Intraoperative Angle Measurement of Anatomical Structures: A Systematic Review
Abstract
1. Introduction
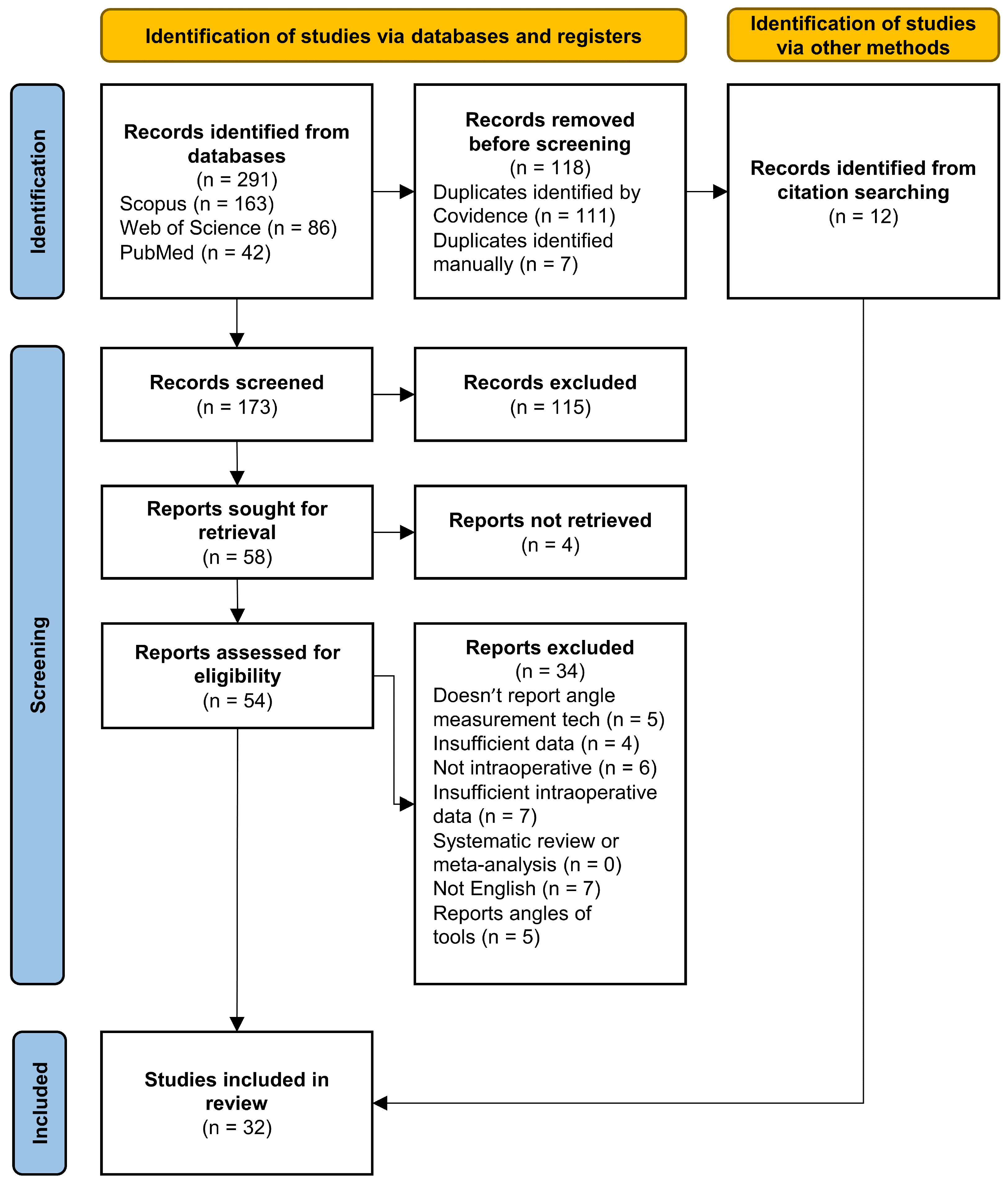
2. Methods
2.1. Search Strategy
2.2. Study Screening and Selection
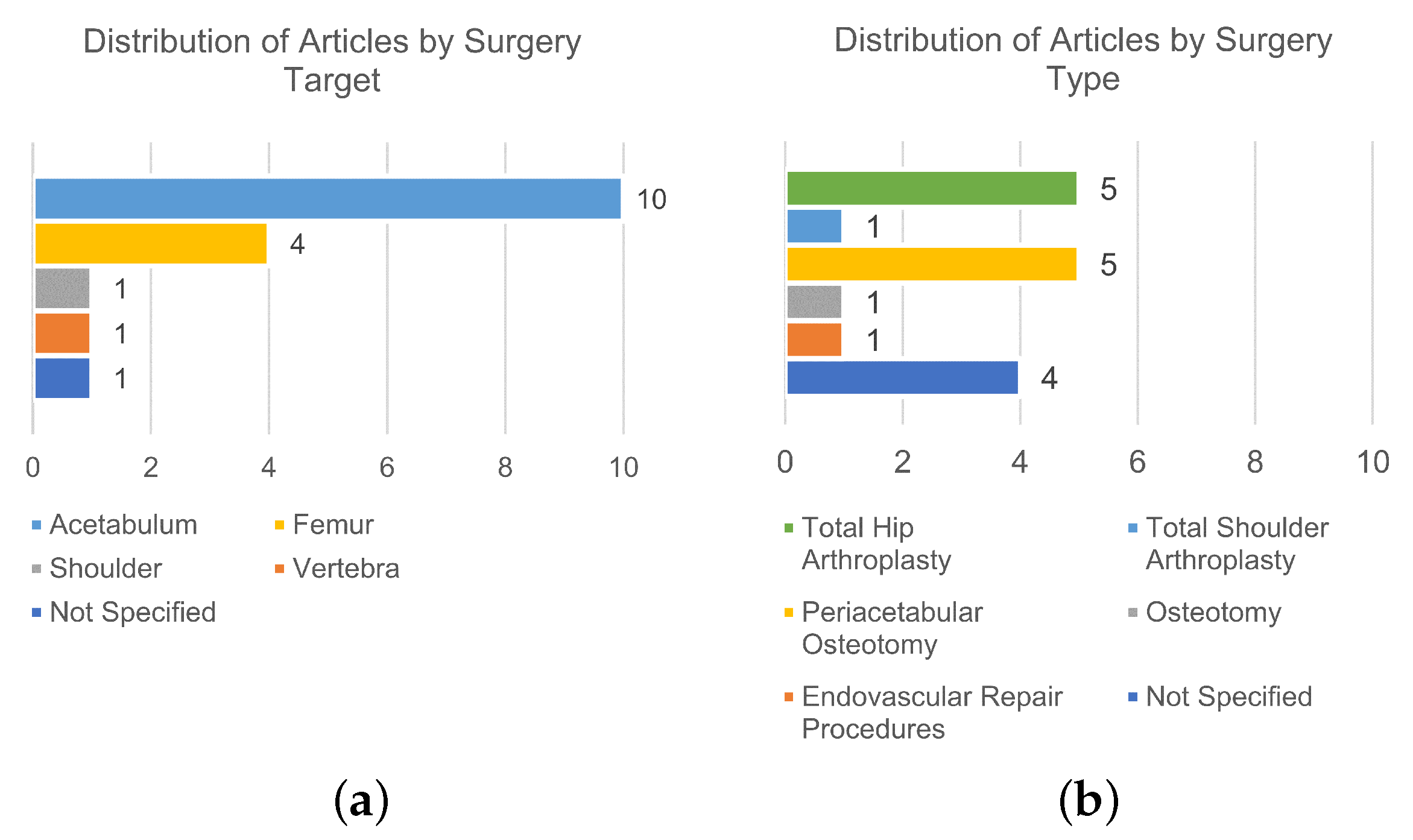
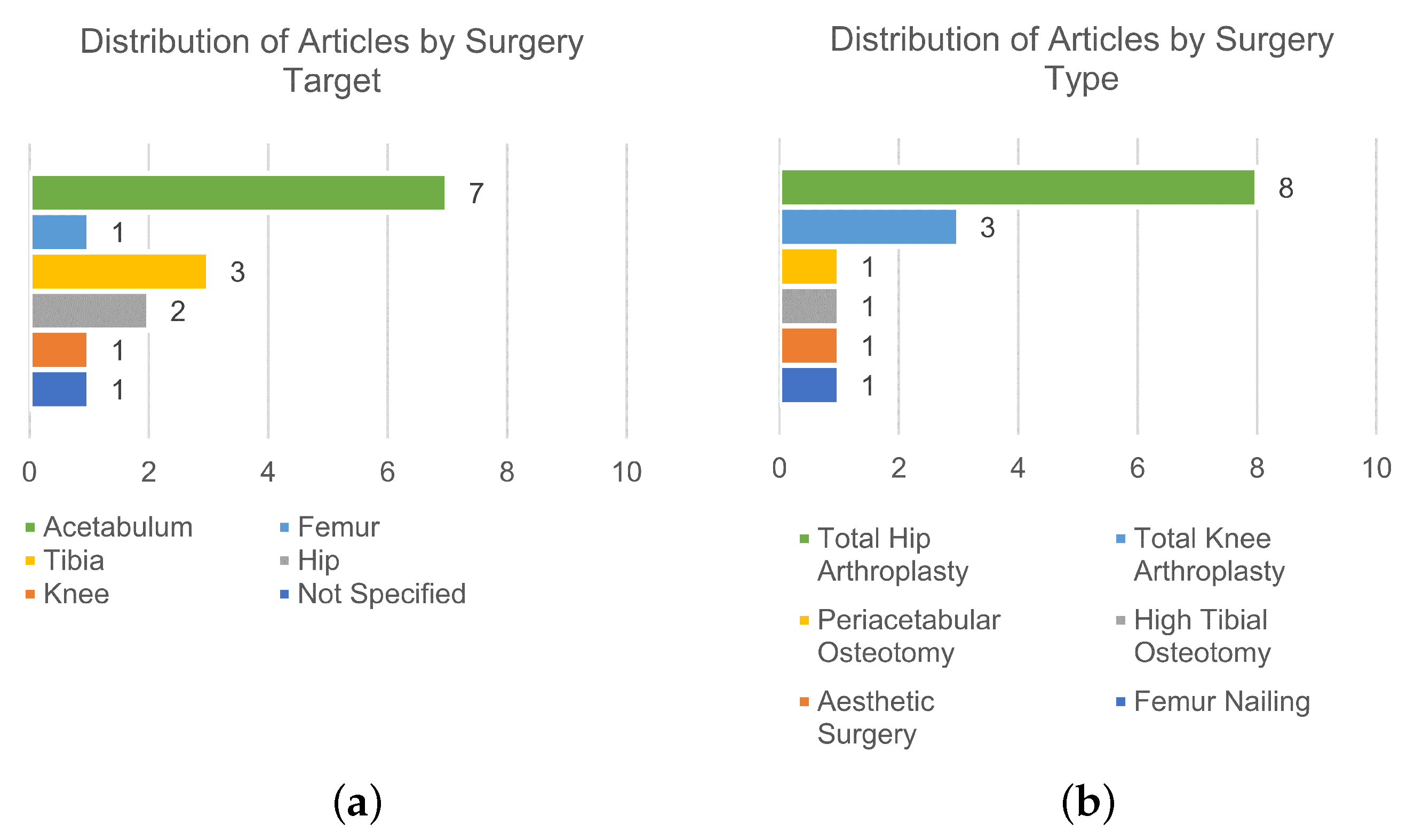
3. Results
3.1. Image-Based Technologies
3.1.1. Fluoroscopy
3.1.2. Camera Tracking Systems
3.1.3. Computed Tomography
3.2. Non-Image-Based Technologies
3.2.1. Manual Instruments
3.2.2. Inertial-Based Instruments
4. Discussion
4.1. Interpretation and Implications of the Results
4.2. Future Trends
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karol, L.A. Rotational deformities in the lower extremities. Curr. Opin. Pediatr. 1997, 9, 77–80. [Google Scholar] [CrossRef]
- Lincoln, T.L.; Suen, P.W. Common rotational variations in children. J. Am. Acad. Orthop. Surg. 2003, 11, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Gruskay, J.A.; Fragomen, A.T.; Rozbruch, S.R. Idiopathic Rotational Abnormalities of the Lower Extremities in Children and Adults. JBJS Rev. 2019, 7, e3. [Google Scholar] [CrossRef] [PubMed]
- Shtarker, H.; Volpin, G.; Stolero, J.; Kaushansky, A.; Samchukov, M. Correction of Combined Angular and Rotational Deformities by the Ilizarov Method. Clin. Orthop. Relat. Res. 2002, 402, 184–195. [Google Scholar] [CrossRef] [PubMed]
- İğrek, S.; Akgülle, A.H.; Kesimer, M.D. Effect of rotational deformities after pediatric femoral fracture on clinical outcome. J. Pediatr. Orthop. B 2022, 31, e130–e134. [Google Scholar] [CrossRef] [PubMed]
- Tarr, R.R.; Garfinkel, A.I.; Sarmiento, A. The effects of angular and rotational deformities of both bones of the forearm. An in vitro study. JBJS 1984, 66, 65. [Google Scholar] [CrossRef]
- Hicks, J.; Arnold, A.; Anderson, F.; Schwartz, M.; Delp, S. The effect of excessive tibial torsion on the capacity of muscles to extend the hip and knee during single-limb stance. Gait Posture 2007, 26, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Trompeter, A.; Dabis, J.; Templeton-Ward, O.; Lacey, A.E.; Narayan, B. The history, evolution and basic science of osteotomy techniques. Strateg. Trauma Limb Reconstr. 2017, 12, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, E.P.; Hyman, C.H. LeFort I Osteotomy. Semin. Plast. Surg. 2013, 27, 149–154. [Google Scholar] [CrossRef]
- Lakin, G.E.; Kawamoto, H.K.J. Le Fort II Osteotomy. J. Craniofacial. Surg. 2012, 23, S22. [Google Scholar] [CrossRef]
- Schlieder, D.; Markiewicz, M.R. Craniofacial Syndromes: The Le Fort III Osteotomy for Correction of Severe Midface Hypoplasia. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2022, 30, 85–99. [Google Scholar] [CrossRef]
- Masry, M.A.E.; Saleh, A.M.; McWilliams, A.B.; Tsiridis, E.; Salah, H.; Hawary, Y.K.E. Concave rib osteotomy: A modified technique revisited. Eur. Spine J. 2007, 16, 1600–1603. [Google Scholar] [CrossRef]
- Arlet, V. Spinal osteotomy in the presence of massive lumbar epidural scarring. Eur. Spine J. 2015, 24 (Suppl. S1), S93–S106. [Google Scholar] [CrossRef]
- Long, Z.; Gong, F.; Xiong, L.; Wen, J.; Chen, G. Modified posterior osteotomy for osteoporotic vertebral collapse with neurological dysfunction in thoracolumbar spine: A preliminary study. J. Orthop. Surg. Res. 2023, 18, 688. [Google Scholar] [CrossRef] [PubMed]
- Rayhack, J.M.; Gasser, S.I.; Latta, L.L.; Anne Ouellette, E.; Milne, E.L. Precision oblique osteotomy for shortening of the ulna. J. Hand Surg. 1993, 18, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, W.L.; Clader, T.J.; Smith, C.; Bayer, M. Supracondylar Humeral Osteotomy for Traumatic Childhood Cubitus Varus Deformity. Clin. Orthop. Relat. Res. 1984, 188, 34. [Google Scholar] [CrossRef]
