Abstract
Whole-body electromyostimulation (WB-EMS) can be considered as a time-efficient, joint-friendly, and highly customizable training technology that attracts a wide range of users. The present evidence map aims to provide an overview of different non-athletic cohorts addressed in WB-EMS research. Based on a comprehensive systematic search according to PRISMA, eighty-six eligible longitudinal trials were identified that correspond with our eligibility criteria. In summary, WB-EMS research sufficiently covers all adult age categories in males and females. Most cohorts addressed (58%) were predominately or exclusively overweight/obese, and in about 60% of them, diseases or conditions were inclusion criteria for the trials. Cohorts specifically enrolled in WB-EMS trials suffer from cancer/neoplasm (n = 7), obesity (n = 6), diabetes mellitus (n = 5), metabolic syndrome (n = 2), nervous system diseases (n = 2), chronic heart failure (n = 4), stroke (n = 1), peripheral arterial diseases (n = 2), knee arthrosis (n = 1), sarcopenia (n = 3), chronic unspecific low back pain (n = 4), and osteopenia (n = 3). Chronic kidney disease was an eligibility criterion in five WB-EMS trials. Finally, three studies included only critically ill patients, and two further studies considered frailty as an inclusion criterion. Of importance, no adverse effects of the WB-EMS intervention were reported. In summary, the evidence gaps in WB-EMS research were particular evident for cohorts with diseases of the nervous and cerebrovascular system.
1. Introduction
Whole-body electromyostimulation (WB-EMS) is a training technology with increasing popularity world-wide. In contrast to the recognized local EMS predominately applied in orthopedic therapy, WB-EMS stimulates most major muscle groups simultaneously but with a dedicated impulse intensity and without the relevant orthopedic demands. Thus, much more than local EMS, WB-EMS can be considered as a time-effective, joint-friendly, and highly customizable alternative to conventional exercise [1]. Whilst this aspect is attractive for athletes looking to improve sport-specific skills, reduce the risk of injuries, or adverse effects (i.e., back pain), the main population for WB-EMS application, however, is sedentary or at least non-athletic adults [2] wanting to increase physical fitness, function, or health-related outcomes. A quick look at the rapidly increasing and very complex research on WB-EMS reveals an unequal addressing of cohorts by present studies. Most of the WB-EMS trials focus on healthy adults, with fewer studies covering participants with specific conditions or diseases. This might be attributable to the rather stringent index of absolute or relative contraindications published by a German expert group in 2019 [3], based in part on an overcautious approach due to a lack of evidence and several adverse effects of intense WB-EMS application reported in the media. In stark contrast, Belt Electrode-Skeletal Muscle Electrical Stimulation (B-SES), a neuromuscular stimulation technique that stimulates large muscle areas and can thus be considered as very closely related to WB-EMS, focuses predominately on frail cohorts in a hospital setting. Adding both systems might increase the evidence for a wider applicability of WB-EMS on different outcomes in varying non-athletic cohorts. Accordingly, in order to provide evidence and identify gaps in knowledge and/or future research on WB-EMS needs [4], we conducted a systematic and comprehensive review of WB-EMS and the eligible B-SES literature. The resulting evidence (gap) map [5] aimed to provide an overview of cohorts enrolled in WB-EMS trials and to support the readjustment of potentially excessive contraindications.
2. Methods
The literature search for this systematic review and evidence map followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.
2.1. Search
Study reports from the five electronic databases (Medline [PubMed], The Cochrane Central Register of Controlled Trials [CENTRAL], Cumulative Index to Nursing & Allied Health [CINAHL via Ebsco Host], SPORTDiscus (via Ebsco Host), and The Physiotherapy Evidence Database [PEDro]), and two study registers (Clinical trial.gov and the WHO’s International Clinical Trials Registry Platform [ICTRP]) published from their incentives up to 6 March 2023 were searched without language restrictions (Figure 1). Strategies were developed applying free-text words as no database-specific key words (e.g., MeSH, Thesaurus) were identified. We piloted our search and found a good balance for maximizing sensitivity and precision by (a) constructing the search around the term whole-body electromyostimulation only and (b) searching the title and abstract fields only in PubMed, CINAHL, and SPORTDiscus, excluding Medline hits in CINAHL and by applying the ‘Trials’ filter in CENTRAL. To identify additional study reports, we used Google Scholar manually on the same date we accessed the medical databases (Figure 1). The full strategies can be found in Supplemental Table S1.
2.2. Selection Process
Titles, abstracts, and full texts were independently screened by two reviewers against the pre-specified eligibility criteria. Disagreements were resolved by discussion or with the help of a third reviewer. The reasons for excluding ineligible studies were recorded. In case of missing data or doubtful information, authors were contacted a maximum of three times within a four-week period.
2.3. Eligibility Criteria
2.3.1. Study Design
We included all longitudinal study designs except single-case studies. Review articles, editorials, conference abstracts, and letters were also not considered. The same criteria were applied for bachelor’s or master’s theses, while doctoral theses (dissertations) were included.
2.3.2. Population
Sedentary or at least non-athletic cohorts, independently of participant characteristics, were included. Cohorts comprised of athletes or sport students were excluded. However, recreational sports persons were accepted.
2.3.3. Comparators
Type or even presence of a control group was not considered as an eligibility criterion.
2.3.4. Intervention
We only included studies that applied whole-body electromyostimulation (WB-EMS [6]) or other kinds of electromyostimulation able to stimulate large muscle areas (≥50% of skeletal muscle mass) simultaneously (This refers solely to the Belt Electrode-Skeletal Muscle Electrical Stimulation (B-SES) approach that stimulates hip and lower extremity muscle groups). Studies that applied local EMS or focused on single muscle groups were not considered.
2.3.5. Outcomes
In the present analysis, we included studies that focus on physical fitness, function, body composition, and health-related outcomes. Special emphasis was further placed on the safety aspects of the WB-EMS intervention and in particular adverse effects. In detail, an “adverse event” (AE) was defined as any untoward medical occurrence, unintended disease or injury, or any untoward clinical signs, including an abnormal laboratory finding related to the WB-EMS application. However, temporary muscular soreness after WB-EMS application was not considered as an adverse effect. A “serious adverse event” was defined as any adverse effects of the WB-EMS application that led to death or serious deterioration in the health of the subject (e.g., life-threatening illness/injury, permanent impairment of a body structure/body function, hospitalization, chronic disease).
2.4. Data Management
Search results were downloaded and imported to Endnote. Duplicates were identified and excluded based on the method proposed by Bramer et al. [7]. Title and abstract screening as well as full-text screening were conducted using Endnote. In cases of multiple publications that addressed an identical cohort but reported varying outcomes (e.g., [8,9,10,11]), only the main publication was included.
2.5. Data Items
A Microsoft Excel table, applied in former studies [12,13] and modified for the present research topic, was used to extract relevant data from the included studies. One author extracted the study, participant, and intervention characteristics, and two other authors checked and confirmed the results. The table was structured into several domains. Publication characteristics include information related to the study type, first author, year, and the country of the publication, while study characteristics listed, for example, the number of study arms, sample size in WB-EMS and control group, comparator (i.e., predominately sedentary control group or active control), and methodologic quality of the studies as determined using the Physiotherapy Evidence Database (PEDro) Scale Risk of Bias Tool.
Intervention characteristics include the following: (1) The mode of application, i.e., isolated WB-EMS or WB-EMS with voluntary movements that should not relevantly affect outcomes, versus superimposed WB-EMS or exercise in addition to WB-EMS. (2) A WB-EMS system including the corresponding manufacturers. (3) The duration of the application (in months), training frequency (sessions/week), and length of the session (in min). (4) The details of the impulse protocol, i.e., impulse type (mono/bipolar), impulse frequency (in Hz), impulse breadth (in µs), impulse intensity, impulse application (continuous or intermittent impulse), length of the impulse phase (in s), and (if applicable) intermittent impulse break (in s).
Due to the topic of the present evidence map, special emphasis was placed on cohort and study characteristics. The cohort and participant characteristics include, in particular, gender, age, BMI, baseline training status, conditions and diseases, drop out, adherence to the WB-EMS protocol, and adverse effects. The trials were categorized into studies with predominately healthy cohorts and studies that focused on participants with specific health-related problems, syndromes (e.g., metabolic syndrome), or diseases. Where applicable, study cohorts were classified according their conditions and diseases by applying the International Statistical Classification of Diseases and Related Health Problems (ICD-10-GM, [14]).
2.6. Quality Assessment
Eligible studies were assessed for risk of bias by two independent reviewers using PEDro [15], specifically dedicated to physiotherapy and/or exercise studies. In case of inconsistencies, a third independent reviewer made the decision. Studies with >7 score points were classified as high, 5–7 score points were classified as moderate, and <5 score points were classified as low methodological quality studies, respectively [16].
