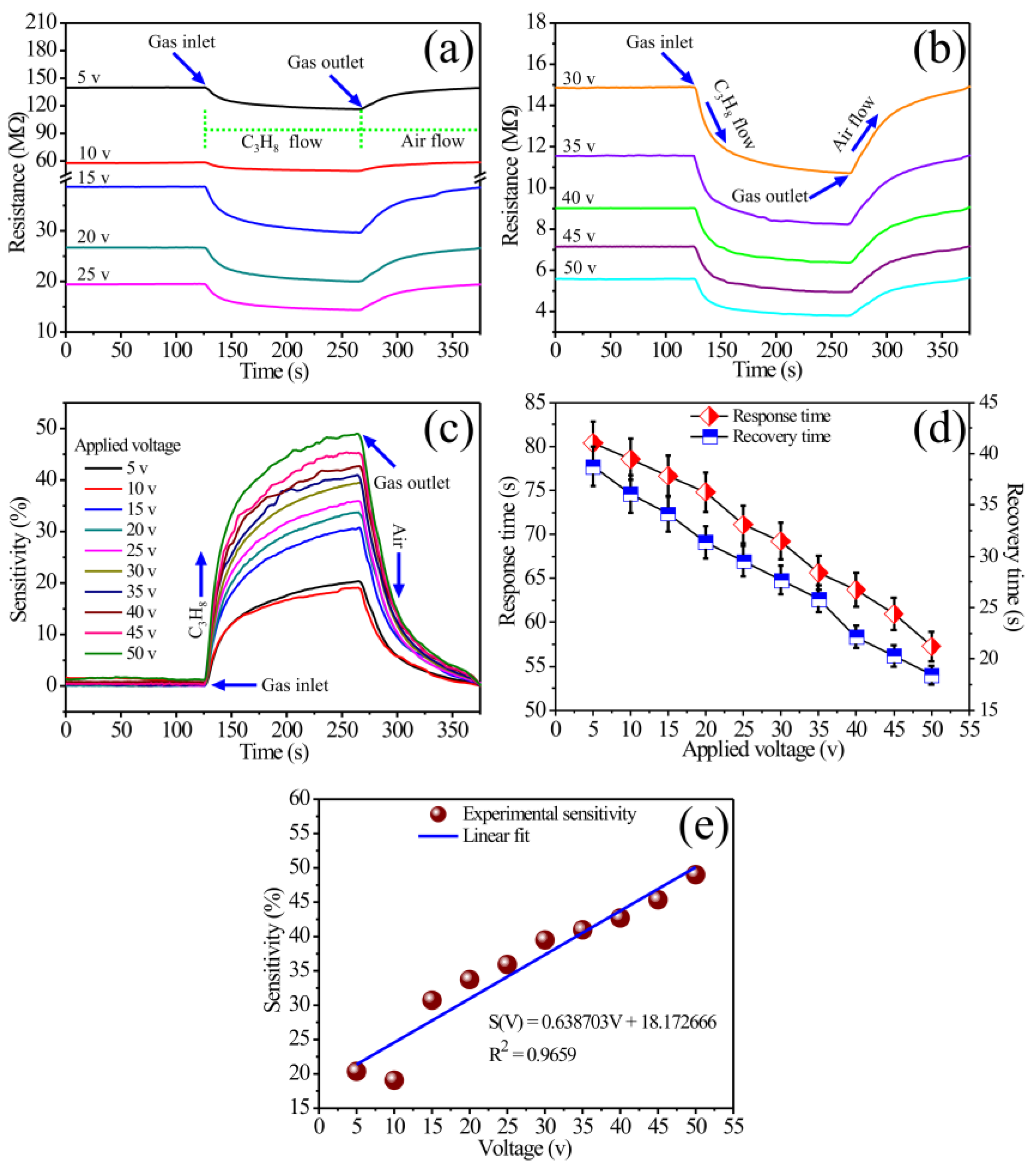
| Concentration (ppm) | ΔR (kΩ) | Sensitivity (%) | Response Time (min) | Recovery Time (min) |
|---|---|---|---|---|
| 150 | 52.50 | 61.09 | 6.15 | 3.22 |
| 300 | 65.95 | 88.80 | 3.10 | 3.08 |
| 400 | 68.90 | 97.65 | 2.40 | 2.97 |
| 600 | 70.59 | 112.81 | 2.13 | 2.85 |
| Voltage (V) | ΔR (MΩ) | Sensitivity (%) | Response Time (s) | Recovery Time (s) |
|---|---|---|---|---|
| 5 | 23.73 | 20.35 | 80.40 | 38.77 |
| 10 | 9.34 | 19.09 | 78.53 | 36.10 |
| 15 | 9.03 | 30.74 | 76.67 | 34.15 |
| 20 | 6.67 | 33.72 | 74.83 | 31.38 |
| 25 | 5.13 | 35.93 | 71.09 | 29.54 |
| 30 | 4.18 | 39.50 | 69.24 | 27.69 |
| 35 | 3.34 | 40.97 | 65.63 | 25.85 |
| 40 | 2.65 | 42.71 | 63.70 | 22.16 |
| 45 | 2.21 | 45.35 | 60.93 | 20.31 |
| 50 | 1.78 | 49.01 | 57.25 | 18.45 |
| Material | Gas | Concentration (ppm) | Sensitivity (%) | Response Time (s) | Recovery Time (s) | Reference |
|---|---|---|---|---|---|---|
| ZnAl2O4 | Propane | 1000 ppm | - | 176.0 | 205.0 | [14] |
| CoSb2O6 | LPG | 5000 ppm | 1.96 | 21.0 | 234.0 | [22] |
| ZnSb2O6 | LPG | 5000 ppm | 1.73 | 41.0 | 95.0 | [26] |
| MgSb2O6 | Propane | 500 ppm | 61.66 | - | - | [28] |
| IrO2 | - | 1000 ppm | - | - | - | [42] |