- Coulet, B.; Boretto, J.G.; Allieu, Y.; Fattal, C.; Laffont, I.; Chammas, M. Pronating osteotomy of the radius for forearm supination contracture in high-level tetraplegic patients: Technique and results. J. Bone Jt. Surg. Br. Vol. 2010, 92-B, 828–834. [Google Scholar] [CrossRef]
- Stirling, P.; Oliver, W.; Ng, N.; Oliver, C.; McQueen, M.; Molyneux, S.; Duckworth, A. Distal radius malunion: Outcomes following an ulnar shortening osteotomy. Eur. J. Orthop. Surg. Traumatol. 2022, 33, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Reif, T.J.; Humphrey, T.J.; Fragomen, A.T. Osteotomies about the Knee: Managing Rotational Deformities. Oper. Tech. Sport. Med. 2022, 30, 150938. [Google Scholar] [CrossRef]
- Koyonos, L.; Slenker, N.; Cohen, S. Complications in brief: Osteotomy for lower extremity malalignment. Clin. Orthop. Relat. Res. 2012, 470, 3630–3636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dowd, G.S.E.; Somayaji, H.S.; Uthukuri, M. High tibial osteotomy for medial compartment osteoarthritis. Knee 2006, 13, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Green, A.H.; Hass, M.I.; Tubridy, S.P.; Goldberg, M.M.; Perry, J.B. Calcaneal osteotomy for retrocalcaneal exostosis. Clin. Podiatr. Med. Surg. 1991, 8, 659–665. [Google Scholar] [CrossRef]
- Mylle, J.; Lammens, J.; Fabry, G. Derotation osteotomy to correct rotational deformities of the lower extremities in children. A comparison of three methods. Acta Orthop. Belg. 1993, 59, 287–292. [Google Scholar] [PubMed]
- Lucas, D.E.; Simpson, G.A.; Philbin, T.M. Comparing Fixation Used for Calcaneal Displacement Osteotomies: A Look at Removal Rates and Cost. Foot Ankle Spec. 2015, 8, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Kunzler, D.; Shazadeh Safavi, P.; Jupiter, D.; Panchbhavi, V.K. A Comparison of Removal Rates of Headless Screws Versus Headed Screws in Calcaneal Osteotomy. Foot Ankle Spec. 2018, 11, 420–424. [Google Scholar] [CrossRef]
- Richter, J.; Ciric, D.; Kalchschmidt, K.; D’Aurelio, C.; Kabir, K.; Dauwe, J.; Gueorguiev, B. Advances in fixation strength of reorienting rectangular triple pelvic innominate osteotomies—A biomechanical investigation of two screw fixation techniques. Clin. Biomech. 2023, 108, 106065. [Google Scholar] [CrossRef]
- Seide, K.; Wolter, D.; Kortmann, H.R. Fracture reduction and deformity correction with the hexapod Ilizarov fixator. Clin. Orthop. Relat. Res. 1999, 363, 186–195. [Google Scholar] [CrossRef]
- Sluga, M.; Pfeiffer, M.; Kotz, R.; Nehrer, S. Lower limb deformities in children: Two-stage correction using the Taylor spatial frame. J. Pediatr. Orthop. B 2003, 12, 123. [Google Scholar] [CrossRef]
- Matsushita, T.; Nakamura, K.; Okazaki, H.; Kurokawa, T. A simple technique for correction of complicated tibial deformity including rotational deformity. Arch. Orthop. Trauma Surg. 1998, 117, 259–261. [Google Scholar] [CrossRef]
- Brorson, S. Management of Fractures of the Humerus in Ancient Egypt, Greece, and Rome: An Historical Review. Clin. Orthop. Relat. Res. 2009, 467, 1907–1914. [Google Scholar] [CrossRef]
- Kfuri, M.; Crist, B.D.; Stannard, J.P. Preoperative Planning and Preservation of the Knee with Complex Osteotomies. Mo. Med. 2022, 119, 144–151. [Google Scholar] [PubMed]
- Mihalko, W.M.; Krackow, K.A. Preoperative planning for lower extremity osteotomies: An analysis using 4 different methods and 3 different osteotomy techniques. J. Arthroplast. 2001, 16, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Shelton, T.J.; Monazzam, S.; Calafi, A.; Leshikar, H.B.; Haus, B.M. Preoperative 3D modeling and printing for guiding periacetabular osteotomy. J. Pediatr. Orthop. 2021, 41, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Paccola, C. Pre-operative planning and surgical technique of the open wedge supracondylar osteotomy for correction of valgus knee and fixation with a fixed-angle implant. Rev. Bras. Ortop. 