2.7. Data Synthesis
Results are displayed for all studies in tables showing the publication and study characteristics, exercise and stimulation characteristics, and cohort and participant characteristics of the studies included. To provide a rapid overview in the present evidence map, bubble charts with 4 dimensions were created on the x-axis, with the health status of participants determined according to ICD-10-GM categories (Figures 2 and 3). The y-axis presents the number of studies that focus on the corresponding cohort, while the color of the bubble represents either WB-EMS vs. B-SES application (Figure 3), or the dedicated health status of the cohort applied as a criterion for inclusion or reported as a simple co-morbidity (Figure 2). Finally, the size of the bubble indicates the methodologic quality according to PEDro. The biggest size indicates at least one study of high methodologic quality (i.e., PEDro Score ≥ 8 score points [16]) in the domain. The lowest size of the bubble chart represents at least one study of low methodologic quality.
3. Results
Of the 1103 records, 86 longitudinal studies/projects with 87 cohorts were finally included in the present evidence map (Figure 1) [11,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101].
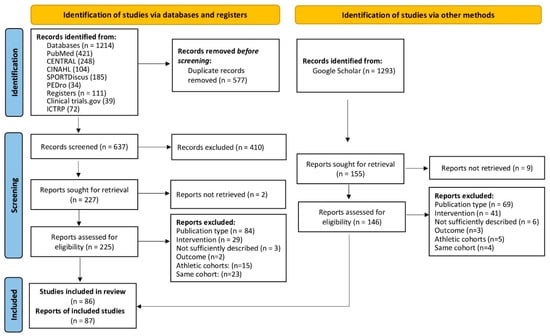
Figure 1.
Flow diagram of search process according to PRISMA [102].
3.1. Publication and Study Characteristics
Table 1 displays the publication and study characteristics of the included trials. The vast majority of the studies were RCTS (69%). Most of the randomized controlled trials (RCTS, 69%) applied a parallel group design, and three short-term studies provided a cross-over design [42,58,93]. Nineteen non-randomized controlled trials (NRCTs, 22%) and eight (9%) intervention studies without control groups [28,30,34,54,63,88,92,96] were also included. Predominately due to the study design, the methodological quality according to PEDro (Table 1) varies considerably. Considering that NRCTs and in particular intervention studies without control groups failed to obtain points for randomization, allocation concealment, blinding, or even group comparison [15], the study design reflects a low methodological quality according to PEDro. Furthermore, considering that the proper blinding of the participants (i.e., a retrospective query of all participants as to which group they think they belonged to) and particularly the caregivers (WB-EMS instructors) in exercise studies is hardly possible, a score index of eight on the ten-point PEDro scale can be considered as the realistic maximum in WB-EMS studies.

Table 1.
Publication and study characteristics of the included studies.
Most studies were conducted in Germany (n = 28), Japan (n = 18), Korea (n = 8), Spain (n = 5), Iran (n = 5), Brazil (n = 4), and Italy (n = 3). The vast majority of studies were published after 2015 (>90%). The number of study arms varied from one [30,34,54,63,88,92,96] to five [62]. The number of participants per study arm varied between three [65] and ninety-six [83] in the WB-EMS group(s), and (if applicable) from three [65] to eighty [56] in the control group(s). The study length varied from 10 days [90] to 12 months [47,98]. Unfortunately, 15 studies (16 subgroups) failed to report the drop-out rate and did not respond to our queries or were unable to calculate the drop-out rate retrospectively. The drop-out rate of the remaining studies varied from 0% to 59%. Of the eleven studies with drop-out rates ≥25%, nine studies focused on patients with severe complaints and diseases (e.g., end-stage kidney disease, stroke, critically illness, cancer) [37,39,42,51,60,80,81,90] (Table 1).
3.2. Exercise and WB-EMS Characteristics
Supplemental Table S2 displays the exercise and WB-EMS characteristics of the studies. Sixty-nine studies applied whole-body electromyostimulation (WB-EMS), and eighteen studies applied Belt Electrode-Skeletal Muscle Electrical Stimulation (B-SES). About two thirds of the WB-EMS studies used miha-bodytec devices (Gersthofen, Germany) while the B-SES technique exclusively used HomerIon (Tokyo, Japan). Although difficult to classify, about 80% of the cohorts conducted predominately isolated WB-EMS, i.e., a protocol without adjuvant or additional movements with relevant effects on the primary study outcome. The remaining studies applied either superimposed WB-EMS (i.e., exercises intensified by WB-EMS) or combined WB-EMS and conventional exercise. In parallel, three quarters of the studies provided an active WB-EMS mode, i.e., predominately movements during the impulse phase. B-SES studies generally focused on a passive EMS application mode. The WB-EMS training frequency varied from daily application [60,61,65] to one session per week [21,46,56,59,79,86,94,95,99,101]. The average training frequency of the B-SES studies was significantly (p < 0.001) higher compared with the WB-EMS studies (4.1 ± 1.7 versus 2.0 ± 0.8 sessions/week). The session length varied between 12 and 20 min [21] and 90 min [17]. Most studies (n = 75) applied WB-EMS or B-SES protocols of 20–30 min (Table 2). All but one study [53] focused on low-frequency stimulation protocols from 4 Hz [42] to 100 Hz [87] and impulse widths of 200–400 µs. The majority of studies applied intermittent WB-EMS protocols, predominately with 4–6 s of impulse and 2–4 s of impulse break; only 4 studies provided a consistently continuous impulse during the session [33,42,57,93] (Table 2). At least seventeen studies [17,20,21,22,23,31,43,44,62,66,67,68,70,75,77,79,87] worked with variable WB-EMS programs, i.e., they applied varying WB-EMS parameters, predominately including impulse frequency, width, or impulse phase/break, during the session or during the intervention. Apart from a few studies that solely evaluated the effects of low impulse intensity [40,46,57] and one study that applied very high impulse intensity [92], all other WB-EMS studies scheduled moderate to high impulse intensities based on the Borg CR-10 (…or rarely CR-20) scale, rate of the maximum impulse tolerance (60–80% 1 MT), or according to the authors’ estimation. In contrast, several B-SES studies applied stimulation protocols up to the maximum tolerable intensity (e.g., [27,36,42,65,93]).

Table 2.
Cohort and participant characteristics of the included studies.
Apart from the WB-EMS application, some studies applied specific diets (e.g., [18,23,35]) or provided protein supplements [11,46,72,100].
3.3. Participant and Cohort Characteristics
Table 2 reports characteristics of the cohorts and study participants. In summary, the studies cover all (adult) age categories in female, male, and mixed gender categories. Most studies (51%) included men and women, 33% focused on female participants, and 16% focused on male participants. About 20% of the studies addressed cohorts largely independently of age. Eleven studies (12%) focused exclusively on cohorts 30 years and younger, and twelve studies (14%) included only participants 70 years and older. With respect to premenopausal women, no longitudinal study focused on issues related to pregnancy, puerperium, or lactation.
Forty-six of the seventy-eight trials that reported corresponding data addressed cohorts that were predominantly or exclusively overweight (i.e., mean BMI ≥ 25.0 kg/m2). However, only about one third of them defined overweight or obesity as a criterion for inclusion.
Diseases or conditions were criteria for inclusion in 60% of the WB-EMS/B-SES studies. Apart from two studies [27,57] that focused on healthy young (22 ± 2) or older people (60–90 years) with limited mobility, all the other B-SES studies addressed predominantly hospitalized people with severe diseases. In contrast, about half of the WB-EMS trials addressed apparently healthy cohorts; further, only one WB-EMS study [91] applied an ambulatory setting. A large variety of conditions and diseases were reported; thus, following the ICD 10 classification [14], the cohorts were categorized into different domains and subcategories (Figure 2 and Figure 3). Due to the critically ill and/or multi-morbid status of some cohorts, the corresponding trials were cited for more than one classification.
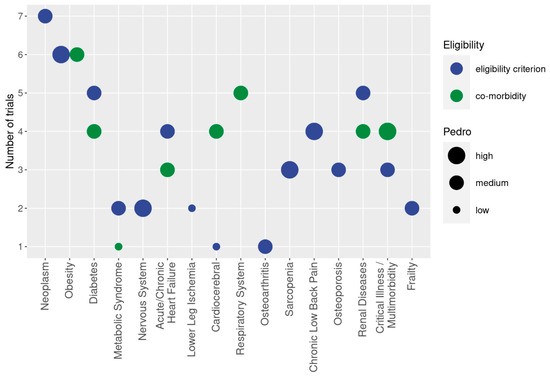
Figure 2.
