Key Contributors to Signal Generation in Frequency Mixing Magnetic Detection (FMMD): An In Silico Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Magnetic Relaxation Theory
2.2. Simulation Implementation & Framework
2.3. Simulation Input: Key Parameters Varied
- The intrinsic physical properties of the MNP: the particle core size, , its size distribution width, , the effective anisotropy constant, , as well as the hydrodynamic size, .
- The external influences the applied field parameters of the AFM: the excitation frequency, , and the drive field amplitude, . Note that the drive frequency, kHz, and excitation field amplitude, mT/µ0, are expected to contribute much less to the FMMD signal generation as they are at least one order of magnitude lower than their respective counterparts [36]; therefore, they are fixed for all simulations.
3. Results
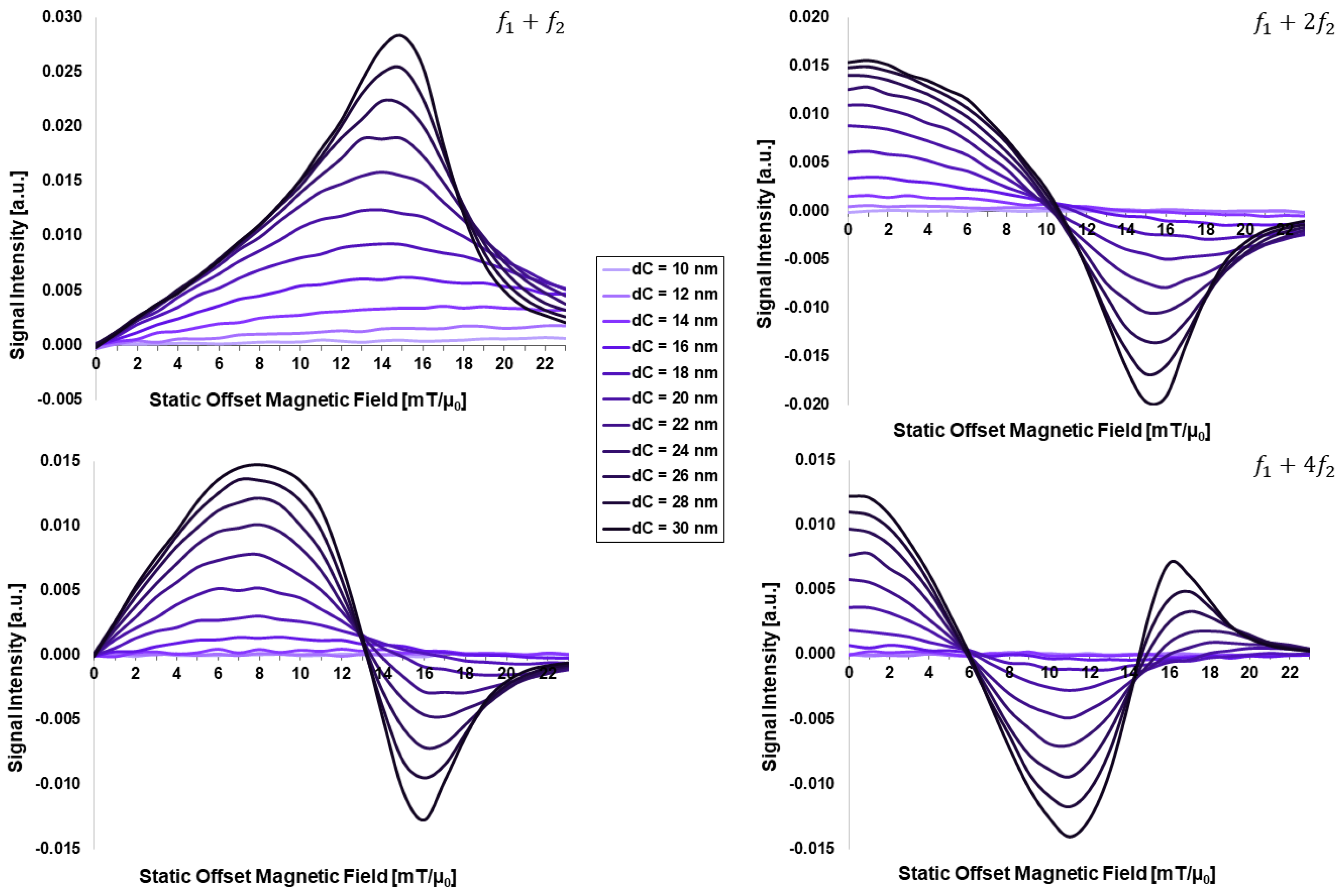
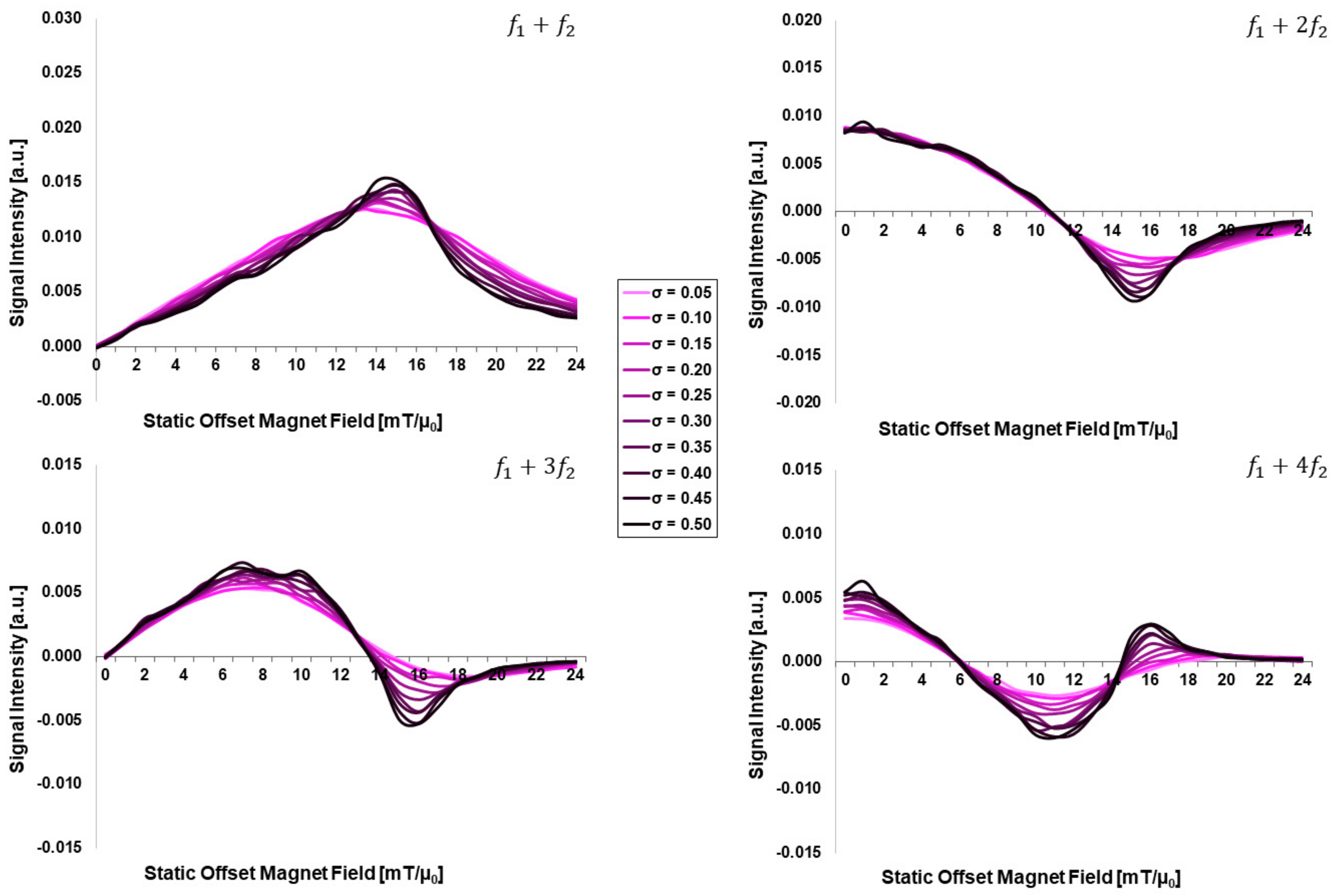
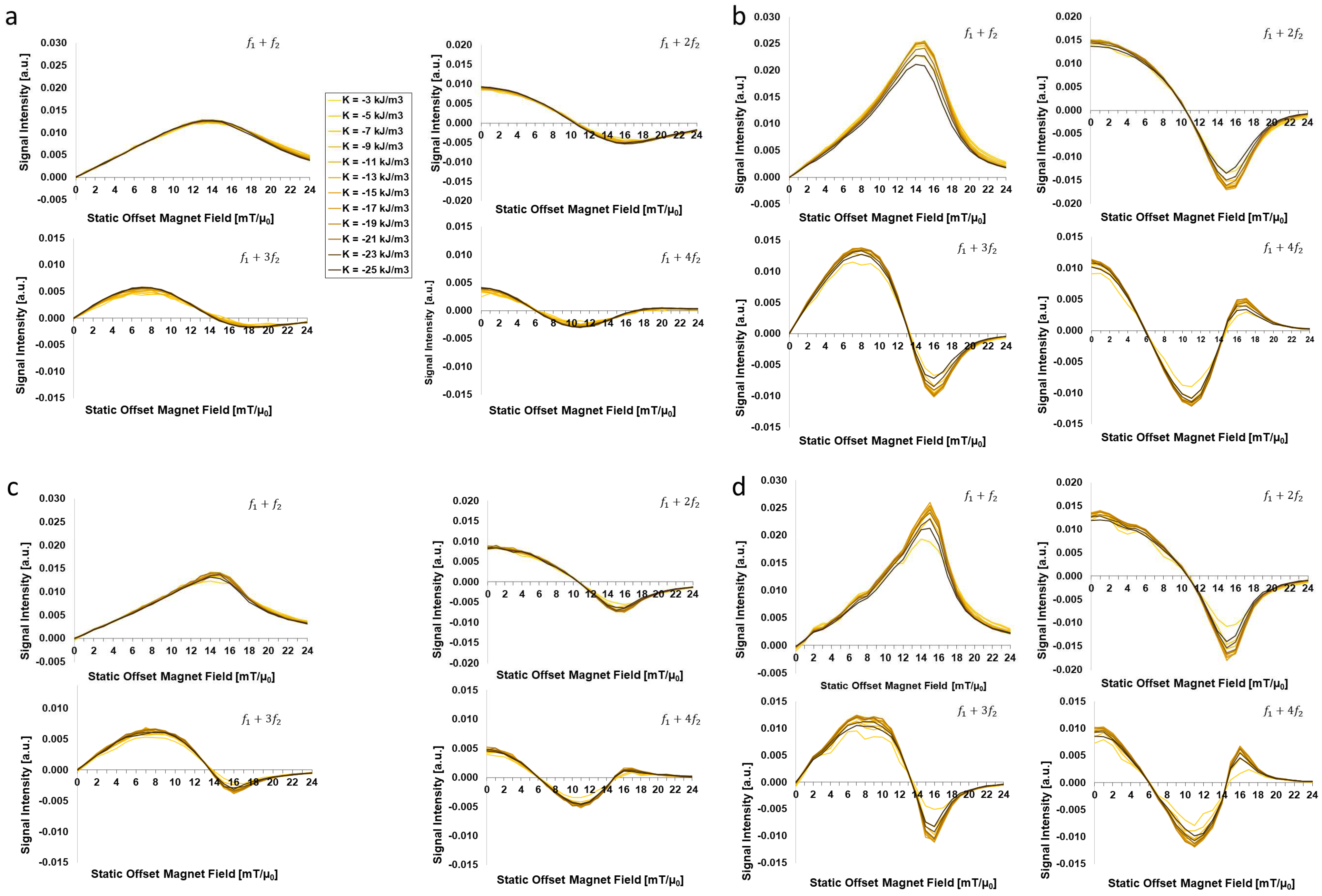
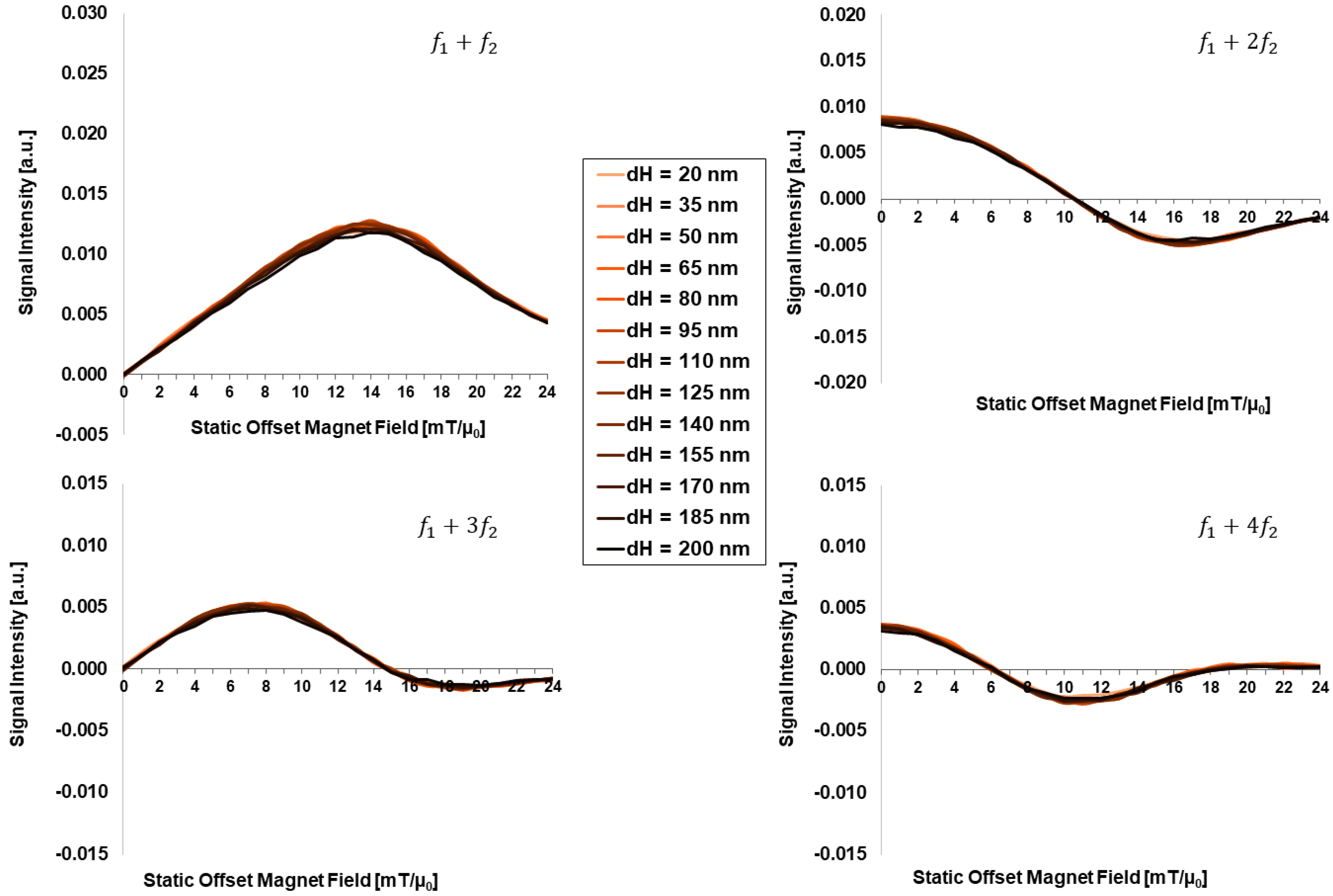
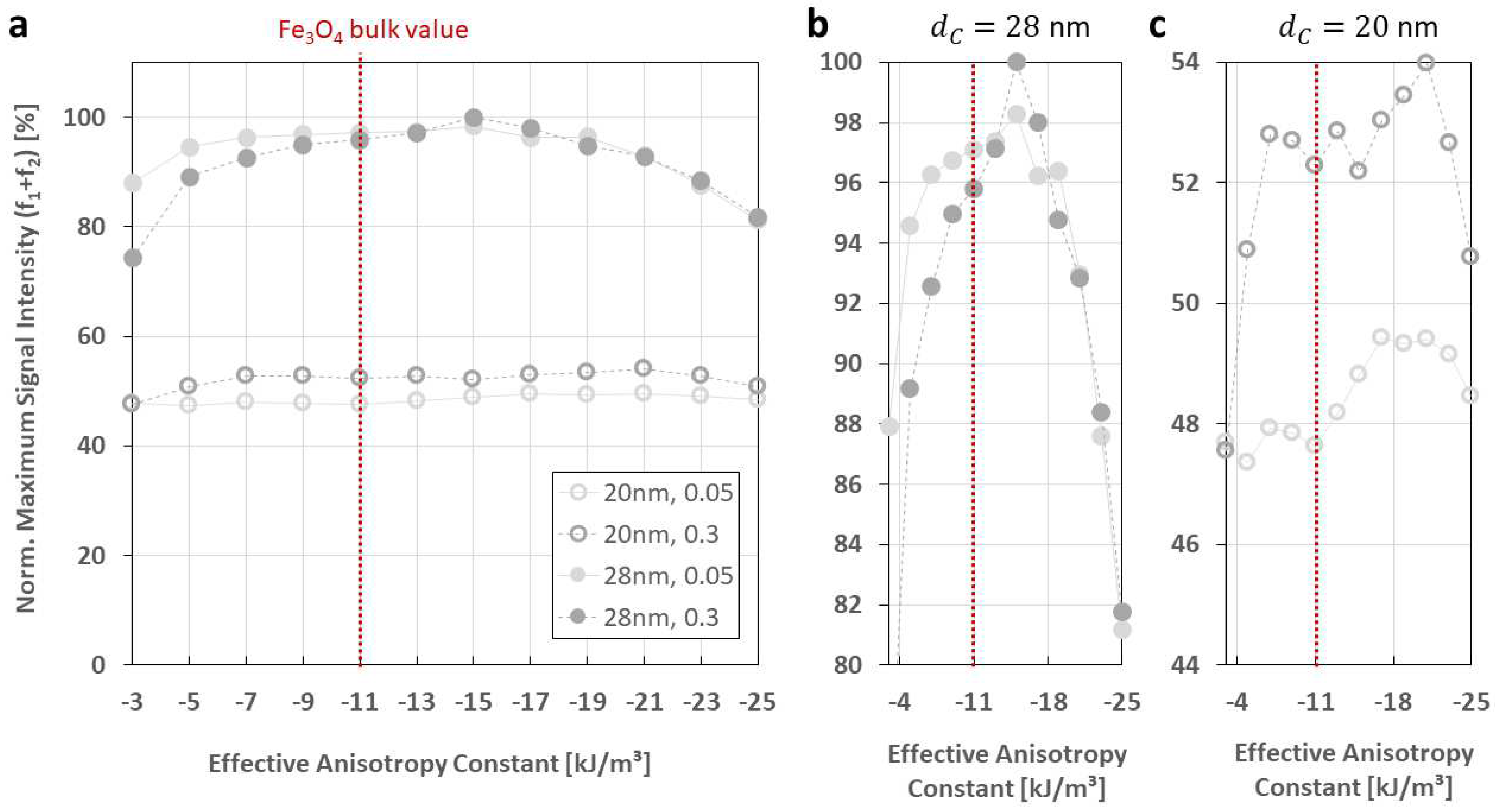
3.1. Dependency on Intrinsic Particle Properties:
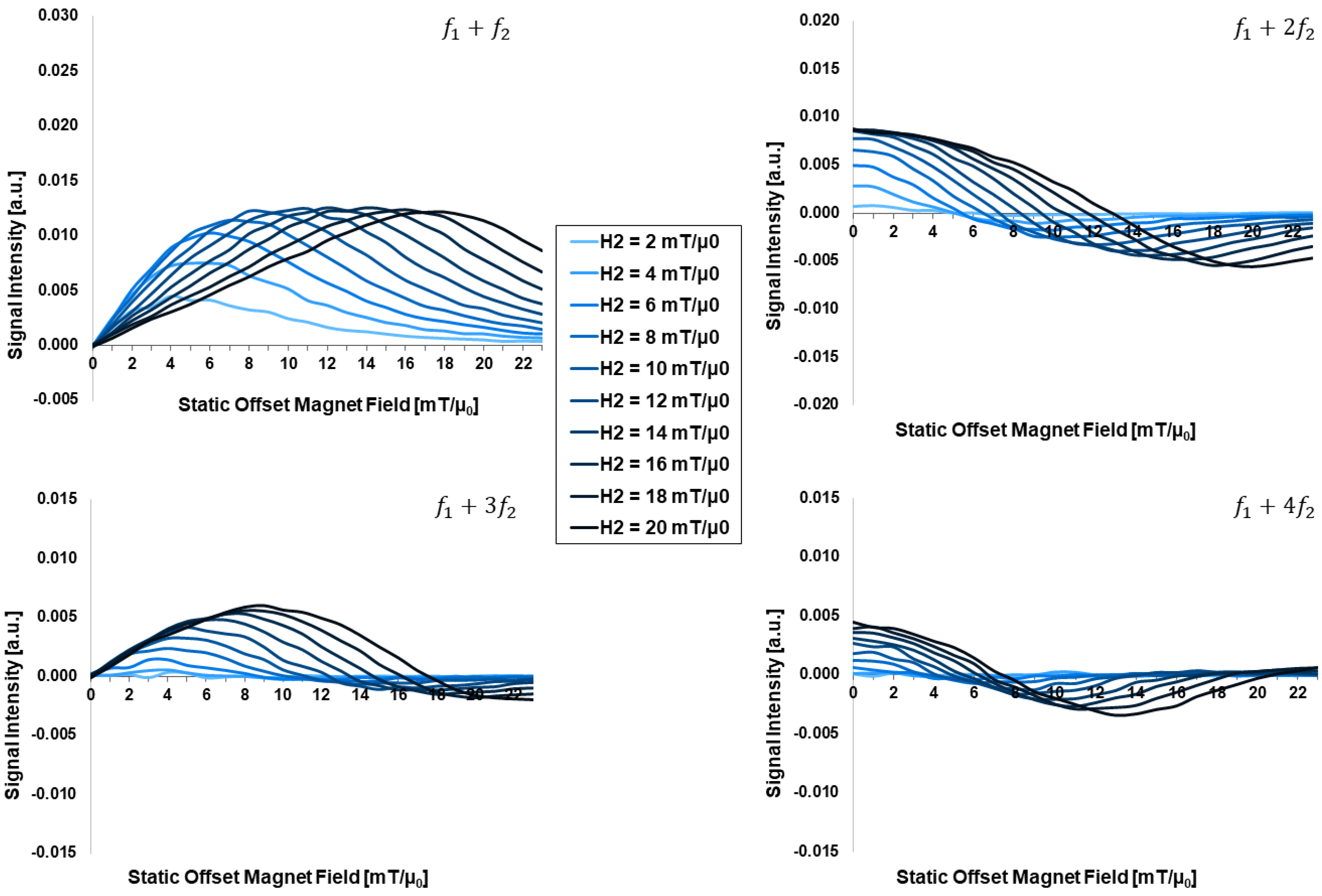
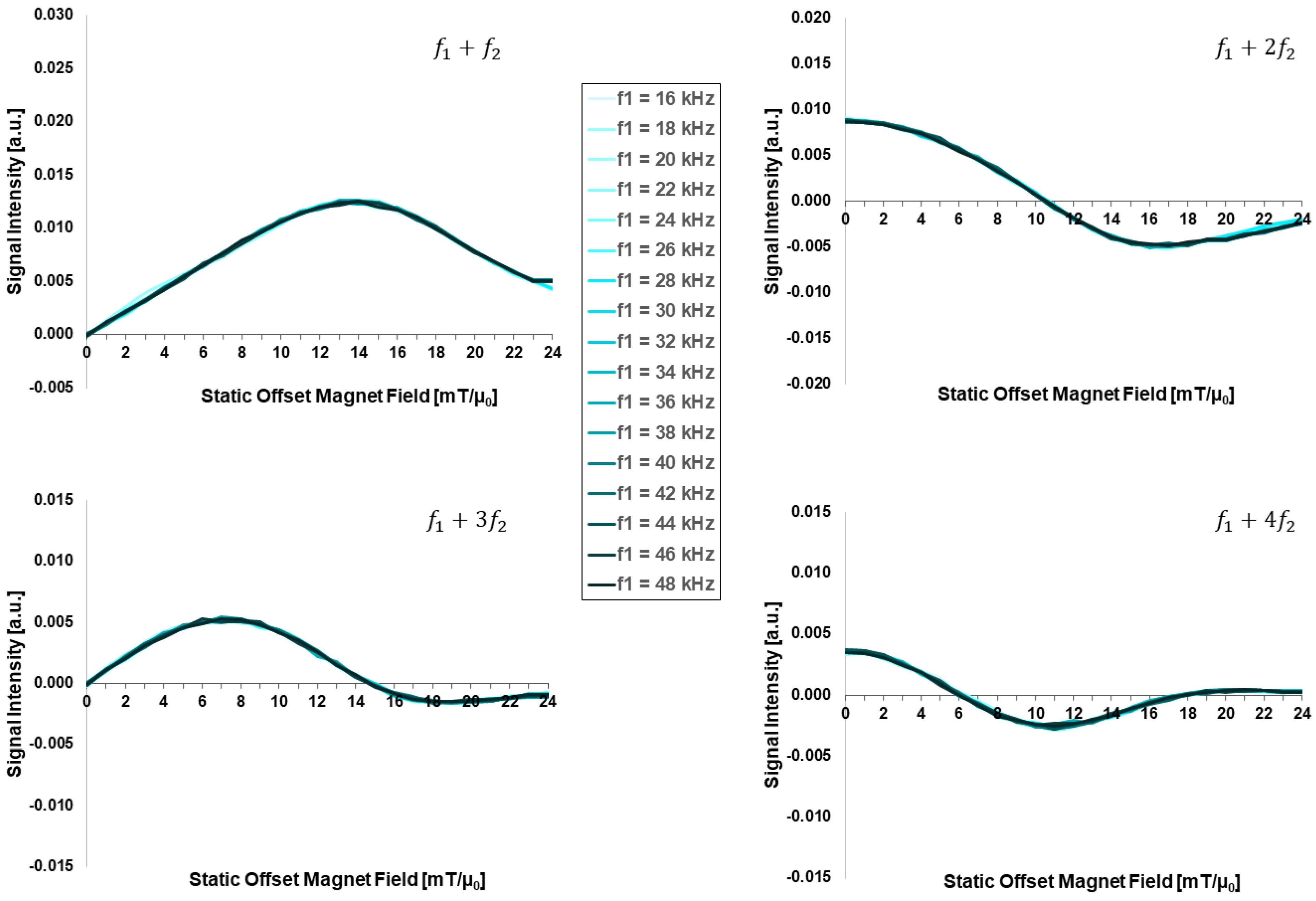
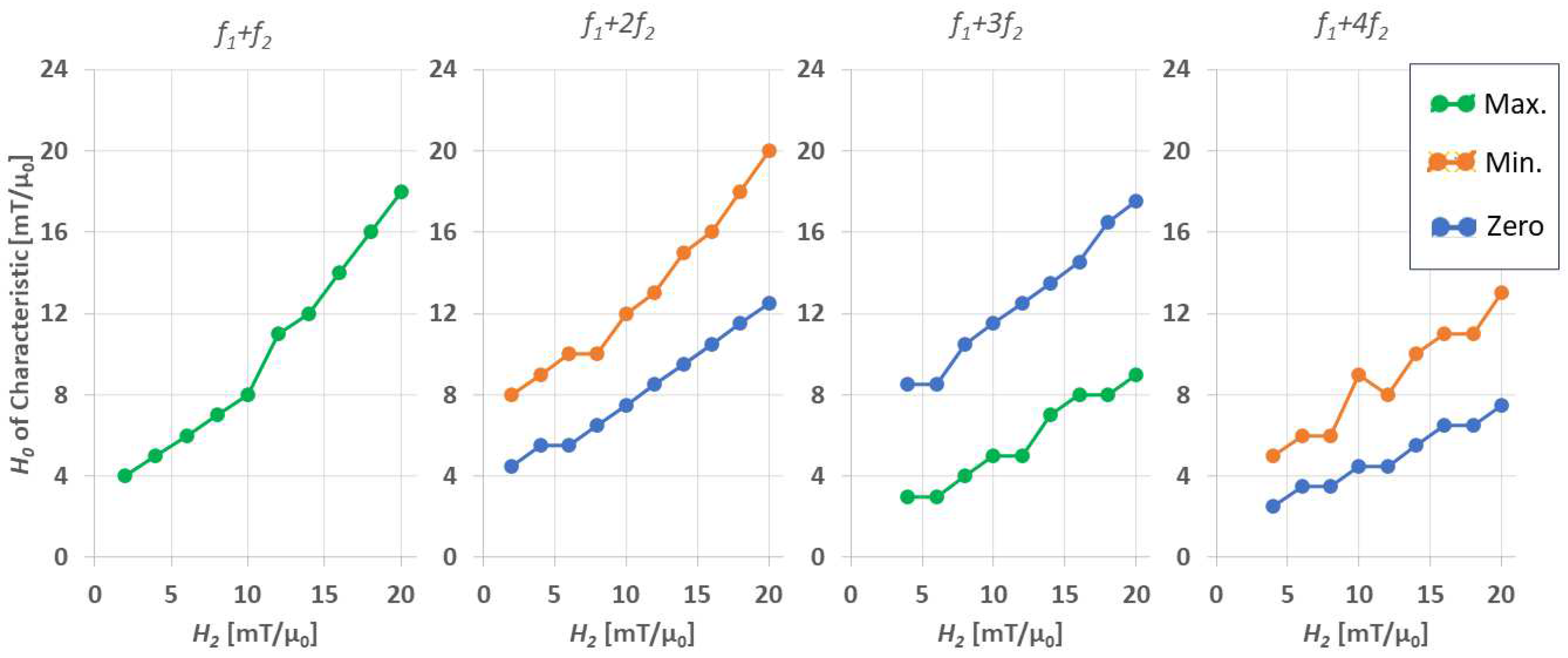
3.2. Dependency on External Applied Field Parameters:
3.3. Summary of Effects of Key Parameters
4. Discussion
4.1. FMMD Dependency on Intrinsic Particle Properties:
4.2. FMMD Dependency on External Applied Field Parameters:
4.3. Limitations and Potential Improvements of the Simulation Framework
- (1)