2015, 45, 627–635. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.E.; Kim, D.H.; Lee, J.I.; Choi, H.G.; Jung, Y.S.; Lee, S.H.; Lee, Y.S. Difference of preoperative varus–valgus stress radiograph is effective for the correction accuracy in the preoperative planning during open-wedge high tibial osteotomy. Knee Surg. Sport. Traumatol. Arthrosc. 2021, 29, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ko, J.; Eom, K.; Kim, J. Preoperative planning using computed tomography in tibial plateau levelling osteotomy: A comparison with conventional radiography. Vet. Med. Sci. 2022, 8, 959–965. [Google Scholar] [CrossRef]
- Grupp, R.B.; Hegeman, R.A.; Murphy, R.J.; Alexander, C.P.; Otake, Y.; McArthur, B.A.; Armand, M.; Taylor, R.H. Pose Estimation of Periacetabular Osteotomy Fragments with Intraoperative X-ray Navigation. IEEE Trans. Biomed. Eng. 2020, 67, 441–452. [Google Scholar] [CrossRef]
- Hudson, D.; Royer, T.; Richards, J. Ultrasound Measurements of Torsions in the Tibia and Femur. J. Bone Jt. Surgery. Am. Vol. 2006, 88, 138–143. [Google Scholar] [CrossRef]
- Guenther, K.P.; Tomczak, R.; Kessler, S.; Pfeiffer, T.; Puhl, W. Measurement of femoral anteversion by magnetic resonance imaging—Evaluation of a new technique in children and adolescents. Eur. J. Radiol. 1995, 21, 47–52. [Google Scholar] [CrossRef]
- Leong, N.L.; Buijze, G.A.; Fu, E.C.; Stockmans, F.; Jupiter, J.B.; the Distal Radius Malunion (DiRaM) collaborative group. Computer-assisted versus non-computer-assisted preoperative planning of corrective osteotomy for extra-articular distal radius malunions: A randomized controlled trial. BMC Musculoskelet. Disord. 2010, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.S.; Lee, E.S.; Lee, Y.S. Disparity between Preoperative Target Correction Amount and Postoperative Correction Amount in Open Wedge High Tibial Osteotomy. Knee Surg. Relat. Res. 2019, 31, 126–131. [Google Scholar] [CrossRef]
- Lee, D.H.; Nha, K.W.; Park, S.J.; Han, S.B. Preoperative and postoperative comparisons of navigation and radiologic limb alignment measurements after high tibial osteotomy. Arthroscopy 2012, 28, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Son, J.M.; Koh, I.J.; Bahk, J.H.; In, Y. Intraoperative adjustment of alignment under valgus stress reduces outliers in patients undergoing medial opening-wedge high tibial osteotomy. Arch. Orthop. Trauma Surg. 2017, 137, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Jiang, Z.; Xu, C.; Shi, W.; Zhang, X.; Wan, X.; Bahat, D.; Li, H.; Senatov, F.; Bulygina, I.; et al. Is Patient-Specific Instrumentation Accurate and Necessary for Open-Wedge High Tibial Osteotomy? A Meta-Analysis. Orthop. Surg. 2023, 15, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Merc, M.; Fokter, S.; Sha, I. Learning curve in relation to radiation exposure, procedure duration and complications rate for Minimally Invasive Chevron Akin (MICA) osteotomy. BMC Musculoskelet. Disord. 2023, 24, 575. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.; Hofer, A.; Matziolis, G.; Wassilew, G. Intraoperative Fluoroscopy Allows the Reliable Assessment of Deformity Correction during Periacetabular Osteotomy. J. Clin. Med. 2022, 11, 4817. [Google Scholar] [CrossRef]
- Grammatopoulos, G.; Alvand, A.; Monk, A.; Mellon, S.; Pandit, H.; Rees, J.; Gill, R.; Murray, D. Surgeons Accuracy in Achieving Their Desired Acetabular Component Orientation. J. Bone Jt. Surg. 2016, 98, e72. [Google Scholar] [CrossRef] [PubMed]
- Ciurana, J. Designing, prototyping and manufacturing medical devices: An overview. Int. J. Comput. Integr. Manuf. 2014, 27, 901–918. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Innovation, V.H. Covidence Systematic Review Software. 2022. Available online: https://www.covidence.org (accessed on 25 April 2023).