Cohorts with diseases and conditions addressed in WB-EMS trails. The y-axis presents the number of studies that focus on the corresponding cohort (x-axis). Different colors indicate whether the health status of the cohort was applied as a criterion for inclusion (blue) or reported as a simple co-morbidity (green). The size of the bubble indicates the methodologic quality according to PEDro. The biggest size indicates at least one study of high methodologic quality in the domain. The lowest size of the bubble chart represents at least one study of low methodologic quality in the domain.
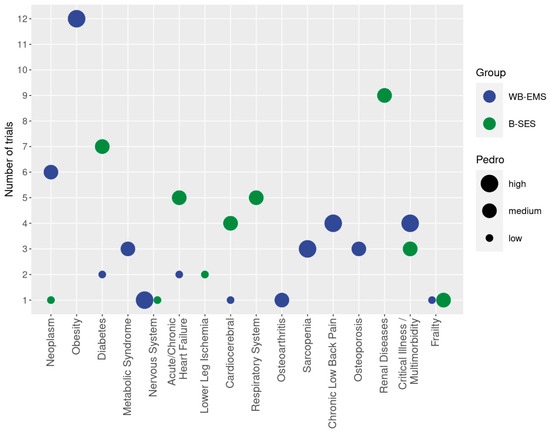
Figure 3.
Cohorts with diseases and conditions addressed in WB-EMS trials classified according to WB-EMS (blue) or B-SES (green) application. The y-axis presents the number of studies that focus on the corresponding cohort (x-axis). The size of the bubble indicates the methodologic quality according to PEDro.
3.4. Neoplasms
In summary, six studies with seven study groups [36,76,80,81,82,83] addressed cohorts with malignant neoplasms. In particular, the research group of Zopf et al. [76,80,81,82,83] focused on this issue, applying WB-EMS for 12 weeks each. So far, the authors have published data on their ongoing advanced cancer project [80] with subgroup analyses on hematological malignancies [81], gastro-intestinal [76], pancreatic [83], prostate, and colorectal cancer [82]. Hamada et al. [36] focused on patients at the early stage of an allogeneic stem cell transplant, predominately in people with acute leukemia applying B-SES for four post-transplantation weeks (A further B-SES case–control study [103] not included in this evidence map focused on the same cohort). Other studies did not focus on but also included cancer patients [88]. Of importance, none of the studies reported adverse effects during the intervention. Evidence for WB-EMS or B-SES application in cancer patients provided by the non-randomized studies and subgroup analysis can be considered moderate (evidence level IIa).
3.5. Endocrine, Nutritional and Metabolic Diseases
A large number of studies focused on cohorts with metabolic disorders and diseases. Apart from two studies with sarcopenic obesity cohorts [11,46], ten further studies addressed cohorts with obesity [17,22,23,38,48,74,75,91,96,100]. However, only six studies considered “obesity” as an eligibility criterion [11,22,46,48,74,75] (Song et al. [85] described his cohort of female students as “obese”; but due to BMI (26.1 kg/m2) or body fat rate (28% as determined by BIA), this cohort can be considered as overweight only. However, this error can be attributable to the translation (Korean–English)). One further study applied abdominal obesity [47] as an eligibility criterion. Apart from one exception with overweight participants [88], all trials on B-SES conducted exercises with participants with a normal BMI or even with severely underweight particpants [42]. Of importance, none of the studies on obesity reported adverse effects during the intervention. Considering the evidence level of the studies, with three RCTs [11,22,75] of high methodologic quality that applied obesity as a criterion for inclusion, the evidence level provided for the EMS application can be classified as high.
Cohorts with non-insulin-dependent diabetes mellitus (NIDDM) were addressed in five randomized and non-randomized trials or intervention studies without CG [38,39,89,93,96] that applied WB-EMS (n = 2) or B-SES (n = 3) for two to four months. Two of the B-SES studies included hospitalized cohorts with diabetic ulcers undergoing minor amputation [39] or end-stage kidney disease [93]. Additionally, four other B-SES studies did not focus on but included a large proportion of participants with diabetes [37,54,55,88]. Of importance, a further three moderate to high quality RCTs [23,43,74] focused on cohorts with metabolic syndrome and applied WB-EMS for 3–6 months. Unfortunately, one [93] study on NIDDM and MetS cohorts failed to report adverse effects. Summarizing the evidence of the studies, with two moderate methodologic quality RCTs [39,93], evidence for EMS application in NIDDM cohorts can be considered moderate–high. Additionally, three low-moderate quality RCTs that applied MetS as a criterion for inclusion [43,74] and did not observe adverse effects might increase the evidence for WB-EMS application in people with cardiometabolic diseases.
3.6. Diseases of the Nervous System
Only a few studies focused on cohorts with diseases of the nervous system [29,58]. While the high-quality RCT of Di Cagno et al. [29] focused on stage 1 (mild)–3 (moderate) Parkinson’s disease in patients 50–80 years old for their 12-week WB-EMS trial, the NRT of Mori et al. [58] addressed Huntington’s disease patients during dialysis with B-SES for 6 weeks (Another case–control study [104] not included in the evidence map focused on B-SES and virtual reality-guided balance training (30 days) for managing paraplegia after spinal cord infarction). While DiCagno et al. [29] observed no adverse effects, unfortunately, Mori et al. [29] did not report the unintended effects of B-SES application.
3.7. Cardiovascular Diseases
The non-controlled cohort 2.5-month WB-EMS study of Fritsche et al. [34] and the 4-month NRCT of van Buuren [97] solely included participants with chronic heart failure [34,97]. Two other moderate quality B-SES studies [55,90] selected acute heart failure as an eligibility criterion and applied 10 and 14 days of B-SES during hospitalization. In parallel, about 50% of the critically ill patients included in the two low and moderate methodologic quality RCTs of Nakamura et al. [60,61], and 70% of the older hemodialysis patients included in the moderate quality RCT of Homma et al. [37], displayed heart failure, cardiopulmonary arrest [60,61], or had a history of ischemic heart disease [37]. Apart from two studies [60,61] with critically ill patients that failed to report unintended side effects related to the intervention, none of the studies reported adverse effects.
Severe ischemia of the lower limbs/peripheral arterial diseases [54,65] was an eligibility criterion in two low methodologic quality B-SES trials. Neither study observed adverse effects related to the intervention.
The low methodologic quality RCT of Lukashevich et al. [53] exclusively addressed patients <6 months after a stroke event with high-frequency WB-EMS for 3 weeks. In parallel, the vast majority (16 of 18) of the bedridden older participants of the moderate quality RCT of Kataoka et al. [42] suffered from cerebral infarction, cerebral or subarachnoid hemorrhage, or hypoxic ischemic encephalopathy (B-SES). Among the two B-SES studies of Nakamura et al. [60,61] with critically ill patients, and the moderate quality B-SES RCT of Homma et al. [37], about half of the patients suffered from stroke [37] or displayed a history of cerebrovascular events/disease. Apart from the two low to moderate quality RCTs of Nakamura et al. [60,61], with their particularly vulnerable cohort that did not report adverse effects, none of the other studies that focused on “stroke patients” reported adverse effects of the EMS intervention.
Surprisingly, hypertonic cohorts were not specifically addressed by longitudinal studies. However, the proportion of study participants with hypertension averaged >50 to >90% in four B-SES trials [37,55,88,90]. None of the four low to moderate quality studies reported adverse effects of the intervention. Nevertheless, due to the high incidence of hypertension in the adult population, a dedicated study that provides evidence for the safe application of WB-EMS in this cohort would be quite welcome.
3.8. Diseases of the Respiratory System
No study has so far applied diseases of the respiratory system as a criterion for inclusion in WB-EMS studies. However, 30% and 60% of the patients in the two low and moderate quality B-SES RCTs on critically ill patients of Nakamura et al. [60,61] suffered from respiratory failure. In parallel, three other low-moderate methodologic quality studies reported the inclusion of patients with respiratory failure or COPD [55,64,90]. While the latter three studies did not observe unintended side effects, Nakamura et al. [60,61] did not report adverse effects in his critically ill patients.
3.9. Musculoskeletal and Connective Tissue Diseases
So far, only one moderate quality WB-EMS RCT of 8 weeks applied (knee) osteoarthritis as the main criterion for inclusion [70]. The study reported no adverse effect of the WB-EMS application.
Sarcopenia, recently included in the ICD 10 GM (M62. 84), was specifically addressed by two high-quality WB-EMS RCTs of 4 and 6 months in an ambulatory setting [11,46], and by one 4-week RCT conducted in a stationary setting [91]. In summary, none of the studies observed adverse effects of the EMS protocol. Considering the poor muscle mass or/and function of patients reported by many B-SES studies (e.g., [37,42,60,61,63,64,90,93]) a large proportion of these cohorts might also suffer from sarcopenia.
Non-specific chronic low back pain was the primary eligibility criterion in two 3-month high-quality WB-EMS studies and two 6- and 8-week NRCTs [51,56,84,99]. None of the trials reported adverse effects during the intervention.
