A Novel Experimental Approach for the Measurement of Vibration-Induced Changes in the Rheological Properties of Ex Vivo Ovine Brain Tissue
Abstract
1. Introduction
2. Background
2.1. Small and Large Animal Models of TBI
2.2. Biological Fatigue
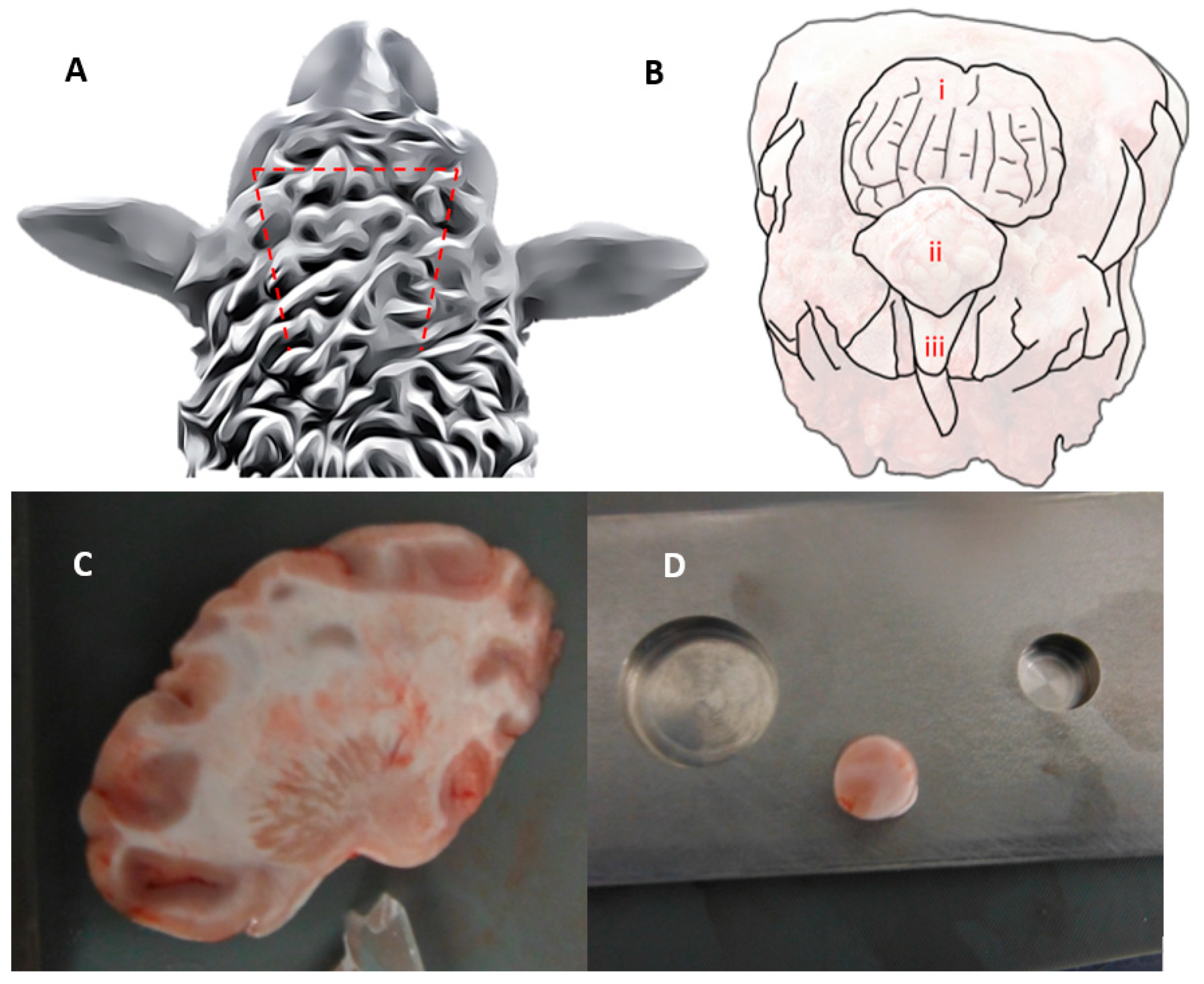
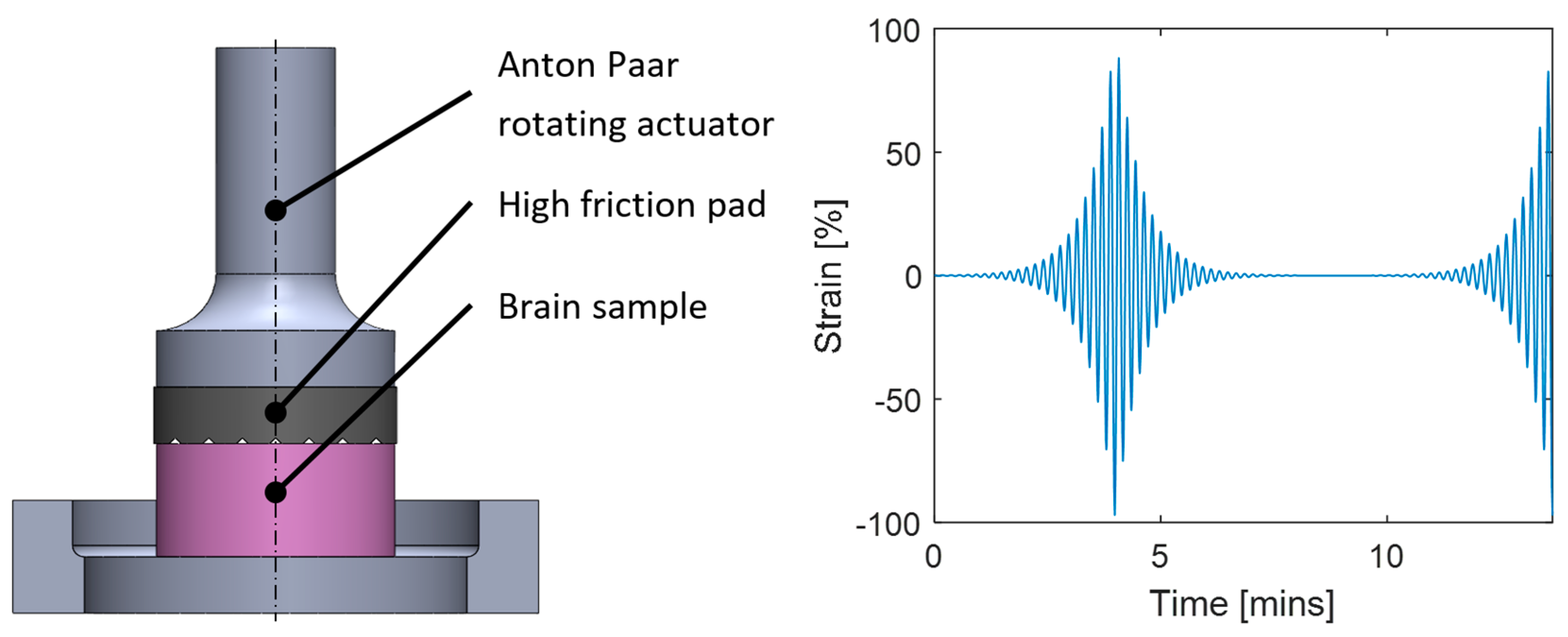
3. Methods
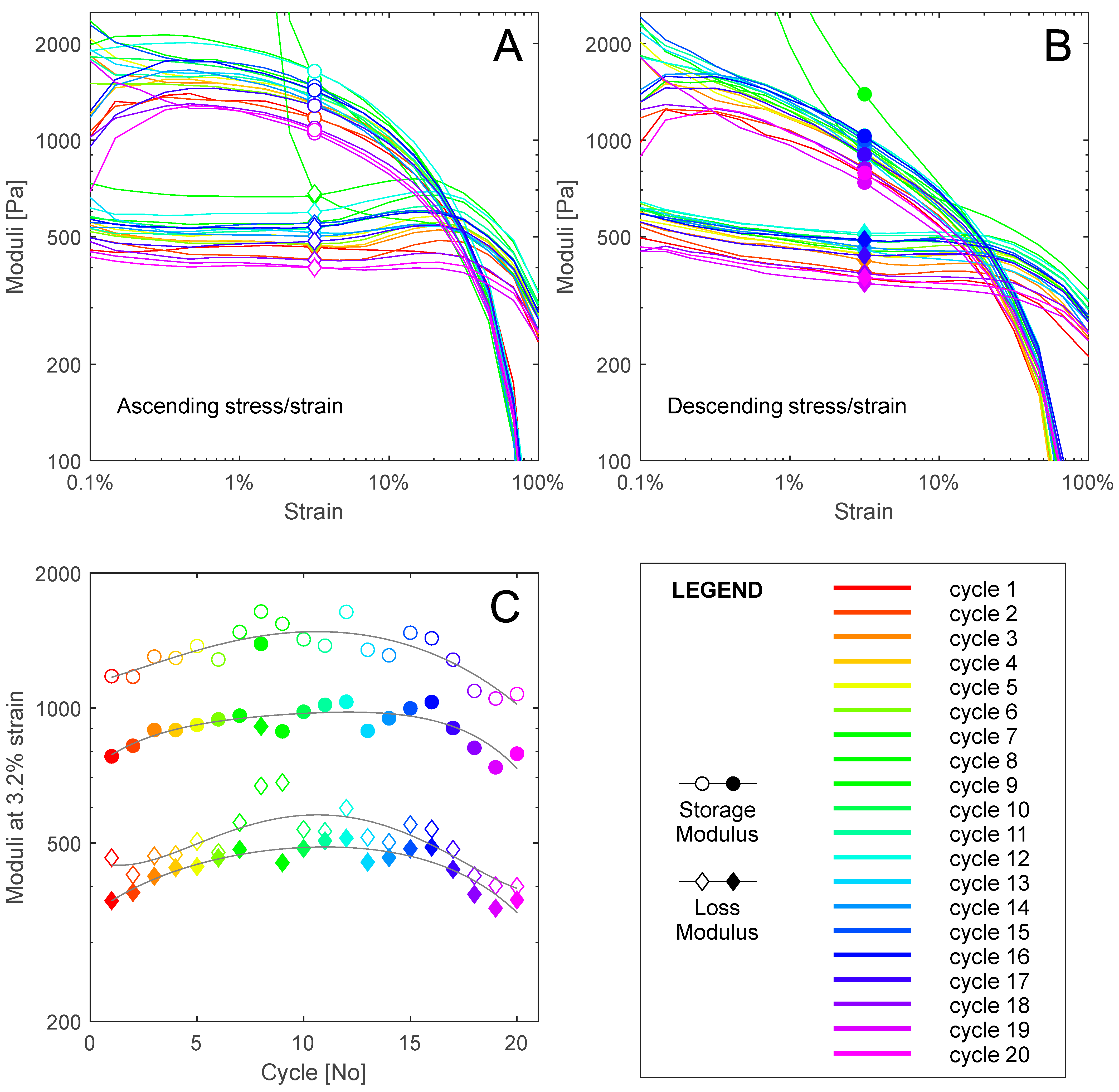
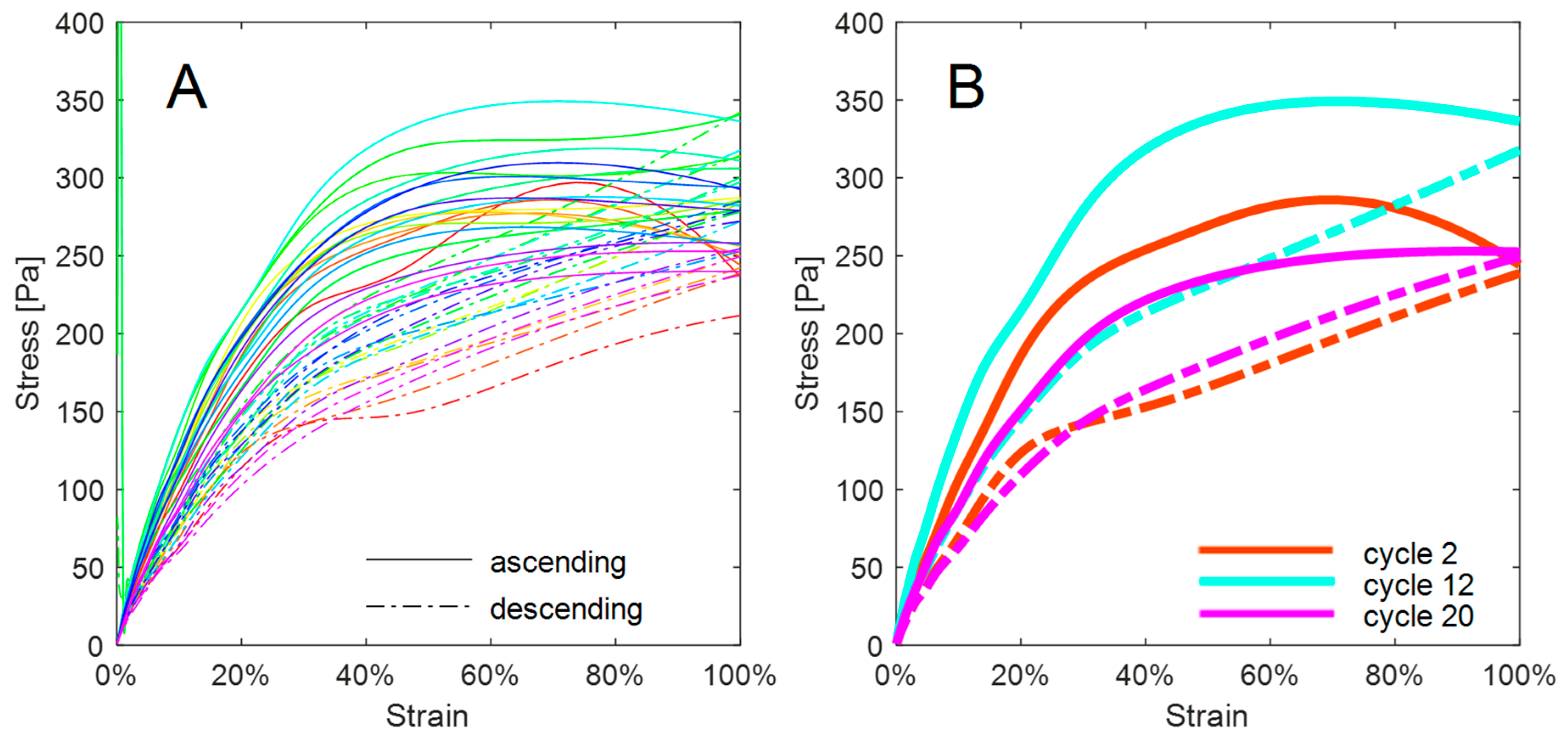
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crisco, J.J.; Wilcox, B.J.; Beckwith, J.G.; Chu, J.J.; Duhaime, A.-C.; Rowson, S.; Duma, S.M.; Maerlender, A.C.; McAllister, T.W.; Greenwald, R.M. Head impact exposure in collegiate football players. J. Biomech. 2011, 44, 2673–2678. [Google Scholar] [CrossRef]
- Nauman, E.A.; Talavage, T.M.; Auerbach, P.S. Mitigating the Consequences of Subconcussive Head Injuries. Annu. Rev. Biomed. Eng. 2020, 22, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhu, W.; Yang, H.-C.; Jang, I.; Vike, N.L.; Svaldi, D.O.; Shenk, T.E.; Poole, V.N.; Breedlove, E.L.; Tamer, G.G.; et al. Development of brain atlases for early-to-middle adolescent collision-sport athletes. Sci. Rep. 2021, 11, 6440. [Google Scholar] [CrossRef] [PubMed]
- Conidi, F.X. Understanding the Cumulative Effects of Concussion. Pract. Neurol. 2015, 15, 6. [Google Scholar]
- Kashyap, P.; Shenk, T.E.; Svaldi, D.O.; Lycke, R.J.; Lee, T.A.; Tamer, G.G.; Nauman, E.A.; Talavage, T.M. Normalized Brain Tissue-Level Evaluation of Volumetric Changes of Youth Athletes Participating in Collision Sports. Neurotrauma Rep. 2022, 3, 57–69. [Google Scholar] [CrossRef] [PubMed]
- McNamee, M.J.; Partridge, B.; Anderson, L. Concussion Ethics and Sports Medicine. Clin. Sports Med. 2016, 35, 257. [Google Scholar] [CrossRef] [PubMed]