- Zhou, Y.J.; Xie, X.L.; Zhou, X.H.; Liu, S.Q.; Bian, G.B.; Hou, Z.G. A Real-Time Multifunctional Framework for Guidewire Morphological and Positional Analysis in Interventional X-ray Fluoroscopy. IEEE Trans. Cogn. Dev. Syst. 2021, 13, 657–667. [Google Scholar] [CrossRef]
- Carli, A.; Aoude, A.; Reuven, A.; Matache, B.; Antoniou, J.; Zukor, D. Inconsistencies between navigation data and radiographs in total knee arthroplasty are system-dependent and affect coronal alignment. Can. J. Surg. 2014, 57, 305–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Citak, M.; Gardner, M.J.; Citak, M.; Krettek, C.; Hüfner, T.; Kendoff, D. Navigated femoral anteversion measurements: A new intraoperative technique. Injury 2008, 39, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Troelsen, A. Surgical advances in periacetabular osteotomy for treatment of hip dysplasia in adults. Acta Orthop. 2009, 80, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Sidon, E.; Steinberg, E.L. Accuracy study of new computer-assisted orthopedic surgery software. Eur. J. Radiol. 2012, 81, 4029–4034. [Google Scholar] [CrossRef] [PubMed]
- Varnavas, A.; Carrell, T.; Penney, G. Fully automated initialisation of 2D-3D image registration. In Proceedings of the 2013 IEEE 10th International Symposium on Biomedical Imaging, San Francisco, CA, USA, 7–11 April 2013; pp. 568–571. [Google Scholar] [CrossRef]
- Apivatthakakul, T.; Duanghakrung, M.; Luevitoonvechkit, S.; Patumasutra, S. Intraoperative panoramic image using alignment grid, is it accurate? Arch. Orthop. Trauma Surg. 2013, 133, 953–959. [Google Scholar] [CrossRef]
- Dalbeth, B.N.; Karlin, W.M.; Lirtzman, R.A.; Kowaleski, M.P. Measurement of Acetabular Component Position in Total Hip Arthroplasty in Dogs: Comparison of a Radio-Opaque Cup Position Assessment Device Using Fluoroscopy with CT Assessment and Direct Measurement. Vet. Comp. Orthop. Traumatol. 2020, 33, 340–347. [Google Scholar] [CrossRef]
- Lin, F.; Lim, D.; Wixson, R.L.; Milos, S.; Hendrix, R.W.; Makhsous, M. Validation of a computer navigation system and a CT method for determination of the orientation of implanted acetabular cup in total hip arthroplasty: A cadaver study. Clin. Biomech. 2008, 23, 1004–1011. [Google Scholar] [CrossRef]
- Chae, Y.S.; Lee, S.H.; Lee, H.K.; Kim, M.Y. Optical coordinate tracking system using afocal optics for image-guided surgery. Int. J. CARS 2015, 10, 231–241. [Google Scholar] [CrossRef]
- Pflugi, S.; Vasireddy, R.; Lerch, T.; Ecker, T.M.; Tannast, M.; Boemke, N.; Siebenrock, K.; Zheng, G. Augmented marker tracking for periacetabular osteotomy surgery. Int. J. CARS 2018, 13, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Kuroda, Y.; Nakano, N.; Matsumoto, T.; Kamenaga, T.; Maeda, T.; Kuroda, R. Comparing the accuracy of three-dimensional mini-optical portable navigation and accelerometer-based portable navigation system for acetabular cup placement during total hip arthroplasty. Arch. Orthop. Trauma Surg. 2022, 143, 3759–3766. [Google Scholar] [CrossRef] [PubMed]
- DiGioia, A.M.; Jaramaz, B.; Blackwell, M.; Simon, D.A.; Morgan, F.; Moody, J.E.; Nikou, C.; Colgan, B.D.; Aston, C.A.; Labarca, R.S.; et al. The Otto Aufranc Award. Image guided navigation system to measure intraoperatively acetabular implant alignment. Clin. Orthop. Relat. Res. 1998, 355, 8–22. [Google Scholar] [CrossRef]
- Armiger, R.S.; Armand, M.; Lepisto, J.; Minhas, D.; Tallroth, K.; Mears, S.C.; Waites, M.D.; Taylor, R.H. Evaluation of a computerized measurement technique for joint alignment before and during periacetabular osteotomy. Comput. Aided Surg. 2007, 12, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Ferreira, L.M.; Brownhill, J.R.; Faber, K.J.; Johnson, J.A. Design and development of a computer assisted glenoid implantation technique for shoulder replacement surgery. Comput. Aided Surg. 2007, 12, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hawi, N.; Suero, E.M.; Liodakis, E.; Decker, S.; Krettek, C.; Citak, M. Intra-operative assessment of femoral antetorsion using ISO-C 3D: A cadaver study. Injury 2014, 45, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.J.; Armiger, R.S.; Lepistö, J.; Mears, S.C.; Taylor, R.H.; Armand, M. Development of a biomechanical guidance system for periacetabular osteotomy. Int. J. CARS 2015, 10, 497–508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogawa, H.; Hasegawa, S.; Tsukada, S.; Matsubara, M. A Pilot Study of Augmented Reality Technology Applied to the Acetabular Cup Placement During Total Hip Arthroplasty. J. Arthroplast. 2018, 33, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- De Raedt, S.; Mechlenburg, I.; Stilling, M.; Rømer, L.; Murphy, R.J.; Armand, M.; Lepistö, J.; de Bruijne, M.; Søballe, K. Reliability of computer-assisted periacetabular osteotomy using a minimally invasive approach. Int. J. CARS 2018, 13, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Jend, H.H. Die computertomographische Antetorsionswinkelbestimmung. Fortschr Röntgenstr 1986, 144, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, R.; Slomczykowski, M.; Sati, M.; Nolte, L.P. Fluoroscopy as an Imaging Means for Computer-Assisted Surgical Navigation. Comput. Aided Surg. 1999, 4, 65–76. [Google Scholar] [CrossRef]
- Ojodu, I.; Ogunsemoyin, A.; Hopp, S.; Pohlemann, T.; Ige, O.; Akinola, O. C-arm fluoroscopy in orthopaedic surgical practice. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 1563–1568. [Google Scholar] [CrossRef]
- Gieroba, T.J.; Bain, G.I.; Cundy, P.J. Review of the Clinical Use of Fluoroscopy in Hand Surgery. Hand Surg. 2015, 20, 228–236. [Google Scholar] [CrossRef]
- Keil, H.; Trapp, O. Fluoroscopic imaging: New advances. Injury 2022, 53 (Suppl. S3), S8–S15. [Google Scholar] [CrossRef] [PubMed]
- Troelsen, A.; Elmengaard, B.; Rømer, L.; Søballe, K. Reliable Angle Assessment During Periacetabular Osteotomy with a Novel Device. Clin. Orthop. Relat. Res. 2008, 466, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, A.; Porfido, M.B.; Mazzoleni, S.; Calvosa, G.; Tenucci, M.; Ciuti, G.; Dario, P. Optical and Electromagnetic Tracking Systems for Biomedical Applications: A Critical Review on Potentialities and Limitations. IEEE Rev. Biomed. Eng. 2020, 13, 212–232. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Huang, T.; Wang, J.; Liao, H. Visualization, registration and tracking techniques for augmented reality guided surgery: A review. Phys. Med. Biol. 2023, 68, 04TR02. [Google Scholar] [CrossRef]
- Keating, T.C.; Jacobs, J.J. Augmented Reality in Orthopedic Practice and Education. Orthop. Clin. N. Am. 2021, 52, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, D.; Boas, F.E. Computed tomography—Old ideas and new technology. Eur. Radiol. 2011, 21, 510–517. [Google Scholar] [CrossRef]
- Chowdhary, A.; Drittenbass, L.; Dubois-Ferrière, V.; Stern, R.; Assal, M. Intraoperative 3-Dimensional Computed Tomography and Navigation in Foot and Ankle Surgery. Orthopedics 2016, 39, e1005–e1010. [Google Scholar] [CrossRef]
- Jones, E.T. Use of computed axial tomography in pediatric orthopedics. J. Pediatr. Orthop. 1981, 1, 329–338. [Google Scholar] [CrossRef]