Osteopenia or osteoporosis was the main criterion for inclusion in three moderate or high-quality WB-EMS studies of 10, 14, and 52 weeks [44,94,98]. None of the studies observed adverse effects of the EMS intervention.
In parallel to sarcopenia, the vast majority of B-SES studies and WB-EMS studies with older people (i.e., 60 years and older) might also include a high proportion of people with osteopenia/osteoporosis; this relates in particular to female cohorts with increased peri- and (early) post-menopausal bone loss [105]. The fact that adverse effects were not observed underscores the safety of EMS application in these older cohorts.
3.10. Diseases of the Genitourinary System
Several 6–12-week low to moderate methodologic quality studies applied B-SES during dialysis in patients with chronic kidney diseases [37,58,63,88,93]. At least two other low to moderate quality B-SES studies included a moderate–large proportion of patients with chronic renal disease [55,90] or post renal replacement therapy [60,61]. Unfortunately, four studies that included patients with renal diseases failed to list adverse effects, while the remaining studies did not observe unintended side effects of the EMS intervention.
3.11. Critical Illness, Multi-Morbidity
Three low to moderate B-SES studies focused on critically ill patients treated in intensive care units [60,61,64] for 10–40 days. While Nakamura et al. [61] did not address this issue, Nonoyama et al. [64] and Nakamura et al. [60] reported no adverse effects of B-SES application in their study.
When defining multi-morbidity as the simultaneous presence of three or more chronic diseases [106], many B-SES studies and at least four WB-EMS studies [11,25,46,91] included multi-morbid cohorts and applied WB-EMS for one to six months. Although not all B-SES studies focused on this issue, no study reported adverse effects of the EMS application.
3.12. Frailty, Functional Limitation
In their moderate quality RCT, Kataoka et al. [42] focused on severely frail, bedridden elderly patients in their 12-week B-SES study. Another two-month low quality WB-EMS pilot study [25] applied frailty as a criterion for inclusion. Boutry-Regard et al. [27] only included older people with limited mobility (…however, the cut-off value for gait speed of 1.5 m/s is considerably above the 0.8 to 1.0 m/s criteria for slow gait speed, e.g., suggested for sarcopenia diagnosis [107,108,109,110]) in their 12-week moderate quality B-SES RCT. None of the studies listed above reported adverse effects of the EMS application.
Apart from these trials, several other studies that focused on critically ill patients [60,61,64], sarcopenia [11,46,91], or end stage kidney disease [93] included a large proportion of frail or physically limited older people. The fact that none of the studies reported unintended side effects might increase the evidence for WB-EMS application in this domain.
3.13. Adverse Effects
Apart from ten studies (WB-EMS n = 6; B-SES n = 4), with three studies [58,61,93] addressing cohorts with conditions and diseases, all other studies reported or submitted the prevalence of adverse effects on request. Besides one study [92], and independently of the cohorts addressed, no study reported side effects of the EMS intervention with WB-EMS or B-SES. The only study that reported acute adverse effects of WB-EMS [92] focused on the effects of very high impulse intensities in novice WB-EMS applicants with rhabdomyolysis effects in a closely medically supervised setting. In summary, the study reported exceptionally high creatine kinase and myoglobin levels 3–4 days after a one-off 20 min WB-EMS application. However, this cannot be considered as an adverse effect, but as the primary study outcome.
4. Discussion
This project aimed to identify and summarize studies that reported data on longitudinal WB-EMS application or closely related techniques able to stimulate large muscle areas in different non-athletic adult cohorts. In summary, the present evidence map provided evidence for the (safe) application of WB-EMS (including B-SES) techniques in several, even critically ill, cohorts covered by the 86 studies included.
With respect to age and gender, most cohorts were addressed by the trials. This particularly includes older women and men who are either institutionalized, hospitalized, or living in community living centers, and who are specifically relevant for joint-friendly, highly customizable, and consistently supervised training technologies. Although WB-EMS-induced reductions of total or regional body fat are limited [111], many studies focused on overweight cohorts. Few of the studies applied a combination of WB-EMS and diet [18,23,35,100]. Although some specific research questions remain, we feel that evidence for WB-EMS application in overweight cohorts is sufficiently provided.
Apart from cohorts with overweight or obese patients, the majority of trials with sedentary, non-athletic adults addressed cohorts with health-related problems and limitations. This refers in particular to B-SES, which is used primarily in hospitals and care facilities. It is of crucial importance that no study, whether it involved advanced cancer, diabetes, stroke, Parkinson’s disease, chronic heart failure, pAVK, COPD, sarcopenia, psteoporosis, pre-frailty or frailty, chronic renal failure, or even critically ill cohorts, observed adverse effects related to WB-EMS or B-SES application. However, one should bear in mind that the studies provided close supervision predominately by medical staff. Summarizing the results of the evidence map for health issues, a sufficient body of evidence for WB-EMS application is available for cohorts with (1) non-specific chronic low back pain, (2) sarcopenia, (3) osteopenia/osteoporosis, (4) obesity, (5) non-insulin-dependent diabetes mellitus and MetS, (6) cancer/neoplasms, (7) chronic renal diseases, (8) multi-morbidity, and (9) critically ill hospitalized patients, although for the latter group, adverse effects were not consistently provided.
Still, insufficient evidence is available for WB-EMS application in cohorts with (1) acute or chronic heart failure, (2) diseases of the respiratory system, or (3) cerebrovascular diseases, a condition particularly promising for WB-EMS due to the lack of other training options.
Our study identified several gaps of WB-EMS research with respect to the cohorts addressed. However, not all health-related domains are equally relevant for dedicated WB-EMS research. This applies in particular for people with local limitations (e.g., arthropathies, spondylopathies) accessible for local EMS-application. On the other hand, cohorts with conditions or diseases that benefit from the simultaneous stimulation of large muscle groups and limited options for conventional exercise training will be particularly important for WB-EMS research. The corresponding gaps in WB-EMS research concerning cohorts with Alzheimer’s diseases, polyneuropathies, myoneural disorders, or multiple sclerosis, but also with stroke or general immobilization, should be addressed with particular emphasis.
Apart from providing evidence for WB-EMS application in varying study cohorts and drawing attention to gaps in the WB-EMS literature, another aim of the present evidence map was to support the considerations of decision makers with respect to future recommendations on absolute and relative contraindications for WB-EMS application. To our best knowledge, only one available publication summarized contraindications for WB-EMS [3]. Although these recommended contraindications focus on the non-medical, commercial German WB-EMS market, most other providers and many researchers consider these recommendations to be mandatory. Briefly addressing the history of these contraindications, the commercial WB-EMS market suffered from a series of adverse effects that resulted in critical discussions in the media and led to a temporary ban in Israel (review in [112]). The lack of mandatory regulations for qualifications for providers and (in particular) instructors led the German expert group on WB-EMS [112] to issue very cautious recommendations in 2019. Meanwhile, a rather dense network of Federal regulations addressed WB-EMS applications (e.g., [113]), which includes the mandatory licensing of WB-EMS instructors/caregivers (e.g., Germany: [114]). Apart from federal regulations, the introduction of “medical WB-EMS”, defined as (1) primarily a therapeutic intervention, (2) based on an existing diagnosis, (3) provided by qualified medical–therapeutic personnel, (4) in compliance with current guidelines, and (5) using medical devices [112], allows for the opening of WB-EMS-applications for previously excluded cohorts [3]. We feel that the present evidence map will be helpful in the elaboration of an updated list of relative and absolute contraindications on WB-EMS-application. However, this approach must be conducted in close liaison with expert groups.
Some features of this evidence map might be irritating or hard to grasp for the reader. First of all, the present evidence map focuses on “cohorts” included by WB-EMS studies and thus differs from most evidence maps that address “study outcomes” (e.g., [115]). Both parameters are similarly important, but since a comprehensive analysis and description of both aspects failed, we decided to give priority to the “cohort aspect”. This reflects our aim to provide timely data for the readjustment of absolute and relative contraindication on WB-EMS.