- Callister, W.D., Jr.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 9th ed.; no. Book, Whole; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Greco, T.; Ferguson, L.; Giza, C.; Prins, M.L. Mechanisms underlying vulnerabilities after repeat mild traumatic brain injuries. Exp. Neurol. 2019, 317, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Koerte, I.K.; Lin, A.P.; Willems, A.; Muehlmann, M.; Hufschmidt, J.; Coleman, M.J.; Green, I.; Liao, H.; Tate, D.F.; Wilde, E.A.; et al. A review of neuroimaging findings in repetitive brain trauma. Brain Pathol. 2015, 25, 318–349. [Google Scholar] [CrossRef]
- Meconi, A.; Wortman, R.C.; Wright, D.K.; Neale, K.J.; Clarkson, M.; Shultz, S.R.; Christie, B.R. Repeated mild traumatic brain injury can cause acute neurologic impairment without overt structural damage in juvenile rats. PLoS ONE 2018, 13, e0197187. [Google Scholar] [CrossRef]
- Prins, M.; Giza, C.C. Repeat traumatic brain injury in the developing brain. Int. J. Dev. Neurosci. 2012, 30, 185–190. [Google Scholar] [CrossRef]
- Sollmann, N.; Echlin, P.S.; Schultz, V.; Viher, P.V.; Lyall, A.E.; Tripodis, Y.; Kaufmann, D.; Hartl, E.; Kinzel, P.; Forwell, L.A.; et al. Sex differences in white matter alterations following repetitive subconcussive head impacts in collegiate ice hockey players. Neuroimage Clin. 2018, 17, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Karton, C.; Hoshizaki, T.B.; Gilchrist, M.D. A novel repetitive head impact exposure measurement tool differentiates player position in National Football League. Sci. Rep. 2020, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- Slobounov, S.; Gay, M.; Johnson, B.; Zhang, K. Concussion in athletics: Ongoing clinical and brain imaging research controversies. Brain Imaging Behav. 2012, 6, 224–243. [Google Scholar] [CrossRef]