- As identified in Section 4.1 (discussing the -dependence), the present simulation framework does not consider particle agglomeration/clustering. However, it is becoming more and more evident that agglomerations (or clusters) play a significant role in MNP systems, either globally (non-directional) [62,63] or as a precondition by purposeful alignment of MNP [64,65]. Recently, it has been demonstrated that agglomeration in a similar simulation framework can be included [66]. However, integration of agglomeration of MNP is indisputably linked to the consideration of magnetic dipole–dipole interactions [67,68], as well as a (more) complex description of the hydrodynamic size [52,69]. Even though the present framework is capable of including magnetic dipole–dipole interactions [27,31], it is not yet sufficiently optimized to be run time-efficiently, since the incorporation of such interactions increases computation time exponentially [34].
- (2)
- As identified in Section 4.2, the variation of core size, , cannot be separated apart from that of effective anisotropy, . Therefore, future investigations shall incorporate Equation (7) in the simulation framework to investigate the core-size dependency of the anisotropy constant further.
5. Conclusions
- (1)
- Core-size effects are strongly dominating the FMMD signal generation, above all other analyzed intrinsic particle properties. This is visible both directly in a steady increase in FMMD signal intensity with increasing , as well as indirectly by increasing the core-size distribution and thereby introducing dominating contributions from few large particles.
- (2)
- The effective anisotropy does have a remarkable effect of FMMD signal generation, but is secondary to that of (larger) core sizes. However, there is evidence that the effective anisotropy itself is core-size-dependent, such that is increasing for smaller sized particles, as summarized in Figure 7.
- (3)
- The drive field amplitude is dominating the shape of the FMMD signal profile. For given magnetic particle ensembles, in case of even mixing terms , , …, the offset field should be zero, and the drive field amplitude should be turned up to the characteristic field of the ensemble. In case of odd terms , , …, the combination of drive field amplitude and static offset field value needs to be optimized, as summarized in Figure 8.