- Arger, P.H.; Coleman, B.G.; Dalinka, M.K. Computed tomography in orthopedics. Orthop. Clin. N. Am. 1983, 14, 217–232. [Google Scholar] [CrossRef]
- Shaye, D.A.; Tollefson, T.T.; Strong, E.B. Use of Intraoperative Computed Tomography for Maxillofacial Reconstructive Surgery. JAMA Facial Plast. Surg. 2015, 17, 113–119. [Google Scholar] [CrossRef]
- Vendittoli, P.A.; Duval, N.; Stitson, D.J.; Mâsse, B. Vertical acetabular positioning with an inclinometer in total hip arthroplasty. J. Arthroplast. 2002, 17, 936–941. [Google Scholar] [CrossRef]
- Sykes, A.M.; Hill, J.C.; Beverland, D.E.; Orr, J.F. A Novel Device to Measure Acetabular Inclination with Patients in Lateral Decubitus. HIP Int. 2012, 22, 683–689. [Google Scholar] [CrossRef] [PubMed]
- McGann, W.; Peter, J.; Currey, J.M.; Buckley, J.M.; Liddle, K.D. A Simple Goniometer for Use Intraoperatively in Total Knee Arthroplasty. J. Med. Devices 2013, 7, 011010. [Google Scholar] [CrossRef]
- Meermans, G.; Goetheer-Smits, I.; Lim, R.; Van Doorn, W.; Kats, J. The difference between the radiographic and the operative angle of inclination of the acetabular component in total hip arthroplasty: Use of a digital protractor and the circumference of the hip to improve orientation. Bone Jt. J. 2015, 97-B, 603–610. [Google Scholar] [CrossRef]
- Jeong, T.K.; Park, S.H. Perioral Ruler in Routine Esthetic Surgery: Convenient and Exact. Aesthetic Plast. Surg. 2020, 44, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Chuaychoosakoon, C.; Parinyakhup, W.; Kwanyuang, A.; Duangnumsawang, Y.; Tangtrakulwanich, B.; Boonriong, T. Coronal Alignment Correction and Maintenance of Tibial Slope in Opening-Wedge Valgus High Tibial Osteotomy Using a 4-Reference Kirschner Wire Technique: A Cadaveric Study. Orthop. J. Sport. Med. 2020, 8, 232596712092360. [Google Scholar] [CrossRef]
- Peters, F.M.; Greeff, R.; Goldstein, N.; Frey, C.T. Improving Acetabular Cup Orientation in Total Hip Arthroplasty by Using Smartphone Technology. J. Arthroplast. 2012, 27, 1324–1330. [Google Scholar] [CrossRef]
- Hawi, N.; Kabbani, A.R.; O’Loughlin, P.; Krettek, C.; Citak, M.; Liodakis, E. Intraoperative measurement of femoral antetorsion using the anterior cortical angle method: A novel use for smartphones. Int. J. Med. Robot. Comput. Assist. Surg. 2013, 9, 29–35. [Google Scholar] [CrossRef]
- Nam, D.; Cody, E.A.; Nguyen, J.T.; Figgie, M.P.; Mayman, D.J. Extramedullary Guides Versus Portable, Accelerometer-Based Navigation for Tibial Alignment in Total Knee Arthroplasty: A Randomized, Controlled Trial: Winner of the 2013 HAP PAUL Award. J. Arthroplast. 2014, 29, 288–294. [Google Scholar] [CrossRef]
- Pflugi, S.; Liu, L.; Ecker, T.M.; Schumann, S.; Larissa Cullmann, J.; Siebenrock, K.; Zheng, G. A cost-effective surgical navigation solution for periacetabular osteotomy (PAO) surgery. Int. J. CARS 2016, 11, 271–280. [Google Scholar] [CrossRef]
- Chen, H.; Cao, Z.; Su, S.; Liu, J.; Wang, Z. Measurement System for Attitude of Anterior Pelvic Plane and Implantation of Prothesis in THR Surgery. IEEE Trans. Instrum. Meas. 2018, 67, 1913–1921. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, Y.; Mai, B.; Zhu, B.; Chen, P.; Fu, Y.; Wang, Z. Monitoring hip posture in total hip arthroplasty using an inertial measurement unit-based hip smart trial system: An in vitro validation experiment using a fixed pelvis model. J. Biomech. 2019, 97, 109415. [Google Scholar] [CrossRef] [PubMed]
- Kamenaga, T.; Hayashi, S.; Hashimoto, S.; Matsumoto, T.; Takayama, K.; Fujishiro, T.; Hiranaka, T.; Niikura, T.; Kuroda, R. Accuracy of cup orientation and learning curve of the accelerometer-based portable navigation system for total hip arthroplasty in the supine position. J. Orthop. Surg. 2019, 27, 230949901984887. [Google Scholar] [CrossRef] [PubMed]
- Takada, R.; Jinno, T.; Miyatake, K.; Hirao, M.; Yoshii, T.; Okawa, A. Portable imageless navigation system and surgeon’s estimate for accurate evaluation of acetabular cup orientation during total hip arthroplasty in supine position. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Kokko, M.A.; Chapman, R.M.; Roche, M.W.; Van Citters, D.W. A gyroscope-based system for intraoperative measurement of tibia coronal plane alignment in total knee arthroplasty. Med. Nov. Technol. Devices 2022, 13, 100112. [Google Scholar] [CrossRef]
- Camarillo, D.B.; Krummel, T.M.; Salisbury, J.K. Robotic technology in surgery: Past, present, and future. Am. J. Surg. 2004, 188, 2–15. [Google Scholar] [CrossRef]
- Vayalapra, S.; Wang, X.; Qureshi, A.; Vepa, A.; Rahman, U.; Palit, A.; Williams, M.A.; King, R.; Elliott, M.T. Repeatability of Inertial Measurement Units for Measuring Pelvic Mobility in Patients Undergoing Total Hip Arthroplasty. Sensors 2023, 23, 377. [Google Scholar] [CrossRef]
- Jost, G.F.; Walti, J.; Mariani, L.; Schaeren, S.; Cattin, P. Inertial Measurement Unit-Assisted Implantation of Pedicle Screws in Combination with an Intraoperative 3-Dimensional/2-Dimensional Visualization of the Spine. Oper. Neurosurg. 2019, 16, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.E.; Stout, A.; Waldon, K.; Kang, S.; Killeen, A.L.; Crisologo, P.A.; Siah, M.; Jupiter, D.; Najafi, B.; Vaziri, A.; et al. Digital Biomarkers of Gait and Balance in Diabetic Foot, Measurable by Wearable Inertial Measurement Units: A Mini Review. Sensors 2022, 22, 9278. [Google Scholar] [CrossRef]
- Saun, T.J.; Grantcharov, T.P. Design and validation of an inertial measurement unit (IMU)-based sensor for capturing camera movement in the operating room. HardwareX 2021, 9, e00179. [Google Scholar] [CrossRef]
- Walmsley, C.P.; Williams, S.A.; Grisbrook, T.; Elliott, C.; Imms, C.; Campbell, A. Measurement of Upper Limb Range of Motion Using Wearable Sensors: A Systematic Review. Sports Med. Open 2018, 4, 53. [Google Scholar] [CrossRef]
- Ahmad, N.; Ghazilla, R.A.R.; Khairi, N.M.; Kasi, V. Reviews on Various Inertial Measurement Unit (IMU) Sensor Applications. Int. J. Signal Process. Syst. 2013, 1, 256–262. [Google Scholar] [CrossRef]
- Merloz, P.; Troccaz, J.; Vouaillat, H.; Vasile, C.; Tonetti, J.; Eid, A.; Plaweski, S. Fluoroscopy-based navigation system in spine surgery. Proc. Inst. Mech. Eng. H 2007, 221, 813–820. [Google Scholar] [CrossRef]
- Kuroda, K.; Kabata, T.; Maeda, T.; Kajino, Y.; Watanabe, S.; Iwai, S.; Kenji, F.; Hasegawa, K.; Inoue, D.; Tsuchiya, H. The value of computed tomography based navigation in revision total hip arthroplasty. Int. Orthop. (SICOT) 2014, 38, 711–716. [Google Scholar] [CrossRef]
- Xie, X.; Zhu, M.; He, B.; Xu, J. Image-guided navigation system for minimally invasive total hip arthroplasty (MITHA) using an improved position-sensing marker. Int. J. CARS 2023, 18, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.H.; Severino, N.R.; de Moraes Barros Fucs, P.M. Preoperative surgical planning versus navigation system in valgus tibial osteotomy: A cross-sectional study. Int. Orthop. 2013, 37, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Lebel, K.; Boissy, P.; Hamel, M.; Duval, C. Inertial measures of motion for clinical biomechanics: Comparative assessment of accuracy under controlled conditions-effect of velocity. PLoS ONE 2013, 8, e79945. [Google Scholar] [CrossRef] [PubMed]