- Thériault, M.; De Beaumont, L.; Tremblay, S.; Lassonde, M.; Jolicoeur, P. Cumulative effects of concussions in athletes revealed by electrophysiological abnormalities on visual working memory. J. Clin. Exp. Neuropsychol. 2011, 33, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Uryu, K.; Laurer, H.; McIntosh, T.; Praticò, D.; Martinez, D.; Leight, S.; Lee, V.M.-Y.; Trojanowski, J.Q. Repetitive mild brain trauma accelerates Aβ deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 2002, 22, 446–454. [Google Scholar] [CrossRef]
- Bailes, J.E.; Petraglia, A.L.; Omalu, B.I.; Nauman, E.; Talavage, T. Role of subconcussion in repetitive mild traumatic brain injury: A review. J. Neurosurg. 2013, 119, 1235–1245. [Google Scholar] [CrossRef]
- Alosco, M.L.; Tripodis, Y.; Baucom, Z.H.; Mez, J.; Stein, T.D.; Martin, B.; Haller, O.; Conneely, S.; McClean, M.; Nosheny, R.; et al. Late contributions of repetitive head impacts and TBI to depression symptoms and cognition. Neurology 2020, 95, e793–e804. [Google Scholar] [CrossRef]
- Manley, G.; Gardner, A.J.; Schneider, K.J.; Guskiewicz, K.M.; Bailes, J.; Cantu, R.C.; Castellani, R.J.; Turner, M.; Jordan, B.D.; Randolph, C.; et al. A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med. 2017, 51, 969–977. [Google Scholar] [CrossRef]
- McAteer, K.M.; Corrigan, F.; Thornton, E.; Turner, R.J.; Vink, R. Short and long term behavioral and pathological changes in a novel rodent model of repetitive mild traumatic brain injury. PLoS ONE 2016, 11, e0160220. [Google Scholar] [CrossRef]
- McKee, A.C.; Alosco, M.L.; Huber, B.R. Repetitive head impacts and chronic traumatic encephalopathy. Neurosurg. Clin. N. Am. 2016, 27, 529–535. [Google Scholar] [CrossRef]
- Pearce, A.J. The neurophysiological response following sub-concussive soccer heading. EBioMedicine 2016, 13, 3–4. [Google Scholar] [CrossRef][Green Version]