- (4)
- The hydrodynamic size, as well as the excitation frequency, does not show any noticeable effect on FMMD signal generation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef]
- Yu, E.Y.; Bishop, M.; Zheng, B.; Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Krishnan, K.M.; Goodwill, P.W.; Conolly, S.M. Magnetic particle imaging: A novel in vivo imaging platform for cancer detection. Nano Lett. 2017, 17, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Zhang, Y.; Steinberg, G.; Qu, H.; Huang, S.; Cheng, M.; Bliss, T.; Du, F.; Rao, J.; Song, G. A review of magnetic particle imaging and perspectives on neuroimaging. Am. J. Neuroradiol. 2019, 40, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Southern, P.; Pankhurst, Q.A. Commentary on the clinical and preclinical dosage limits of interstitially administered magnetic fluids for therapeutic hyperthermia based on current practice and efficacy models. Int. J. Hyperth. 2018, 34, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Hedayatnasab, Z.; Abnisa, F.; Daud, W.M.A.W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Wu, K.; Liu, J.; Chugh, V.K.; Liang, S.; Saha, R.; Krishna, V.D.; Cheeran, M.C.J.; Wang, J.-P. Magnetic Nanoparticles and Magnetic Particle Spectroscopy-based Bioassays: A 15 year Recap. Nano Futures 2022, 6, 022001. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Saha, R.; Wong, D.; Wang, J.-P. Magnetic particle spectroscopy-based bioassays: Methods, applications, advances, and future opportunities. J. Phys. D Appl. Phys. 2019, 52, 173001. [Google Scholar] [CrossRef]
- Hong, H.B.; Krause, H.-J.; Nam, I.H.; Choi, C.J.; Shin, S.W. Magnetic immunoassay based on frequency mixing magnetic detection and magnetic particles of different magnetic properties. Anal. Methods 2014, 6, 8055–8058. [Google Scholar] [CrossRef]
- Hong, H.; Lim, J.; Choi, C.-J.; Shin, S.-W.; Krause, H.-J. Magnetic particle imaging with a planar frequency mixing magnetic detection scanner. Rev. Sci. Instrum. 2014, 85, 13705. [Google Scholar] [CrossRef]
- Krause, H.-J.; Wolters, N.; Zhang, Y.; Offenhäusser, A.; Miethe, P.; Meyer, M.H.F.; Hartmann, M.; Keusgen, M. Magnetic particle detection by frequency mixing for immunoassay applications. J. Magn. Magn. Mater. 2007, 311, 436–444. [Google Scholar] [CrossRef]
- Pourshahidi, A.M.; Achtsnicht, S.; Nambipareechee, M.M.; Offenhäusser, A.; Krause, H.-J. Multiplex Detection of Magnetic Beads Using Offset Field Dependent Frequency Mixing Magnetic Detection. Sensors 2021, 21, 5859. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Saha, R.; Su, D.; Krishna, V.D.; Liu, J.; Cheeran, M.C.-J.; Wang, J.-P. Magnetic-nanosensor-based virus and pathogen detection strategies before and during COVID-19. ACS Appl. Nano Mater. 2020, 3, 9560–9580. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, J.; Saha, R.; Su, D.; Krishna, V.D.; Cheeran, M.C.-J.; Wang, J.-P. Magnetic particle spectroscopy for detection of influenza A virus subtype H1N1. ACS Appl. Mater. Interfaces 2020, 12, 13686–13697. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.-B.; Krause, H.-J.; Song, K.-B.; Choi, C.-J.; Chung, M.-A.; Son, S.; Offenhäusser, A. Detection of two different influenza A viruses using a nitrocellulose membrane and a magnetic biosensor. J. Immunol. Methods 2011, 365, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, J.; Dittmann, D.; Spiegel, H.; Krause, H.-J.; Schröper, F. A Novel Method for Antibiotic Detection in Milk Based on Competitive Magnetic Immunodetection. Foods 2020, 9, 1773. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, J.; Spiegel, H.; Krause, H.-J.; Schillberg, S.; Schröper, F. Sensitive aflatoxin B1 detection using nanoparticle-based competitive magnetic immunodetection. Toxins 2020, 12, 337. [Google Scholar] [CrossRef]
- Pourshahidi, A.M.; Engelmann, U.M.; Offenhäusser, A.; Krause, H.-J. Resolving ambiguities in core size determination of magnetic nanoparticles from magnetic frequency mixing data. J. Magn. Magn. Mater. 2022, 563, 169969. [Google Scholar] [CrossRef]
- Mamiya, H.; Jeyadevan, B. Hyperthermic effects of dissipative structures of magnetic nanoparticles in large alternating magnetic fields. Sci. Rep. 2011, 1, 157. [Google Scholar] [CrossRef]
- Reeves, D.B. Nonequilibrium Dynamics of Magnetic Nanoparticles in Biomedical Applications; Dartmouth College: Hanover, NH, USA, 2015; ISBN 1321824769. [Google Scholar]
- Reeves, D.B.; Weaver, J.B. Combined Néel and Brown rotational Langevin dynamics in magnetic particle imaging, sensing, and therapy. Appl. Phys. Lett. 2015, 107, 223106. [Google Scholar] [CrossRef]
- Kratz, H.; Taupitz, M.; Ariza de Schellenberger, A.; Kosch, O.; Eberbeck, D.; Wagner, S.; Trahms, L.; Hamm, B.; Schnorr, J. Novel magnetic multicore nanoparticles designed for MPI and other biomedical applications: From synthesis to first in vivo studies. PLoS ONE 2018, 13, e0190214. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.-P.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, 2304848. [Google Scholar] [CrossRef]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C. Magnetic particle imaging-guided heating in vivo using gradient fields for arbitrary localization of magnetic hyperthermia therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef]
- Lu, Y.; Rivera-Rodriguez, A.; Tay, Z.W.; Hensley, D.; Fung, K.B.; Colson, C.; Saayujya, C.; Huynh, Q.; Kabuli, L.; Fellows, B. Combining magnetic particle imaging and magnetic fluid hyperthermia for localized and image-guided treatment. Int. J. Hyperth. 2020, 37, 141–154. [Google Scholar] [CrossRef]
- Buchholz, O.; Sajjamark, K.; Franke, J.; Wei, H.; Behrends, A.; Münkel, C.; Grüttner, C.; Levan, P.; von Elverfeldt, D.; Graeser, M. In situ theranostic platform combining highly localized magnetic fluid hyperthermia, magnetic particle imaging, and thermometry in 3D. Theranostics 2024, 14, 324. [Google Scholar] [CrossRef]