One may argue that combining WB-EMS, defined as the “simultaneous application of electric stimuli via at least six current channels or participation of all major muscle groups with a current impulse effective to trigger muscular adaptations” [6], with the B-SES technique might not be reliable. While many features are comparable (Supplemental Table S2), B-SES neuromuscular stimulation uses a monophasic, exponentially climbing pulse. Further, depending of the B-SES device, five (e.g., [37]) or six (e.g., [36,42]) electrodes are fixed at the waist/lower back and/or thigh and ankles, resulting in a lower stimulation area compared to WB-EMS. Additionally, in contrast to the usual WB-EMS application in an upright standing position, all included trials applied B-SES in a sitting [27] or a (mainly) supine position, predominately in a passive mode, i.e., without voluntary movements during the impulse phase. While the duration of the WB-EMS or B-SES sessions are largely similar, the training frequency of B-SES is significantly higher. The stimulus intensity of B-SES was consistently described as the maximum tolerable impulse intensity without pain (or discomfort); i.e., largely in line with the specification applied by WB-EMS. For both methods, acute stimulation effects on deeper muscle layers of the thigh and lower legs were reported [116,117]. We based our criteria of “safety” on missing adverse effects. We agree with the objection that this did not necessarily indicate that WB-EMS is a harmless exercise technology for every cohort. This particularly refers to applications that were too intense for novice users, which have resulted in severe rhabdomyolysis [92,118]. However, considering recently updated guidelines [119] and the rather restrictive contraindications on WB-EMS [3], we conclude that the safety standards for WB-EMS application are exceptionally high, at least compared with other types of exercise or exercise technologies. Nevertheless, no WB-EMS study exceeded the length of 12 months [98] (and thus long-term adverse effects were not recorded), which indicates the need for the scientific long-term monitoring of WB-EMS application. Further, although not addressed by the present work but nevertheless important for increasing safety, more research on the customization of WB-EMS protocols is required to meet the specific demands, particularities, and preferences of different populations.
Due to the large number of studies, poor information provided, difficulties in proper translation, and partially missing author responses, we might have failed to identify all eligible articles, or always correctly classify or describe the included articles. This may also be attributable to the approach of including all kinds of longitudinal (full-text) studies irrespective of their design. We agree that this would be a limitation when addressing “study outcomes”; however, when addressing “cohorts”, the study design might be of lesser relevance. Nevertheless, it is important to classify the contribution of the single studies for evidence and relevance of the domain. This was covered by considering whether the corresponding trial considered the dedicated disease/condition as a criterion for inclusion or as a simple co-morbidity. In parallel, methodologic quality was rated by the PEDro scale, which is specifically dedicated to clinical physiotherapy and exercise studies. However, this score is not perfectly suitable for non-randomized controlled trials; nevertheless, our approach allows for a rough overview of this important aspect.
Finally, a relevant limitation of the present review is the missing data, which are particularly important in the domain of adverse effects related to WB-EMS application. Although we contacted the corresponding authors several times by email or phone, we failed to obtain data of 10 studies. Unfortunately, these included particularly important studies with vulnerable cohorts [58,61,93]. Apart from the failure of several trails to report adverse effects at all, considering the scarce data provided by all other studies, the monitoring and reporting of adverse effects seem to be a neglected domain in clinical WB-EMS trials overall.
Finally, and of minor importance for the presence study, albeit relevant for studies that focus on effects, many researchers do not report the WB-EMS intervention comprehensibly or completely.
5. Conclusions
The present work provides evidence for the application of WB-EMS techniques in a wide range of human cohorts. We conclude that priority should be given to WB-EMS research in people with neurological and cerebrovascular diseases to address existing evidence gaps. This does not exclude advanced research on cohorts repeatedly addressed by WB-EMS studies, however. Nevertheless, the unique selling points of WB-EMS, i.e., its ability to involuntarily stimulate large muscle groups simultaneously with adequate intensity but low orthopedic stress, should be considered in when making decisions about WB-EMS application in eligible cohorts. Another demand related to WB-EMS application in vulnerable cohorts is that ongoing or at least long-running projects should address the long-term safety of WB-EMS. Addressing the safety of WB-EMS applications, although a few articles failed to report adverse effects, none of the identified trials, whether they were conducted with advanced cancer, diabetes, stroke, Parkinson, chronic heart failure, pAVK, COPD, sarcopenia, osteoporosis, frailty, chronic renal failure, or even critically ill cohorts, observed adverse effects related to WB-EMS or B-SES application. Although this of course did not indicate the complete harmlessness of WB-EMS, advanced federal regulations and mandatory qualifications and education for WB-EMS providers and trainers suggest that an easing of the very restrictive contraindications of WB-EMS, at least in consistently supervised settings, should be considered in the near future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s24030972/s1, Table S1: Search strategies and their results; Table S2: Exercise and stimulation characteristics of the included studies.
Author Contributions
Conceptualization, W.K., D.S., S.v.S., M.K., M.B. and M.U.; methodology, W.K., D.S., M.K., S.v.S., M.B. and M.U.; software, M.K.; validation, M.B., W.K. and M.K.; formal analysis, M.K. and W.K.; investigation, W.K., D.S., S.v.S., M.K., M.B. and M.U.; resources, W.K. and M.U.; data curation, W.K., M.B. and D.S.; writing—original draft preparation, M.B. and W.K.; writing—review and editing, W.K., D.S., S.v.S., M.K., M.B. and M.U.; visualization, M.K. and W.K.; supervision, W.K.; project administration, W.K., M.B. and D.S.; funding acquisition, W.K. and M.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank all the authors who provided missing data. The present study was performed in (partial) fulfillment of the requirements for Miriam Beier obtaining the degree Dr. med. dent.
Conflicts of Interest
Miriam Beier, Daniel Schoene, Matthias Kohl, Simon von Stengel, Michael Uder, and Wolfgang Kemmler declare no conflicts of interest.
References
- Kemmler, W.; Kleinoder, H.; Fröhlich, M. Editorial: Whole-body electromyostimulation: A training technology to improve health and performance in humans? volume II. Front. Physiol. 2022, 13, 972011. [Google Scholar] [CrossRef]
- Eifler, C. Marktsituation, Trends und Entwicklungen. In Ganzkörper-EMS; Kemmler, W., Fröhlich, M., Eifler, C., Eds.; Springer Spektrum: Wiesbaden, Germany, 2022; Volume Essentials. [Google Scholar]
- Kemmler, W.; Weissenfels, A.; Willert, S.; Fröhlich, M.; Ludwig, O.; Berger, J.; Zart, S.; Becker, S.; Backfisch, M.; Kleinöder, H.; et al. Recommended Contraindications for the Use of Non-Medical WB-Electromyostimulation. Dtsch Z Sport. 2019, 70, 278–281. [Google Scholar] [CrossRef]
- Miake-Lye, I.M.; Hempel, S.; Shanman, R.; Shekelle, P.G. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst. Rev. 2016, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Schmucker, C.; Motschall, E.; Antes, G.; Meerpohl, J.J. Methods of evidence mapping. A systematic review. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013, 56, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Kleinoder, H.; Fröhlich, M. Editorial: Whole-Body Electromyostimulation: A Training Technology to Improve Health and Performance in Humans? Front. Physiol. 2020, 11, 523. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef]
- Kemmler, W.; Kohl, M.; Freiberger, E.; Sieber, C.; von Stengel, S. Effect of whole-body electromyostimulation and/or protein supplementation on obesity and cardiometabolic risk in older men with sarcopenic obesity: The randomized controlled FranSO trial. BMC Geriatr. 2018, 18, 70. [Google Scholar] [CrossRef]
- Kemmler, W.; von Stengel, S.; Kohl, M.; Rohleder, N.; Bertsch, T.; Sieber, C.C.; Freiberger, E.; Kob, R. Safety of a Combined WB-EMS and High-Protein Diet Intervention in Sarcopenic Obese Elderly Men. Clin. Interv. Aging 2020, 15, 953–967. [Google Scholar] [CrossRef]
- Kemmler, W.; von Stengel, S.; Teschler, M.; Weissenfels, A.; Bebenek, M.; Freiberger, E.; Sieber, C.; Kohl, M. Ganzkörper-Elektromyostimulation, Sarkopenie und Adipositas. Ergebnisse der randomisierten kontrollierten “Franconia Sarcopenic Obesity Study” (FRANSO). Osteoporose Rheuma Aktuell 2017, 15, 12–18. [Google Scholar]
- Kemmler, W.; Weissenfels, A.; Teschler, M.; Willert, S.; Bebenek, M.; Shojaa, M.; Kohl, M.; Freiberger, E.; Sieber, C.; von Stengel, S. Whole-body Electromyostimulation and protein supplementation favorably affect Sarcopenic Obesity in community-dwelling older men at risk. The Randomized Controlled FranSO Study. Clin. Interv. Aging 2017, 12, 1503–1513. [Google Scholar] [CrossRef]
- Mages, M.; Shojaa, M.; Kohl, M.; von Stengel, S.; Becker, C.; Gosch, M.; Jakob, F.; Kerschan-Schindl, K.; Kladny, B.; Klockner, N.; et al. Exercise Effects on Bone Mineral Density in Men. Nutrients 2021, 13, 4244. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, R.; Shojaa, M.; Kohl, M.; von Stengel, S.; Jakob, F.; Kerschan-Schindl, K.; Lange, U.; Peters, S.; Thomasius, F.; Uder, M.; et al. Exercise training and bone mineral density in postmenopausal women: An updated systematic review and meta-analysis of intervention studies with emphasis on potential moderators. Osteo. Int. 2023, 4, 1145–1178. [Google Scholar] [CrossRef] [PubMed]
- ICD-10-GM. International Statistical Classification of Diseases and Related Health Problems, 10. Revision, German Modification. 2022. Available online: https://www.bfarm.de/EN/Code-systems/Classifications/ICD/ICD-10-GM/_node.html (accessed on 11 October 2023).