- Bey, T.; Ostick, B. Second impact syndrome. West. J. Emerg. Med. 2009, 10, 6. [Google Scholar]
- Cantu, R.C.; Voy, R. Second impact syndrome: A risk in any contact sport. Phys. Sportsmed. 1995, 23, 27–34. [Google Scholar] [CrossRef]
- McCrory, P. Does second impact syndrome exist? Clin. J. Sport Med. 2001, 11, 144–149. [Google Scholar] [CrossRef] [PubMed]
- McCrory, P.; Feddermann-Demont, N.; Dvořák, J.; Cassidy, J.D.; McIntosh, A.; Vos, P.E.; Echemendia, R.J.; Meeuw, W. What is the definition of sports-related concussion: A systematic review. Br. J. Sports Med. 2017, 51, 877–887. [Google Scholar] [CrossRef] [PubMed]
- McCrory, P.R.; Berkovic, S.F. Second impact syndrome. Neurology 1998, 50, 677–683. [Google Scholar] [CrossRef]
- McCrea, H.J.; Perrine, K.; Niogi, S.; Härtl, R. Concussion in sports. Sports Health 2013, 5, 160–164. [Google Scholar] [CrossRef]
- McLendon, L.A.; Kralik, S.F.; Grayson, P.A.; Golomb, M.R. The controversial second impact syndrome: A review of the literature. Pediatr. Neurol. 2016, 62, 9–17. [Google Scholar] [CrossRef]
- Dadas, A.; Washington, J.; Diaz-Arrastia, R.; Janigro, D. Biomarkers in traumatic brain injury (TBI): A review. Neuropsychiatr. Dis. Treat. 2018, 14, 2989–3000. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.I.; Garimella, H.T.; Kraft, R.H. Computation of history-dependent mechanical damage of axonal fiber tracts in the brain: Towards tracking sub-concussive and occupational damage to the brain. bioRxiv 2018, 346700. [Google Scholar]
- Graham, R.; Rivara, F.P.; Ford, M.A.; Spicer, C.M.; Committee on Sports-Related Concussions in Youth; Board on Children, Youth, and Families; Institute of Medicine; National Research Council. Concussion recognition, diagnosis, and acute management. In Sports-Related Concussions in Youth: Improving the Science, Changing the Culture; National Academies Press: Washington, DC, USA, 2014. [Google Scholar]