| MgSb2O6 MgSb2O6 | Propane Propane | 600 ppm 560 ppm | 112.81 49.01 | 127.8 57.2 | 171 18.5 | This work This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillén-Bonilla, H.; Guillén-Bonilla, J.T.; Rodríguez-Betancourtt, V.-M.; Ramírez-Ortega, J.A.; Morán Lázaro, J.P.; Guillén-Bonilla, A. Synthesis and Sensing Response of Magnesium Antimoniate Oxide (MgSb2O6) in the Presence of Propane Atmospheres at Different Operating Voltages. Sensors 2024, 24, 2147. https://doi.org/10.3390/s24072147
Guillén-Bonilla H, Guillén-Bonilla JT, Rodríguez-Betancourtt V-M, Ramírez-Ortega JA, Morán Lázaro JP, Guillén-Bonilla A. Synthesis and Sensing Response of Magnesium Antimoniate Oxide (MgSb2O6) in the Presence of Propane Atmospheres at Different Operating Voltages. Sensors. 2024; 24(7):2147. https://doi.org/10.3390/s24072147
Chicago/Turabian StyleGuillén-Bonilla, Héctor, José Trinidad Guillén-Bonilla, Verónica-María Rodríguez-Betancourtt, Jorge Alberto Ramírez-Ortega, Juan Pablo Morán Lázaro, and Alex Guillén-Bonilla. 2024. "Synthesis and Sensing Response of Magnesium Antimoniate Oxide (MgSb2O6) in the Presence of Propane Atmospheres at Different Operating Voltages" Sensors 24, no. 7: 2147. https://doi.org/10.3390/s24072147
APA StyleGuillén-Bonilla, H., Guillén-Bonilla, J. T., Rodríguez-Betancourtt, V.-M., Ramírez-Ortega, J. A., Morán Lázaro, J. P., & Guillén-Bonilla, A. (2024). Synthesis and Sensing Response of Magnesium Antimoniate Oxide (MgSb2O6) in the Presence of Propane Atmospheres at Different Operating Voltages. Sensors, 24(7), 2147. https://doi.org/10.3390/s24072147