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro de Avila, V.; Bento, T.; Gomes, W.; Leitao, J.; Fortuna de Sousa, N. Functional Outcomes and Quality of Life After Ankle Fracture Surgically Treated: A Systematic Review. J. Sport Rehabil. 2018, 27, 274–283. [Google Scholar] [CrossRef]
- Afsharnezhad, T.; Soumander, S. The Effects of Resistance Training With and Without Electrical Muscle Stimulation on Body Composition of Obese Women. Iran. J. Health Sci. 2022, 10, 51–62. [Google Scholar] [CrossRef]
- Akçay, N.; Güney, H.; Kaplan, S.; Akgül, M. Electromyostimulation Exercise with Diet Program is More Effective on Body Composition than its Exercise without Diet. Mediterr. J. Sport Sci. 2022, 4, 814–822. [Google Scholar] [CrossRef]
- Almada, R.; Molina Martín, J.J.; Tregón, P.S.; García, J.L. Comparación Entre los Efectos de un Programa de Entrenamiento de Fuerza Explosiva Mediante Bandas Elásticas y un Programa de Entrenamiento con Electro-Estimulación de Cuerpo Completo. Rev. Kronos 2016, 15, 1. [Google Scholar]
- Amaro-Gahete, F.J.; De-la, O.A.; Jurado-Fasoli, L.; Dote-Montero, M.; Gutierrez, A.; Ruiz, J.R.; Castillo, M.J. Changes in Physical Fitness After 12 Weeks of Structured Concurrent Exercise Training, High Intensity Interval Training, or Whole-Body Electromyostimulation Training in Sedentary Middle-Aged Adults: A Randomized Controlled Trial. Front. Physiol. 2019, 10, 451. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; De-la, O.A.; Sanchez-Delgado, G.; Robles-Gonzalez, L.; Jurado-Fasoli, L.; Ruiz, J.R.; Gutierrez, A. Functional Exercise Training and Undulating Periodization Enhances the Effect of Whole-Body Electromyostimulation Training on Running Performance. Front. Physiol. 2018, 9, 720. [Google Scholar] [CrossRef]
- Andre, L.D.; Basso-Vanelli, R.P.; Ricci, P.A.; Di Thommazo-Luporini, L.; de Oliveira, C.R.; Haddad, G.F.; Haddad, J.M.; Parizotto, N.A.; de Vieira, R.; Arena, R.; et al. Whole-body electrical stimulation as a strategy to improve functional capacity and preserver lean mass after bariatric surgery: A randomized triple-blind controlled trial. Int. J. Obes. 2021, 45, 1476–1487. [Google Scholar] [CrossRef]
- Bellia, A.; Ruscello, B.; Bolognino, R.; Briotti, G.; Gabrielli, P.R.; Silvestri, A.; Rosazza, C.; Ambruoso, F.; Lombardo, M.; Bernardini, A.; et al. Whole-body Electromyostimulation plus Caloric Restriction in Metabolic Syndrome. Int. J. Sports Med. 2020, 41, 751–758. [Google Scholar] [CrossRef]
- Berger, J.; Ludwig, O.; Becker, S.; Backfisch, M.; Kemmler, W.; Frohlich, M. Effects of an Impulse Frequency Dependent 10-Week Whole-body Electromyostimulation Training Program on Specific Sport Performance Parameters. J. Sports Sci. Med. 2020, 19, 271–281. [Google Scholar]
- Bloeckl, J.; Raps, S.; Weineck, M.; Kob, R.; Bertsch, T.; Kemmler, W.; Schoene, D. Feasibility and Safety of Whole-Body Electromyostimulation in Frail Older People-A Pilot Trial. Front. Physiol. 2022, 13, 856681. [Google Scholar] [CrossRef]
- Bostan, G.; Gümüş, M. Effects of fitness and electromyostimulation (EMS) training techniques on body composition [Antrenman Tekniklerinin Vücut Kompozisyonu Üzerine Etkileri]. Turk. J. Diabetes Obes. 2022, 6, 149–158. [Google Scholar] [CrossRef]
- Boutry-Regard, C.; Vinyes-Pares, G.; Breuille, D.; Moritani, T. Supplementation with Whey Protein, Omega-3 Fatty Acids and Polyphenols Combined with Electrical Muscle Stimulation Increases Muscle Strength in Elderly Adults with Limited Mobility: A Randomized Controlled Trial. Nutrients 2020, 12, 1866. [Google Scholar] [CrossRef]
- Çetin, E.; Özdol Pinar, Y.; Deniz, S. Effects of Whole-Body Electromyostimulation on body composition in women of different age [Tüm Beden Elektromiyostimülasyon Uygulamasinin Farkli Yaş Gruplarindaki Kadinlarda Beden Kompozisyonu Üzerine Etkisi]. J. Phys. Educ. Sports Sci. 2017, 15, 173–177. [Google Scholar]
- di Cagno, A.; Buonsenso, A.; Centorbi, M.; Manni, L.; Di Costanzo, A.; Casazza, G.; Parisi, A.; Guerra, G.; Calcagno, G.; Iuliano, E.; et al. Whole body-electromyostimulation effects on serum biomarkers, physical performances and fatigue in Parkinson’s patients: A randomized controlled trial. Front. Aging Neurosci. 2023, 15, 1086487. [Google Scholar] [CrossRef] [PubMed]
- Dyaksa, R.S.; Susilo, E.A.; Virdianto, A.W. The Effect of EMS Exercise on Body Circumstances in Sedentary Women [Pengaruh Latihan Ems Terhadap Lingkar Tubuh Pada Wanita Sedentary]. J. Phys. Educ. Sports Health 2022, 5, 264–270. [Google Scholar] [CrossRef]
- Ethem, H.; Orhan, İ.; ÇAnakci, G. Investigation of the Effect of 6 Weeks Whole-Body Electromyostimulation and with Body Weight Strength Training on some Motoric Properties in Sedantery Women [Sedanter Kadınlarda Tüm Beden Elektromyostimülasyonla Kombine Dinamik Kuvvet Alıştırmalarının Bazı Motorik Özellikler Üzerine Etkisinin İncelenmesi]. Eurasian Res. Sport Sci. 2019, 2, 83–96. [Google Scholar] [CrossRef]
- Evangelista, A.L.; Alonso, A.C.; Ritti-Dias, R.M.; Barros, B.M.; de Souza, C.R.; Braz, T.V.; Bocalini, D.S.; Greve, J.M.D. Effects of Whole Body Electrostimulation Associated With Body Weight Training on Functional Capacity and Body Composition in Inactive Older People. Front. Physiol. 2021, 12, 638936. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.L.; Teixeira, C.V.S.; Barros, B.M.; de Azevedo, J.B.; Paunksnis, M.R.R.; Souza, C.R.; Wadhi, T.; Rica, R.L.; Braz, T.V.; Bocalini, D.S. Does whole-body electrical muscle stimulation combined with strength training promote morphofunctional alterations? Clinics 2019, 74, e1334. [Google Scholar] [CrossRef]
- Fritzsche, D.; Fruend, A.; Schenk, S.; Mellwig, K.; Keinöder, H.; Gummert, J.; Horstkotte, D. Elektromyostimulation (EMS) bei kardiologischen Patienten. Wird das EMS-Training bedeutsam für die Sekundärprävention? Herz 2010, 35, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ghannadi, S.; Halabchi, F.; Maleklou, F.; Tavakol, Z.; Rajabian Tabesh, M.; Bala, D.; Alizadeh, Z. The effect of 6 weeks electrical muscle stimulation training and aerobic exercise on body composition of overweight women: A randomized controlled study. Sport Sci. Health 2022, 18, 1387–1395. [Google Scholar] [CrossRef]
- Hamada, R.; Sato, S.; Miyasaka, J.; Murao, M.; Matsushita, M.; Kajimoto, T.; Otagaki, A.; Asano, T.; Nankaku, M.; Kondo, T.; et al. Belt Electrode-Skeletal Muscle Electrical Stimulation During Early Hematopoietic Post-Transplantation To Prevent Skeletal Muscle Atrophy and Weakness. Transpl. Cell Ther. 2023, 29, 51 e51–51 e57. [Google Scholar] [CrossRef] [PubMed]
- Homma, M.; Miura, M.; Hirayama, Y.; Takahashi, T.; Miura, T.; Yoshida, N.; Miyata, S.; Kohzuki, M.; Ebihara, S. Belt Electrode-Skeletal Muscle Electrical Stimulation in Older Hemodialysis Patients with Reduced Physical Activity: A Randomized Controlled Pilot Study. J. Clin. Med. 2022, 11, 622–630. [Google Scholar] [CrossRef]
- Houdijk, A.P.J.; Bos, N.; Verduin, W.M.; Hijdendaal, M.M.; Zwartkruis, M.A.L. Visceral fat loss by whole-body electromyostimulation is attenuated in male and absent in female older Non-Insulin-Dependent diabetes patients. Endocrinol. Diabetes Metab. 2022, 5, e377. [Google Scholar] [CrossRef]