- McAllister, T.; McCrea, M. Long-term cognitive and neuropsychiatric consequences of repetitive concussion and head-impact exposure. J. Athl. Train. 2017, 52, 309–317. [Google Scholar] [CrossRef]
- Meaney, D.F.; Morrison, B.; Bass, C.D. The mechanics of traumatic brain injury: A review of what we know and what we need to know for reducing its societal burden. J. Biomech. Eng. 2014, 136, 021008. [Google Scholar] [CrossRef]
- Mullally, W.J. Concussion. Am. J. Med. 2017, 130, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M. The Effects of Concussive and Sub-Concussive Head Impacts on Brain Activity. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2016. [Google Scholar]
- Miller, L.E.; Urban, J.E.; Davenport, E.M.; Powers, A.K.; Whitlow, C.T.; Maldjian, J.A.; Stitzel, J.D. Brain strain: Computational model-based metrics for head impact exposure and injury correlation. Ann. Biomed. Eng. 2021, 49, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Tyler, W.J. The mechanobiology of brain function. Nat. Rev. Neurosci. 2012, 13, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Schechtman, H.; Bader, D.L. Dynamic Characterization of Human Tendons. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1994, 208, 241–248. [Google Scholar] [CrossRef]
- Gilpin, C.M. Cyclic Loading of Porcine Coronary Arteries. Master’s Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2005. [Google Scholar]
- Vappou, J.; Breton, E.; Choquet, P.; Goetz, C.; Willinger, R.; Constantinesco, A. Magnetic resonance elastography compared with rotational rheometry for in vitro brain tissue viscoelasticity measurement. Magma 2007, 20, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Bayly, P.V.; Alshareef, A.; Knutsen, A.K.; Upadhyay, K.; Okamoto, R.J.; Carass, A.; Butman, J.A.; Pham, D.L.; Prince, J.L.; Ramesh, K.T.; et al. MR Imaging of Human Brain Mechanics In Vivo: New Measurements to Facilitate the Development of Computational Models of Brain Injury. Ann. Biomed. Eng. 2021, 49, 2677–2692. [Google Scholar] [CrossRef]