- Saggio, G.; Tombolini, F.; Ruggiero, A. Technology-based complex motor tasks assessment: A 6-DOF inertial-based system versus a gold-standard optoelectronic-based one. IEEE Sens. J. 2020, 21, 1616–1624. [Google Scholar] [CrossRef]
- Krille, L.; Hammer, G.P.; Merzenich, H.; Zeeb, H. Systematic review on physician’s knowledge about radiation doses and radiation risks of computed tomography. Eur. J. Radiol. 2010, 76, 36–41. [Google Scholar] [CrossRef]
- Roupa, I.; da Silva, M.R.; Marques, F.; Gonçalves, S.B.; Flores, P.; da Silva, M.T. On the Modeling of Biomechanical Systems for Human Movement Analysis: A Narrative Review. Arch. Computat. Methods Eng. 2022, 29, 4915–4958. [Google Scholar] [CrossRef]
- McFadden, C.; Daniels, K.; Strike, S. The effect of simulated marker misplacement on the interpretation of inter-limb differences during a change of direction task. J. Biomech. 2021, 116, 110184. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Li, J.; Huang, Y.; Mittauer, K.; Lu, B.; Liu, C. Ghost marker detection and elimination in marker-based optical tracking systems for real-time tracking in stereotactic body radiotherapy. Med. Phys. 2014, 41, 101713. [Google Scholar] [CrossRef] [PubMed]
- Menolotto, M.; Komaris, D.S.; Tedesco, S.; O’Flynn, B.; Walsh, M. Motion capture technology in industrial applications: A systematic review. Sensors 2020, 20, 5687. [Google Scholar] [CrossRef] [PubMed]
- Andria, G.; Attivissimo, F.; Nisio, A.D.; Lanzolla, A.M.L.; Larizza, P.; Selicato, S. Development and performance evaluation of an electromagnetic tracking system for surgery navigation. Measurement 2019, 148, 106916. [Google Scholar] [CrossRef]
- Gonçalves, S.B.; Lama, S.B.C.; da Silva, M.T. Three decades of gait index development: A comparative review of clinical and research gait indices. Clin. Biomech. 2022, 96, 105682. [Google Scholar] [CrossRef]
- He, A.; Mao, Y.; Zhou, Y.; Kong, Q.; Zhang, H.; Chen, Y.; Liu, W.; Zhang, X. Preoperative planning by osteotomy master software helps to improve the accuracy of target limb alignment in high tibial osteotomy. J. Orthop. Surg. Res. 2020, 15, 504. [Google Scholar] [CrossRef]
- Audenaert, E.; Smet, B.; Pattyn, C.; Khanduja, V. Imageless versus image-based registration in navigated arthroscopy of the hip: A cadaver-based assessment. J. Bone Jt. Surg. Br. Vol. 2012, 94-B, 624–629. [Google Scholar] [CrossRef]
- Zurmühle, C.A.; Zickmantel, B.; Christen, M.; Christen, B.; Zheng, G.; Schwab, J.M.; Tannast, M.; Steppacher, S.D. Image-Less THA Cup Navigation in Clinical Routine Setup: Individual Adjustments, Accuracy, Precision, and Robustness. Medicina 2022, 58, 832. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Cai, L. Imageless navigation versus traditional method in total hip arthroplasty: A meta-analysis. Int. J. Surg. 2015, 21, 122–127. [Google Scholar] [CrossRef]
- Migliorini, F.; Cuozzo, F.; Oliva, F.; Eschweiler, J.; Hildebrand, F.; Maffulli, N. Imageless navigation for primary total hip arthroplasty: A meta-analysis study. J. Orthop. Traumatol. 2022, 23, 21. [Google Scholar] [CrossRef] [PubMed]
- Wahdan, A.; Georgy, J.; Abdelfatah, W.F.; Noureldin, A. Magnetometer Calibration for Portable Navigation Devices in Vehicles Using a Fast and Autonomous Technique. IEEE Trans. Intell. Transp. Syst. 2014, 15, 2347–2352. [Google Scholar] [CrossRef]
- Laidig, D.; Weygers, I.; Seel, T. Self-Calibrating Magnetometer-Free Inertial Motion Tracking of 2-DoF Joints. Sensors 2022, 22, 9850. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Engels, S.; Goos, P.; Süykers, M.C.; Gudenkauf, S.; Henke, R.P.; Wawroschek, F. Accuracy of Magnetometer-Guided Sentinel Lymphadenectomy after Intraprostatic Injection of Superparamagnetic Iron Oxide Nanoparticles in Prostate Cancer: The SentiMag Pro II Study. Cancers 2020, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Woodford, S.; Senanayake, D.; Ackland, D. Conversion of Upper-Limb Inertial Measurement Unit Data to Joint Angles: A Systematic Review. Sensors 2023, 23, 6535. [Google Scholar] [CrossRef] [PubMed]
- Aligia, D.A.; Roccia, B.A.; Angelo, C.H.D.; Magallán, G.A.; González, G.N. An orientation estimation strategy for low cost IMU using a nonlinear Luenberger observer. Measurement 2021, 173, 108664. [Google Scholar] [CrossRef]
- Alatise, M.B.; Hancke, G.P. Pose estimation of a mobile robot based on fusion of IMU data and vision data using an extended Kalman filter. Sensors 2017, 17, 2164. [Google Scholar] [CrossRef]
- Kok, M.; Hol, J.D.; Schön, T.B. Using inertial sensors for position and orientation estimation. Found. Trends Signal Process. 2017, 11, 1–153. [Google Scholar] [CrossRef]
- Hol, J.D.; Schön, T.B.; Luinge, H.; Slycke, P.J.; Gustafsson, F. Robust real-time tracking by fusing measurements from inertial and vision sensors. J. Real-Time Image Process. 2007, 2, 149–160. [Google Scholar] [CrossRef]
- Kok, M.; Hol, J.D.; Schön, T.B. Indoor positioning using ultrawideband and inertial measurements. IEEE Trans. Veh. Technol. 2015, 64, 1293–1303. [Google Scholar] [CrossRef]
- Zimmermann, T.; Taetz, B.; Bleser, G. IMU-to-Segment Assignment and Orientation Alignment for the Lower Body Using Deep Learning. Sensors 2018, 18, 302. [Google Scholar] [CrossRef] [PubMed]
- Zabat, M.; Ababou, A.; Ababou, N.; Dumas, R. IMU-based sensor-to-segment multiple calibration for upper limb joint angle measurement—A proof of concept. Med. Biol. Eng. Comput. 2019, 57, 2449–2460. [Google Scholar] [CrossRef]
- Maruyama, T.; Toda, H.; Ishii, W.; Tada, M. Inertial Measurement Unit to Segment Calibration Based on Physically Constrained Pose Generation. SICE J. Control. Meas. Syst. Integr. 2020, 13, 122–130. [Google Scholar] [CrossRef]
- Vargas-Valencia, L.S.; Elias, A.; Rocon, E.; Bastos-Filho, T.; Frizera, A. An IMU-to-body alignment method applied to human gait analysis. Sensors 2016, 16, 2090. [Google Scholar] [CrossRef]
- Zheng, T.; Xu, A.; Xu, X.; Liu, M. Modeling and Compensation of Inertial Sensor Errors in Measurement Systems. Electronics 2023, 12, 2458. [Google Scholar] [CrossRef]
- Du, S.; Sun, W.; Gao, Y. MEMS IMU Error Mitigation Using Rotation Modulation Technique. Sensors 2016, 16, 2017. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, H.; Fang, D.; Wang, Y.; Zhang, H.; Zhang, X. Auxiliary Error Correction Method for High Precision IMU. IOP Conf. Ser. Mater. Sci. Eng. 2019, 612, 042063. [Google Scholar] [CrossRef]
- Jones, C.L. Surgical Instruments: History and Historiography. In The Palgrave Handbook of the History of Surgery; Schlich, T., Ed.; Palgrave Macmillan: London, UK, 2018; pp. 235–257. [Google Scholar] [CrossRef]
- Yoshii, Y.; Ogawa, T.; Hara, Y.; Totoki, Y.; Ishii, T. An image fusion system for corrective osteotomy of distal radius malunion. BioMed. Eng. OnLine 2021, 20, 66. [Google Scholar] [CrossRef]
- Vroemen, J.C.; Dobbe, J.G.; Strackee, S.D.; Streekstra, G.J. Positioning evaluation of corrective osteotomy for the malunited radius: 3-D CT versus 2-D radiographs. Orthopedics 2013, 36, e193–e199. [Google Scholar] [CrossRef]