- Imaoka, S.; Kudou, G.; Tsugiyama, K.; Minata, S.; Teroh, T.; Ootsuka, M.; Furukawa, M.; Higashi, T.; Okita, M. Efficacy of Belt Electrode Skeletal Muscle Electrical Stimulation in the Postoperative Rest Period in Patients with Diabetes who Have Undergone minor Amputations: A Randomized Controlled Trial. Int. J. Low. Extrem. Wounds 2022, 15347346221077491. [Google Scholar] [CrossRef]
- Jee, Y.-S. The effect of high-impulse-electromyostimulation on adipokine profiles, body composition and strength: A pilot study. J. Isokinet. 2019, 27, 163–176. [Google Scholar] [CrossRef]
- Junger, J.; Junger, A.; Ostrowski, P. Body composition of trainees undergoing EMS training with respect to their nutrition. J. Phys. Educ. Sport 2020, 20, 97–101. [Google Scholar] [CrossRef]
- Kataoka, H.; Nakashima, S.; Aoki, H.; Goto, K.; Yamashita, J.; Honda, Y.; Kondo, Y.; Hirase, T.; Sakamoto, J.; Okita, M. Electrical stimulation in addition to passive exercise has a small effect on spasticity and range of motion in bedridden elderly patients: A pilot randomized crossover study. Health 2019, 11, 1072–1086. [Google Scholar] [CrossRef][Green Version]
- Kemmler, W.; Birlauf, A.; von Stengel, S. Einfluss von Ganzkörper-Elektromyostimulation auf das Metabolische Syndrom bei älteren Männern mit metabolischem Syndrom. Dtsch. Z. Sportmed. 2010, 61, 117–123. [Google Scholar]
- Kemmler, W.; Schliffka, R.; Mayhew, J.L.; von Stengel, S. Effects of Whole-Body-Electromyostimulation on Resting Metabolic Rate, Anthropometric and Neuromuscular Parameters in the Elderly. The Training and ElectroStimulation Trial (TEST). J. Strength Cond. Res. 2010, 24, 1880–1886. [Google Scholar] [CrossRef]
- Kemmler, W.; Teschler, M.; Weissenfels, A.; Bebenek, M.; Frohlich, M.; Kohl, M.; von Stengel, S. Effects of Whole-Body Electromyostimulation versus High-Intensity Resistance Exercise on Body Composition and Strength: A Randomized Controlled Study. Evid. -Based Complement. Altern. Med. Ecam 2016, 2016, 9236809. [Google Scholar] [CrossRef]
- Kemmler, W.; Teschler, M.; Weissenfels, A.; Bebenek, M.; von Stengel, S.; Kohl, M.; Freiberger, E.; Goisser, S.; Jakob, F.; Sieber, C.; et al. Whole-body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. Resultsof the randomized controlled FORMOsA-sarcopenic obesity study. Osteoporos. Int. 2016, 27, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; von Stengel, S. Whole-body electromyostimulation as a means to impact muscle mass and abdominal body fat in lean, sedentary, older female adults: Subanalysis of the TEST-III trial. Clin. Interv. Aging 2013, 8, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jee, Y. EMS-effect of Exercises with Music on Fatness and Biomarkers of Obese Elderly Women. Medicina 2020, 56, 156. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Eun, D.; Jee, Y.S. Higher Impulse Electromyostimulation Contributes to Psychological Satisfaction and Physical Development in Healthy Men. Medicina 2021, 57, 197. [Google Scholar] [CrossRef]
- Kirişcioğlu, M.; Bicer, M.; Pancar, Z.; Doğan, İ. Effects of electromyostımulatıon traınıng on body composıtıon. Turk. J. Sport Exerc. 2019, 21, 34–37. [Google Scholar] [CrossRef]
- Konrad, K.L.; Baeyens, J.-P.; Birkenmaier, C.; Ranker, A.H.; Widmann, J.; Leukert, J.; Wenisch, L.; Kraft, E.; Jansson, V.; Wegener, B. The effects of whole-body electromyostimulation (WB-EMS) in comparison to a multimodal treatment concept in patients with non-specific chronic back pain—A prospective clinical intervention study. PLoS ONE 2020, 15, e0236780. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, O.; Berger, J.; Becker, S.; Kemmler, W.; Frohlich, M. The Impact of Whole-Body Electromyostimulation on Body Posture and Trunk Muscle Strength in Untrained Persons. Front. Physiol. 2019, 10, 1020. [Google Scholar] [CrossRef]
- Lukashevich, U.A.; Ponomarev, V.V.; Tarasevich, M.I.; Zhivolupov, S.A. Functional reciprocal neuromuscular electric stimulation in adaptive kinesitherapy in post-stress patients. Sci. Healthc. 2020, 22, 80–88. [Google Scholar] [CrossRef]
- Matsumoto, J.M.; Terabe, S.Y.; Sakaki, R.H. Experience of Belt Electrode Skeletal Muscle Electrical Stimulation Method for Severe Lower Limb Ischemic Patients: A Case Report. Phys. Ther. Clin. Pract. Res. Educ. 2020, 27, 81–85. [Google Scholar]
- Matsuo, K.; Yoneki, K.; Tatsuki, H.; Mibu, K.; Furuzono, K.; Kobayashi, K.; Yasuda, S.; Tamiya, S. Effect of Electrical Muscle Stimulation on the Reduction of Muscle Volume Loss in Acute Heart Failure Patients. Int. Heart J. 2022, 63, 1141–1149. [Google Scholar] [CrossRef]
- Micke, F.; Weissenfels, A.; Wirtz, N.; Von Stengel, S.; Dörmann, U.; Kohl, M.; Kleinöder, H.; Donath, L.; Kemmler, W. Similar Pain Intensity Reductions and Trunk Strength Improvements following Whole-Body Electromyostimulation vs. Whole-Body Vibration vs. Conventional Back-Strengthening Training in Chronic Non-specific Low Back Pain Patients: A 3-armed randomized controlled trial. Front. Physiol. 2021, 13, 664991. [Google Scholar] [CrossRef]
- Miyamoto, T.; Kamada, H.; Tamaki, A.; Moritani, T. Low-intensity electrical muscle stimulation induces significant increases in muscle strength and cardiorespiratory fitness. Eur. J. Sport Sci. 2016, 16, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Tamura, Y.; Deguchi, K.; Miura, Y.; Yura, Y.T.K. Effect of belt electrode skeletal muscle electrical stimulation during hemodialysis on the endothelial function in hemodialysis. Jpn. J. Electrophysical Agents 2020, 27, 78–81. [Google Scholar]
- Müllerová, M.; Vaculíková, P.; Potúčková, A.; Struhár, I.; Balousová, D.N. Impact of Whole-Body Electromyostimulation and Resistance Training Programme on Strength Parameters and Body Composition in Group of Elderly Women at Risk of Sarcopenia. Stud. Sport. 2022, 16, 292–304. [Google Scholar] [CrossRef]
- Nakamura, K.; Kihata, A.; Naraba, H.; Kanda, N.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Morimura, N. Efficacy of belt electrode skeletal muscle electrical stimulation on reducing the rate of muscle volume loss in critically ill patients: A randomized controlled trial. J. Rehabil. Med. 2019, 51, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakano, H.; Naraba, H.; Mochizuki, M.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Morimura, N. High protein versus medium protein delivery under equal total energy delivery in critical care: A randomized controlled trial. Clin. Nutr. 2021, 40, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Nejad, M.D.; Nikbakht, M.; Ghanbarzadeh, M.; Ranjbar, R. Effect of Concurrent Training Order With Electromyostimulation on Physical Performance in Young Elderly Women. Arch. Rehabil. 2021, 21, 508–525. [Google Scholar] [CrossRef]
- Noguchi, Y.; Hirano, H.; Mizutani, C.; Ito, T.; Kawamura, N. Die Wirkung der elektrischen Stimulation der Skelettmuskulatur mit Gürtelelektroden während der Hämodialyse auf die körperliche Funktion von Hämodialysepatienten. J. Dial. Soc. 2018, 51, 87–91. [Google Scholar] [CrossRef]
- Nonoyama, T.; Shigemi, H.; Kubota, M.; Matsumine, A.; Shigemi, K.; Ishizuka, T. Neuromuscular electrical stimulation in the intensive care unit prevents muscle atrophy in critically ill older patients: A retrospective cohort study. Medicine 2022, 101, e29451. [Google Scholar] [CrossRef]