- Shasha, C.; Krishnan, K.M. Nonequilibrium Dynamics of Magnetic Nanoparticles with Applications in Biomedicine. Adv. Mater. 2021, 33, 1904131. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, U.M.; Shasha, C.; Teeman, E.; Slabu, I.; Krishnan, K.M. Predicting size-dependent heating efficiency of magnetic nanoparticles from experiment and stochastic Néel-Brown Langevin simulation. J. Magn. Magn. Mater. 2019, 471, 450–456. [Google Scholar] [CrossRef]
- Engelmann, U.M.; Shalaby, A.; Shasha, C.; Krishnan, K.M.; Krause, H.-J. Comparative Modeling of Frequency Mixing Measurements of Magnetic Nanoparticles Using Micromagnetic Simulations and Langevin Theory. Nanomaterials 2021, 11, 1257. [Google Scholar] [CrossRef]
- Engelmann, U.M.; Pourshahidi, A.M.; Shalaby, A.; Krause, H.-J. Probing particle size dependency of frequency mixing magnetic detection with dynamic relaxation simulation. J. Magn. Magn. Mater. 2022, 563, 169965. [Google Scholar] [CrossRef]
- Shasha, C. Nonequilibrium Nanoparticle Dynamics for the Development of Magnetic Particle Imaging. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 2019. [Google Scholar]
- Gilbert, T.L. Classics in Magnetics A Phenomenological Theory of Damping in Ferromagnetic Materials. IEEE Trans. Magn. 2004, 40, 3443–3449. [Google Scholar] [CrossRef]
- Usov, N.A.; Liubimov, B.Y. Dynamics of magnetic nanoparticle in a viscous liquid: Application to magnetic nanoparticle hyperthermia. J. Appl. Phys. 2012, 112, 23901. [Google Scholar] [CrossRef]
- Engelmann, U.M.; Shasha, C.; Slabu, I. Magnetic nanoparticle relaxation in biomedical application: Focus on simulating nanoparticle heating. In Magnetic Nanoparticles in Human Health and Medicine: Current Medical Applications and Alternative Therapy of Cancer; Wiley: Hoboken, NJ, USA, 2021; pp. 327–354. [Google Scholar]
- Shah, S.A.; Reeves, D.B.; Ferguson, R.M.; Weaver, J.B.; Krishnan, K.M. Mixed Brownian alignment and Néel rotations in superparamagnetic iron oxide nanoparticle suspensions driven by an ac field. Phys. Rev. B 2015, 92, 94438. [Google Scholar] [CrossRef]
- Achtsnicht, S. Multiplex Magnetic Detection of Superparamagnetic Beads for the Identification of Contaminations in Drinking Water. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2020. [Google Scholar]
- Thanh, N.T.K. Magnetic Nanoparticles: From Fabrication to Clinical Applications; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Philip, J. Magnetic nanofluids (Ferrofluids): Recent advances, applications, challenges, and future directions. Adv. Colloid Interface Sci. 2023, 311, 102810. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Thanh, N.T.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef]
- Krishnan, K.M. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef]
- Ludwig, F.; Remmer, H.; Kuhlmann, C.; Wawrzik, T.; Arami, H.; Ferguson, R.M.; Krishnan, K.M. Self-consistent magnetic properties of magnetite tracers optimized for magnetic particle imaging measured by ac susceptometry, magnetorelaxometry and magnetic particle spectroscopy. J. Magn. Magn. Mater. 2014, 360, 169–173. [Google Scholar] [CrossRef]
- Mamiya, H.; Fukumoto, H.; Cuya Huaman, J.L.; Suzuki, K.; Miyamura, H.; Balachandran, J. Estimation of Magnetic Anisotropy of Individual Magnetite Nanoparticles for Magnetic Hyperthermia. ACS Nano 2020, 14, 8421–8432. [Google Scholar] [CrossRef]
- Aali, H.; Mollazadeh, S.; Khaki, J.V. Single-phase magnetite with high saturation magnetization synthesized via modified solution combustion synthesis procedure. Ceram. Int. 2018, 44, 20267–20274. [Google Scholar] [CrossRef]
- Pourshahidi, A.M.; Achtsnicht, S.; Offenhäusser, A.; Krause, H.-J. Frequency Mixing Magnetic Detection Setup Employing Permanent Ring Magnets as a Static Offset Field Source. Sensors 2022, 22, 8776. [Google Scholar] [CrossRef]
- Kemp, S.J.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Monodisperse magnetite nanoparticles with nearly ideal saturation magnetization. RSC Adv. 2016, 6, 77452–77464. [Google Scholar] [CrossRef]
- Arami, H.; Teeman, E.; Troksa, A.; Bradshaw, H.; Saatchi, K.; Tomitaka, A.; Gambhir, S.S.; Häfeli, U.O.; Liggitt, D.; Krishnan, K.M. Tomographic magnetic particle imaging of cancer targeted nanoparticles. Nanoscale 2017, 9, 18723–18730. [Google Scholar] [CrossRef]
- Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Arami, H.; Saritas, E.U.; Croft, L.R.; Konkle, J.; Goodwill, P.W.; Halkola, A.; Rahmer, J. Magnetic particle imaging with tailored iron oxide nanoparticle tracers. IEEE Trans. Med. Imaging 2014, 34, 1077–1084. [Google Scholar] [CrossRef]
- Shasha, C.; Teeman, E.; Krishnan, K.M. Nanoparticle core size optimization for magnetic particle imaging. Biomed. Phys. Eng. Express 2019, 5, 55010. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Teran, F.J.; Ortega, D. Chapter 8—Current Outlook and Perspectives on Nanoparticle-Mediated Magnetic Hyperthermia. In Iron Oxide Nanoparticles for Biomedical Applications: Metal Oxides; Mahmoudi, M., Laurent, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 197–245. ISBN 978-0-08-101925-2. [Google Scholar]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Ilg, P.; Kröger, M. Dynamics of interacting magnetic nanoparticles: Effective behavior from competition between Brownian and Néel relaxation. Phys. Chem. Chem. Phys. 2020, 22, 22244–22259. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, F.; Balceris, C.; Viereck, T.; Posth, O.; Steinhoff, U.; Gavilan, H.; Costo, R.; Zeng, L.; Olsson, E.; Jonasson, C.; et al. Size analysis of single-core magnetic nanoparticles. J. Magn. Magn. Mater. 2017, 427, 19–24. [Google Scholar] [CrossRef]