- Grewal, B.; Kianercy, A.; Gerrah, R. Characterization of Surgical Movements As a Training Tool for Improving Efficiency. J. Surg. Res. 2024, 296, 411–417. [Google Scholar] [CrossRef]
- Lin, W.; Xie, F.; Zhao, S.; Lin, S.; He, C.; Wang, Z. Novel Pedicle Navigator Based on Micro Inertial Navigation System (MINS) and Bioelectric Impedance Analysis (BIA) to Facilitate Pedicle Screw Placement in Spine Surgery: Study in a Porcine Model. Spine 2022, 47, 1172. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, D.; Li, R.; Liu, Z.; Sun, W. Gyro-Net: IMU Gyroscopes Random Errors Compensation Method Based on Deep Learning. IEEE Robot. Autom. Lett. 2022, 8, 1471–1478. [Google Scholar] [CrossRef]
- Burström, G.; Persson, O.; Edström, E.; Elmi-Terander, A. Augmented reality navigation in spine surgery: A systematic review. Acta Neurochir. 2021, 163, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Batailler, C.; Fernandez, A.; Swan, J.; Servien, E.; Haddad, F.S.; Catani, F.; Lustig, S. MAKO CT-based robotic arm-assisted system is a reliable procedure for total knee arthroplasty: A systematic review. Knee Surgery Sport. Traumatol. Arthrosc. 2021, 29, 3585–3598. [Google Scholar] [CrossRef]
- Hu, X.; y Baena, F.R.; Cutolo, F. Head-mounted augmented reality platform for markerless orthopaedic navigation. IEEE J. Biomed. Health Inform. 2021, 26, 910–921. [Google Scholar] [CrossRef]
- Golse, N.; Petit, A.; Lewin, M.; Vibert, E.; Cotin, S. Augmented reality during open liver surgery using a markerless non-rigid registration system. J. Gastrointest. Surg. 2021, 25, 662–671. [Google Scholar] [CrossRef]
- Sadeghi-Niaraki, A.; Choi, S.M. A survey of marker-less tracking and registration techniques for health & environmental applications to augmented reality and ubiquitous geospatial information systems. Sensors 2020, 20, 2997. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Sakura, T.; Ueda, K.; Omura, L.; Kimura, A.; Iino, Y.; Fukashiro, S.; Yoshioka, S. Evaluation of 3D markerless motion capture accuracy using OpenPose with multiple video cameras. Front. Sport. Act. Living 2020, 2, 50. [Google Scholar] [CrossRef]
- Kocabas, M.; Athanasiou, N.; Black, M.J. Vibe: Video inference for human body pose and shape estimation. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Seattle, WA, USA, 13–19 June 2020; pp. 5253–5263. [Google Scholar] [CrossRef]
- Kocabas, M.; Huang, C.H.P.; Hilliges, O.; Black, M.J. PARE: Part attention regressor for 3D human body estimation. In Proceedings of the IEEE/CVF International Conference on Computer Vision, Montreal, BC, Canada, 11–17 October 2021; pp. 11127–11137. [Google Scholar] [CrossRef]
- Roupa, I.F.; Gonçalves, S.B.; Silva, M.T.d.; Neptune, R.R.; Lopes, D.S. Motion envelopes: Unfolding longitudinal rotation data from walking stick-figures. Comput. Methods Biomech. Biomed. Eng. 2022, 25, 1459–1470. [Google Scholar] [CrossRef]
- Fernandes, F.; Roupa, I.; Gonçalves, S.B.; Moita, G.; da Silva, M.T.; Pereira, J.; Jorge, J.; Neptune, R.R.; Lopes, D.S. Sticks and STONES may build my bones: Deep learning reconstruction of limb rotations in stick figures. Pattern Recognit. Lett. 2023, 165, 138–145. [Google Scholar] [CrossRef]

| Surgery | Angle | Measurement | Intraoperative |
|---|---|---|---|
| surgical operation | orientation | determination | perioperative |
| surgical procedure | pose | calculation | in-surgery |
| surgical intervention | angular position | computation | intrasurgical |
| surgical technique | angular displacement | assessment | operating room |
| osteotomy | evaluation | real-time | |
| estimation | |||
| mensuration | |||
| quantification | |||
| valuation |
| First Author (Year) | Technology | Target | Main Results |
|---|---|---|---|
| Citak (2008) [53] | Fluoroscopy images Software calculated the angles | Femoral anteversion | Mean differences of 1.4° and 0.3° on the conventional and new techniques, respectively |
| Troelsen (2009) [54] | Fluoroscopy images Measurements obtained by a device with adjustable measuring discs | Acetabular reorientation during periacetabular osteotomy | Angle measurements differed less than ±5° from radiograph measurements |
| Sidon (2012) [55] | Panoramic view image from fluoroscopy images Software measures the angles | Varus or valgus angles during femur osteotomy | No significant difference between the different techniques |
| Varnavas (2013) [56] | Generalized Hough Transform to fluoroscopy images Maximize the Gradient Difference Similarity Measure to establish initial pose | Endovascular procedures | Final registration within ±2.5° of ground truth |
| Apivatthakakul (2013) [57] | Alignment grid stitches fluoroscopy images Obtain the coronal plane angulation | Coronal plane alignment of femur fractures | No significant difference between the different techniques |
| Dalbeth (2020) [58] | Fluoroscopy images Digital calipers derive the angles | Acetabular component position in total hip arthroplasty in dogs | No significant difference between the different techniques |
| First Author (Year) | Technology | Target | Main Results |
|---|---|---|---|
| Lin (2008) [59] | Rigidly fixed trackers, communicate via infra-red signals with camera system and computer Orientation is determined in real-time | Acetabular cup orientation during total hip arthroplasty | Average difference for anteversion and abduction were 3.3 ± 3.5° and 0.6 ± 3.7°, respectively |
| Chae (2015) [60] | Marker with a lens and micro-engraved data-coded patterns captured by a camera on an afocal image Orientation-tracking algorithm measures the angles | Position and orientation during surgical navigation | The afocal optical system provided accuracy equal to or better Orientation error of 0.093° |
| Pflugi (2018) [61] | Tracking unit and augmented marker with an IMU Kalman filter fuses the marker tracking and IMU data | Acetabular orientation during periacetabular osteotomy | Mean absolute differences for inclination and anteversion were 1.34 ± 1.50° and 1.21 ± 1.07°, respectively, for the cadaver study 1.63 ± 1.48° and 1.55 ± 1.49°, respectively, for the plastic bone study |
| Hayashi (2022) [62] | Camera captures the movements of a tracker placed on the base unit Three anatomical landmarks are registered System provides real-time data | Cup positioning during a total hip arthroplasty | Similar results comparing optical versus accelerometer-based: 2.8° ± 1.7° versus 2.8° ± 1.9° for inclination 2.6° ± 2.3° vs. 2.5° ± 1.9° for ante version, respectively |
| First Author (Year) | Technology | Target | Main Results |
|---|---|---|---|
| DiGioia (1998) [63] | CT preoperative data matched with the intraoperative tracking data | Acetabular alignment during total hip replacement | The system was successfully used in operating room with minimal impact on surgical routine |
| Armiger (2007) [64] | CT scans Lunate–Trace algorithm to CT images | Joint alignment during periacetabular osteotomy | Minor discrepancies between manual and computerized technique. The measurement error for the proposed computer method was −1.30 ± 3.30° |