- Petrov, A.; Chase, J.G.; Sellier, M.; Docherty, P.D. Non-identifiability of the Rayleigh damping material model in Magnetic Resonance Elastography. Math. Biosci. 2013, 246, 191–201. [Google Scholar] [CrossRef]
- Petrov, A.Y.; Docherty, P.D.; Sellier, M.; Chase, J.G. Multi-frequency inversion in Rayleigh damped Magnetic Resonance Elastography. Biomed. Signal Process. Control 2014, 13, 270–281. [Google Scholar] [CrossRef]
- Chan, D.D.; Knutsen, A.K.; Lu, Y.-C.; Yang, S.H.; Magrath, E.; Wang, W.-T.; Bayly, P.V.; Butman, J.A.; Pham, D.L. Statistical Characterization of Human Brain Deformation During Mild Angular Acceleration Measured In Vivo by Tagged Magnetic Resonance Imaging. J. Biomech. Eng. 2018, 140, 10. [Google Scholar] [CrossRef]
- Gomez, A.D.; Bayly, P.V.; Butman, J.A.; Pham, D.L.; Prince, J.L.; Knutsen, A.K. Group characterization of impact-induced, in vivo human brain kinematics. J. R. Soc. Interface 2021, 18, 20210251. [Google Scholar] [CrossRef]
- Martin, C.; Sun, W. Fatigue Modeling of Collagenous Soft Tissue; Springer: New York, NY, USA, 2011; pp. 561–568. [Google Scholar]
- Huber, B.R.; Alosco, M.L.; Stein, T.D.; McKee, A.C. Potential long-term consequences of concussive and subconcussive injury. Phys. Med. Rehabil. Clin. 2016, 27, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.H. Brain Damage in Boxers: A Study of the Prevalence of Traumatic Encephalopathy Among Ex-Professional Boxers; Pitman Medical & Scientific Pub. Co.: London, UK, 1969. [Google Scholar]