- Engelmann, U.M.; Buhl, E.M.; Draack, S.; Viereck, T.; Ludwig, F.; Schmitz-Rode, T.; Slabu, I. Magnetic relaxation of agglomerated and immobilized iron oxide nanoparticles for hyperthermia and imaging applications. IEEE Magn. Lett. 2018, 9, 1507305. [Google Scholar] [CrossRef]
- Aharoni, A. Introduction to the Theory of Ferromagnetism; Clarendon Press: Oxford, UK, 2000; ISBN 0198508085. [Google Scholar]
- Krishnan, K.M. Fundamentals and Applications of Magnetic Materials; Oxford University Press: Oxford, UK, 2016; ISBN 0199570442. [Google Scholar]
- Salazar-Alvarez, G.; Qin, J.; Sepelak, V.; Bergmann, I.; Vasilakaki, M.; Trohidou, K.N.; Ardisson, J.D.; Macedo, W.A.; Mikhaylova, M.; Muhammed, M. Cubic versus spherical magnetic nanoparticles: The role of surface anisotropy. J. Am. Chem. Soc. 2008, 130, 13234–13239. [Google Scholar] [CrossRef]
- Bødker, F.; Mørup, S.; Linderoth, S. Surface effects in metallic iron nanoparticles. Phys. Rev. Lett. 1994, 72, 282. [Google Scholar] [CrossRef]
- Dieckhoff, J.; Eberbeck, D.; Schilling, M.; Ludwig, F. Magnetic-field dependence of Brownian and Néel relaxation times. J. Appl. Phys. 2016, 119, 43903. [Google Scholar] [CrossRef]
- Yoshida, T.; Enpuku, K. Simulation and Quantitative Clarification of AC Susceptibility of Magnetic Fluid in Nonlinear Brownian Relaxation Region. Jpn. J. Appl. Phys. 2009, 48, 127002. [Google Scholar] [CrossRef]
- Hensley, D.; Tay, Z.W.; Dhavalikar, R.; Zheng, B.; Goodwill, P.; Rinaldi, C.; Conolly, S. Combining magnetic particle imaging and magnetic fluid hyperthermia in a theranostic platform. Phys. Med. Biol. 2017, 62, 3483. [Google Scholar] [CrossRef]
- Healy, S.; Bakuzis, A.F.; Goodwill, P.W.; Attaluri, A.; Bulte, J.W.M.; Ivkov, R. Clinical magnetic hyperthermia requires integrated magnetic particle imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1779. [Google Scholar] [CrossRef]
- Egler-Kemmerer, A.-N.; Baki, A.; Löwa, N.; Kosch, O.; Thiermann, R.; Wiekhorst, F.; Bleul, R. Real-time analysis of magnetic nanoparticle clustering effects by inline-magnetic particle spectroscopy. J. Magn. Magn. Mater. 2022, 564, 169984. [Google Scholar] [CrossRef]
- Gavilán, H.; Avugadda, S.K.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic nanoparticles and clusters for magnetic hyperthermia: Optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef]
- Niculaes, D.; Lak, A.; Anyfantis, G.C.; Marras, S.; Laslett, O.; Avugadda, S.K.; Cassani, M.; Serantes, D.; Hovorka, O.; Chantrell, R. Asymmetric assembling of iron oxide nanocubes for improving magnetic hyperthermia performance. ACS Nano 2017, 11, 12121–12133. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsugi, Y.; Tsujimura, N.; Sasayama, T.; Enpuku, K.; Viereck, T.; Schilling, M.; Ludwig, F. Effect of alignment of easy axes on dynamic magnetization of immobilized magnetic nanoparticles. J. Magn. Magn. Mater. 2017, 427, 162–167. [Google Scholar] [CrossRef]
- Durhuus, F.L.; Wandall, L.H.; Boisen, M.H.; Kure, M.; Beleggia, M.; Frandsen, C. Simulated clustering dynamics of colloidal magnetic nanoparticles. Nanoscale 2021, 13, 1970–1981. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Saha, R.; Liu, J.; Wang, J.-P. Investigating the effect of magnetic dipole–dipole interaction on magnetic particle spectroscopy: Implications for magnetic nanoparticle-based bioassays and magnetic particle imaging. J. Phys. D Appl. Phys. 2019, 52, 335002. [Google Scholar] [CrossRef]
- Ilg, P.; Kröger, M. Field- and concentration-dependent relaxation of magnetic nanoparticles and optimality conditions for magnetic fluid hyperthermia. Sci. Rep. 2023, 13, 16523. [Google Scholar] [CrossRef]
- Ivanov, A.O.; Camp, P.J. Effects of interactions, structure formation, and polydispersity on the dynamic magnetic susceptibility and magnetic relaxation of ferrofluids. J. Mol. Liq. 2022, 356, 119034. [Google Scholar] [CrossRef]
| Parameter | [nm] | [--] | [nm] | [kJ/m3] | [mT/µ0] | [kHz] |
|---|---|---|---|---|---|---|
| Core size [nm] | 10, 12, …, 30 | 0.05 | 130 | −11 | 16 | 40 |
| Core-size distribution width [--] | 20 | 0.05, 0.1, …, 0.5 | 130 | −11 | 16 | 40 |
| Anisotropy constant [kJ/m3] | 20, 28 | 0.05, 0.3 | 130 | −3, −5, … −25 | 16 | 40 |
| Hydrodynamic size [nm] | 20 | 0.05 | 20, 35, …, 200 | −11 | 16 | 40 |
| Drive field amplitude [mT/µ0] | 20 | 0.05 | 130 | −11 | 2, 4, …, 20 | 40 |
| Excitation frequency [kHz] | 20 | 0.05 | 130 | −11 | 16 | 16, 18, …, 48 |
| Parameter ↓ | Effect → | Peak Intensity | Peak Width | Shape of Profile |
|---|---|---|---|
| Core size [nm] | Strong | Strong | Moderate |
| Core-size distribution width [--] | Weak | Moderate | None |
| Anisotropy constant [kJ/m3] | Moderate | None | None |
| Hydrodynamic size [nm] | None | None | None |
| Drive field amplitude [mT/µ0] | Moderate | None | Strong |
| Excitation frequency [kHz] | None | None | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engelmann, U.M.; Simsek, B.; Shalaby, A.; Krause, H.-J. Key Contributors to Signal Generation in Frequency Mixing Magnetic Detection (FMMD): An In Silico Study. Sensors 2024, 24, 1945. https://doi.org/10.3390/s24061945
Engelmann UM, Simsek B, Shalaby A, Krause H-J. Key Contributors to Signal Generation in Frequency Mixing Magnetic Detection (FMMD): An In Silico Study. Sensors. 2024; 24(6):1945. https://doi.org/10.3390/s24061945
Chicago/Turabian StyleEngelmann, Ulrich M., Beril Simsek, Ahmed Shalaby, and Hans-Joachim Krause. 2024. "Key Contributors to Signal Generation in Frequency Mixing Magnetic Detection (FMMD): An In Silico Study" Sensors 24, no. 6: 1945. https://doi.org/10.3390/s24061945
APA StyleEngelmann, U. M., Simsek, B., Shalaby, A., & Krause, H.-J. (2024). Key Contributors to Signal Generation in Frequency Mixing Magnetic Detection (FMMD): An In Silico Study. Sensors, 24(6), 1945. https://doi.org/10.3390/s24061945