| Nguyen (2007) [65] | 3D models created from CT slice data Tracking system obtains angles | Placement of the glenoid component during total shoulder arthroplasty | Statistically significant differences were found, however, in the 1° range The authors assume it is unlikely to be clinically significant |
| Hawi (2014) [66] | Mobile image intensifier with CT (ISO-C 3D) images Previously established method for calculating angles [70] | Femoral antetorsion | No significant difference between different techniques. Mean time to perform scan: 9 ± 3 min. Mean time to measure ante torsion: 8 ± 2 min |
| Murphy (2015) [67] | 3D models created from preoperative CT images Biomechanical Guidance System, camera-based tracking | Characterization of the acetabulum during periacetabular osteotomy | Computed measures differed from measured fiducial transformations by 1.0° and 2.2° in rotations, in two cases |
| Ogawa (2018) [68] | CT images and multiplanar reconstruction obtains 3D coordinate points Alignes the superimposed image on the view of actual cup | Acetabular cup placement during total hip arthroplasty | AR-HIP was significantly more accurate measuring anteversion 2.7° versus 6.8° Not significantly different measuring inclination 2.1° versus 2.6° |
| De Raedt (2018) [69] | CT data obtained preoperatively Lunate–Trace algorithm and Biomechanical Guidance System with CT data | Characterization of the acetabulum during periacetabular osteotomy | Intra-operative reported angle measurements showed good agreement with manual angle measurements |
| First Author (Year) | Technology | Target | Main Results |
|---|---|---|---|
| Vendittoli (2002) [84] | Inclinometer with acetabular insertion rods by hand-tightening a single screw Scaled at 2° intervals from 0° to 70° | Vertical acetabular positioning during total hip arthroplasty | Position precision for the inclinometer is 42.2 ± 3.8° compared with 44.4 ± 11.4° |
| Sykes (2012) [85] | The closed-tube inclinometer used with the transverse acetabular ligament | Acetabular inclination during total hip arthroplasty in the lateral decubitus position | Two trials were performed for three techniques (freehand, mechanical guide and inclinometer): In the first one, the mean errors were: 5.2 ± 4.3°, 3.6 ± 3.9°, 0.5 ± 0.4° In the second they were: 6.2 ± 4.2°, 3.8 ± 3.3°, 0.6 ± 0.5° |
| McGann (2013) [86] | Knee goniometer: digital level mounted to a base attached to two needles | Knee flexion and extension during total knee arthroplasty | Systematic error ranged from −9.1° to 3° Measurement error was 1.5 ± 1° |
| Meermans (2015) [87] | Digital inclinometer | Inclination of the acetabular component during total hip arthroplasty | Significantly reduced the number of acetabular component inclination outliers compared with freehand positioning |
| Jeong (2020) [88] | Ruler with differently colored lines | Perioral ruler for routine aesthetic surgery | A woman received corner mouth lift where the angle of the corner of the mouth measured from the stomion changed from −6° to 3° |
| Chuaychoosakoon (2020) [89] | Two coronal K-wires placed at an angle measured using a goniometer Two sagittal K-wires placed parallel to each other to ensure tibial slope is maintained | Coronal alignment correction during opening-wedge valgus high tibial osteotomy | No statistically significant differences were found between the desired amount of alignment correction and the corrections achieved |
| First Author (Year) | Technology | Target | Main Results |
|---|---|---|---|
| Peters (2012) [90] | Level indicator and a protractor application using accelerometer and the camera | Acetabular cup orientation during total hip arthroplasty | All cups were placed within a narrow range in the safe zone and less than 5% difference between pre-, intra- and postoperative inclinations |
| Hawi (2013) [91] | Standard goniometer application for smartphones using gyroscope | Femoral antetorsion during femoral nailing | Found a fair or good correlation between the new method and the traditional ones in all scenarios with no statistically significant differences |
| Nam (2014) [92] | KneeAlign: disposable display console and a reference sensor both containing a 3-axial accelerometer | Tibial alignment during total knee arthroplasty | 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis and 95.0% were within 2° of a 3° slope, for the device, compared with 68.1% and 72.1% for the extramedullary guide |
| Pflugi (2016) [93] | Two IMUs fuse data using a variation of the Kalman filter | Orientation of the acetabular fragment during periacetabular osteotomy | No statistically significant difference was found on the measurement of acetabular component reorientation |
| Chen (2018) [94] | 9-DOF IMU with a quaternion-based extended Kalman filter | Implantation angles during total hip replacement | RMSE of attitude and acetabular orientation are less than 1.6° and 3° with uncertainty of less than 0.22° and 0.17°, respectively |
| Tang (2019) [95] | 9-DOF IMU with Kalman filtering | Hip posture during total hip arthroplasty | Mean absolute errors in measuring in the 3 axes: 2.5 ± 4.9° for flexion/extension; 2.5 ± 4.4° for adduction/abduction; and 1.0 ± 2.0° for internal/external rotation. |
| Kamenaga (2019) [96] | HipAlign: accelerometer-based device with a disposable computer display unit and a reference sensor | Acetabular cup orientation during total hip arthroplasty in the supine position | The average absolute error in measurement compared with postoperative CT navigation: 2.6 ± 2.7° for inclination 2.8 ± 2.7° for anteversion |
| Takada (2020) [97] | HipAlign: accelerometer-based device with a disposable computer display unit and a reference sensor | Acetabular cup orientation during total hip arthroplasty in supine position | Achieved similar errors between methods: with the HipAlign registering 3.3 ± 2.7° and 3.8 ± 3.4° and the manual goniometer 3.0 ± 2.5° and 6.0 ± 3.7° |
| Kokko (2022) [98] | ST LSM6DS3 IMU evaluation board using the gyroscope output and custom MATLAB® scripts | Tibia coronal plane alignment during total knee arthroplasty | Average accuracy was estimated within ±1° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, J.; Gonçalves, S.B.; Neves, M.C.; Silva, H.P.; Silva, M.T. Intraoperative Angle Measurement of Anatomical Structures: A Systematic Review. Sensors 2024, 24, 1613. https://doi.org/10.3390/s24051613
Cruz J, Gonçalves SB, Neves MC, Silva HP, Silva MT. Intraoperative Angle Measurement of Anatomical Structures: A Systematic Review. Sensors. 2024; 24(5):1613. https://doi.org/10.3390/s24051613
Chicago/Turabian StyleCruz, João, Sérgio B. Gonçalves, Manuel Cassiano Neves, Hugo Plácido Silva, and Miguel Tavares Silva. 2024. "Intraoperative Angle Measurement of Anatomical Structures: A Systematic Review" Sensors 24, no. 5: 1613. https://doi.org/10.3390/s24051613
APA StyleCruz, J., Gonçalves, S. B., Neves, M. C., Silva, H. P., & Silva, M. T. (2024). Intraoperative Angle Measurement of Anatomical Structures: A Systematic Review. Sensors, 24(5), 1613. https://doi.org/10.3390/s24051613