- Bajwa, N.M.; Halavi, S.; Hamer, M.; Semple, B.D.; Noble-Haeusslein, L.J.; Baghchechi, M.; Hiroto, A.; Hartman, R.E.; Obenaus, A. Mild Concussion, but Not Moderate Traumatic Brain Injury, Is Associated with Long-Term Depression-like Phenotype in Mice. PLoS ONE 2016, 11, e0146886. [Google Scholar] [CrossRef][Green Version]
- Hoogenboom, W.S.; Rubin, T.G.; Ambadipudi, K.; Cui, M.-H.; Ye, K.; Foster, H.; Elkouby, E.; Liu, J.; A Branch, C.; Lipton, M.L. Evolving brain and behaviour changes in rats following repetitive subconcussive head impacts. Brain Commun. 2023, 5, fcad316. [Google Scholar] [CrossRef] [PubMed]
- Donovan, V.; Kim, C.; Anugerah, A.K.; Coats, J.S.; Oyoyo, U.; Pardo, A.C.; Obenaus, A. Repeated Mild Traumatic Brain Injury Results in Long-Term White-Matter Disruption. J. Cereb. Blood Flow Metab. 2014, 34, 715–723. [Google Scholar] [CrossRef] [PubMed]
- DeFord, S.M.; Wilson, M.S.; Rice, A.C.; Clausen, T.; Rice, L.K.; Barabnova, A.; Bullock, R.; Hamm, R.J. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J. Neurotrauma 2002, 19, 427–438. [Google Scholar] [CrossRef]
- Vink, R. Large animal models of traumatic brain injury. J. Neurosci. Res. 2018, 96, 527–535. [Google Scholar] [CrossRef]
- Anderson, R.W.G.; Sandoz, B.; Dutschke, J.; Finnie, J.; Turner, R.; Blumbergs, P.; Manavis, J.; Vink, R. Biomechanical studies in an ovine model of non-accidental head injury. J. Biomech. 2014, 47, 2578–2583. [Google Scholar] [CrossRef]
- Cernak, I. Animal Models of Head Trauma. NeuroRX 2005, 2, 410–422. [Google Scholar] [CrossRef]
- Wang, X.T.; Ker, R.F.; Alexander, R.M. Fatigue rupture of wallaby tail tendons. J. Exp. Biol. 1995, 198, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Anton Paar GmbH. Modular Compact Rheometer: MCR 102e/302e/502e; Technical Specifications. Available online: https://www.anton-paar.com/nz-en/products/details/rheometer-mcr-102-302-502/ (accessed on 1 December 2023).
- Hrapko, M.; van Dommelen, J.A.W.; Peters, G.W.M.; Wismans, J.S.H.M. The influence of test conditions on characterization of the mechanical properties of brain tissue. J. Biomech. Eng. 2008, 130, 031003. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y. Dynamic Deformation and Mechanical Properties of Brain Tissue. Ph.D. Thesis, Washington University, St. Louis, MO, USA, 2012. [Google Scholar]
- Bilston, L.E.; Liu, Z.; Phan-Thien, N. Linear viscoelastic properties of bovine brain tissue in shear. Biorheology 1997, 34, 377. [Google Scholar] [CrossRef]
- Bilston, L.E.; Liu, Z.; Phan-Thien, N. Large strain behaviour of brain tissue in shear: Some experimental data and differential constitutive model. Biorheology 2001, 38, 335. [Google Scholar]
- Fehily, B.; Fitzgerald, M. Repeated Mild Traumatic Brain Injury: Potential Mechanisms of Damage. Cell Transpl. 2017, 26, 1131–1155. [Google Scholar] [CrossRef]
- Chen, Z.; Shin, D.; Chen, S.; Mikhail, K.; Hadass, O.; Tomlison, B.N.; Korkin, D.; Shyu, C.-R.; Cui, J.; Anthony, D.C.; et al. Histological quantitation of brain injury using whole slide imaging: A pilot validation study in mice. PLoS ONE 2014, 9, e92133. [Google Scholar] [CrossRef]
- Al-Sarraj, S. The pathology of traumatic brain injury (TBI): A practical approach. Diagn. Histopathol. 2016, 22, 318–326. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lilley, R.L.; Kabaliuk, N.; Reynaud, A.; Devananthan, P.; Smith, N.; Docherty, P.D. A Novel Experimental Approach for the Measurement of Vibration-Induced Changes in the Rheological Properties of Ex Vivo Ovine Brain Tissue. Sensors 2024, 24, 2022. https://doi.org/10.3390/s24072022
Lilley RL, Kabaliuk N, Reynaud A, Devananthan P, Smith N, Docherty PD. A Novel Experimental Approach for the Measurement of Vibration-Induced Changes in the Rheological Properties of Ex Vivo Ovine Brain Tissue. Sensors. 2024; 24(7):2022. https://doi.org/10.3390/s24072022
Chicago/Turabian StyleLilley, Rebecca L., Natalia Kabaliuk, Antoine Reynaud, Pavithran Devananthan, Nicole Smith, and Paul D. Docherty. 2024. "A Novel Experimental Approach for the Measurement of Vibration-Induced Changes in the Rheological Properties of Ex Vivo Ovine Brain Tissue" Sensors 24, no. 7: 2022. https://doi.org/10.3390/s24072022
APA StyleLilley, R. L., Kabaliuk, N., Reynaud, A., Devananthan, P., Smith, N., & Docherty, P. D. (2024). A Novel Experimental Approach for the Measurement of Vibration-Induced Changes in the Rheological Properties of Ex Vivo Ovine Brain Tissue. Sensors, 24(7), 2022. https://doi.org/10.3390/s24072022