- Ochiai, K.; Tamura, Y.; Ehara, K.; Shimizu, R.; Matushita, Y.; Yasu, T. Bridging Therapy Using B-SES for Peripheral Arterial Disease Patients with Severe Lower Limb Ischemia. J. Phys. Ther. Sci. 2018, 33, 545–548. [Google Scholar] [CrossRef]
- Öktem, U.; Akin, M. Investigation of the Effects of Electrical Muscle Stimulation (EMS) and Traditional Training on Strength Gain and Anthropometric Properties in Sedentary Women [Sedanter Kadınlarda Elektriksel Kas Uyarımı (EMS) ve Geleneksel Antrenmanın Kuvvet Kazanımı ve Antropometrik Özellikler Üzerine Etkisinin İncelenmesi]. CBU J. Phys. Educ. Sport Sci. 2022, 17, 70–79. [Google Scholar]
- Özdal, M.; Bostanci, Ö. Effects of whole-body electromyostimulation with and without voluntary muscular contractions on total and regional fat mass of women. Arch. Appl. Sci. Res. 2016, 8, 75–79. [Google Scholar]
- Pano-Rodriguez, A.; Beltran-Garrido, J.V.; Hernandez-Gonzalez, V.; Reverter-Masia, J. Effects of Whole-Body Electromyostimulation on Physical Fitness in Postmenopausal Women: A Randomized Controlled Trial. Sensors 2020, 20, 1482. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Na, S.M.; Choi, S.L.; Seon, J.K.; Do, W.H. Physiological Effect of Exercise Training with Whole Body Electric Muscle Stimulation Suit on Strength and Balance in Young Women: A Randomized Controlled Trial. Chonnam Med. J. 2021, 57, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Min, S.; Park, S.H.; Yoo, J.; Jee, Y.S. Influence of Isometric Exercise Combined with Electromyostimulation on Inflammatory Cytokine Levels, Muscle Strength, and Knee Joint Function in Elderly Women with Early Knee Osteoarthritis. Front. Physiol. 2021, 12, 688260. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, J.; Ham, J.A.; Jee, Y. Effects of aerobic dance with electrical stimulant on body composition and radiological circumference of obese elderly women. Gazz. Medica Ital. Arch. Sci. Mediche 2021, 180, 87–95. [Google Scholar] [CrossRef]
- Park, W.; Lee, J.; Hong, K.; Park, H.Y.; Park, S.; Kim, N.; Park, J. Protein-Added Healthy Lunch-Boxes Combined with Exercise for Improving Physical Fitness and Vascular Function in Pre-Frail Older Women: A Community-Based Randomized Controlled Trial. Clin. Interv. Aging 2023, 18, 13–27. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, H.; Liu, X.; Wu, J.; Zhang, Y. Effects of whole-body electromyostimulation training on upper limb muscles strength and body composition in moderately trained males: A randomized controlled study. Front. Public Health 2022, 10, 982062. [Google Scholar] [CrossRef]
- Reljic, D.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Iron Beats Electricity: Resistance Training but not Whole-Body Electromyostimulation Improves Cardiometabolic Health in Obese Metabolic Syndrome Patients during Caloric Restriction-A Randomized-Controlled Study. Nutrients 2021, 13, 1640. [Google Scholar] [CrossRef]
- Ricci, P.A.; Di Thommazo-Luporini, L.; Jurgensen, S.P.; Andre, L.D.; Haddad, G.F.; Arena, R.; Borghi-Silva, A. Effects of Whole-Body Electromyostimulation Associated with Dynamic Exercise on Functional Capacity and Heart Rate Variability After Bariatric Surgery: A Randomized, Double-Blind, and Sham-Controlled Trial. Obes. Surg. 2020, 30, 3862–3871mats. [Google Scholar] [CrossRef]
- Richter, H. Effect of Electromyostimulation Training and High-Protein Diet on Gastrointestinal Tumor Patients in Palliative and Curative Treatment Settings. [Einfluss von Elektromyostimulationstraining und Proteinreicher Ernährung auf Gastrointestinale Tumorpatienten in Palliativer und Kurativer Behandlungssituation]; Friedrich-Alexander-University Erlangen-Nürnberg: Erlangen, Germany, 2019. [Google Scholar]
- Sadeghipour, S.; Mirzaei, B. Effects of whole-body electromyostimulation with two different frequencies and combined training on lipid profile and body composition in overweight women. Physiother. Q. 2022, 30, 79–85. [Google Scholar] [CrossRef]
- Sadeghipour, S.; Mirzaei, B.; Korobeynikov, G.; Tropin, Y. Effects of Whole-Body Electromyostimulation and Resistance Training on Body Composition and Maximal Strength in Trained Women. Health Sport Rehabil. 2021, 7, 18–28. [Google Scholar] [CrossRef]
- Sánchez-Infante, J.; Bravo-Sáncheza, A.; Abiánb, P.; Estebana, P.; Jimeneza, J.; Abián-Vicén, J. The influence of whole-body electromyostimulation training in middle-aged women. Isokinet. Exerc. Sci. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Schink, K.; Herrmann, H.J.; Schwappacher, R.; Meyer, J.; Orlemann, T.; Waldmann, E.; Wullich, B.; Kahlmeyer, A.; Fietkau, R.; Lubgan, D.; et al. Effects of whole-body electromyostimulation combined with individualized nutritional support on body composition in patients with advanced cancer: A controlled pilot trial. BMC Cancer 2018, 18, 886. [Google Scholar] [CrossRef] [PubMed]
- Schink, K.; Reljic, D.; Herrmann, H.J.; Meyer, J.; Mackensen, A.; Neurath, M.F.; Zopf, Y. Whole-Body Electromyostimulation Combined With Individualized Nutritional Support Improves Body Composition in Patients With Hematological Malignancies—A Pilot Study. Front. Physiol. 2018, 9, 1808. [Google Scholar] [CrossRef] [PubMed]
- Schwappacher, R.; Schink, K.; Sologub, S.; Dieterich, W.; Reljic, D.; Friedrich, O.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Physical activity and advanced cancer: Evidence of exercise-sensitive genes regulating prostate cancer cell proliferation and apoptosis. J. Physiol. 2020, 598, 3871–3889. [Google Scholar] [CrossRef] [PubMed]
- Schwappacher, R.; Dieterich, W.; Reljic, D.; Pilarsky, C.; Mukhopadhyay, D.; Chang, D.K.; Biankin, A.V.; Siebler, J.; Herrmann, H.J.; Neurath, M.F.; et al. Muscle-Derived Cytokines Reduce Growth, Viability and Migratory Activity of Pancreatic Cancer Cells. Cancers 2021, 13, 3820. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, A.; Ruscello, B.; Rosazza, C.; Briotti, G.; Gabrielli, P.R.; Tudisco, C.; D’Ottavio, S. Acute Effects of Whole-Body Electrostimulation Combined with Stretching on Lower Back Pain. Int. J. Sports Med. 2023, 44, 820–829. [Google Scholar] [CrossRef]
- Song, J.; Heo, S.Y.K. Effects of short-term whole-body electrical stimulation training on metabolic syndrome risk factors and fitness in obese female college students J. Coach. Dev. 2020, 22, 140–148. [Google Scholar] [CrossRef]
- Stephan, H.; Wehmeier, U.F.; Forster, T.; Tomschi, F.; Hilberg, T. Additional Active Movements Are Not Required for Strength Gains in the Untrained during Short-Term Whole-Body Electromyostimulation Training. Healthcare 2023, 11, 741. [Google Scholar] [CrossRef]
- Struhár, I.; Vaculíková, P.; Gimunová, M.; Minster, D.; Körnerová, V. Effects of whole-body electrostimulation and acroyoga based exercise programme on blood pressure in a group of young women. J. Phys. Educ. Sport 2019, 19, 49–57. [Google Scholar]
- Suzuki, T.; Ikeda, M.; Minami, M.; Matayoshi, Y.; Nakao, M.; Nakamura, T.; Abo, M. Beneficial Effect of Intradialytic Electrical Muscle Stimulation in Hemodialysis Patients: A Randomized Controlled Trial. Artif. Organs 2018, 42, 899–910. [Google Scholar] [CrossRef]
- Suzuki, Y.; Suzuki, H.; Yato, S.; Iwasaki, H.; Eguchi, K.; Haneda, K.; Shimano, H. Mittelfristige Auswirkungen der Elektrotherapie der Skeletmuskulatur mit Gürtelelektroden auf den Glukose und Fettstoffwechsel die Körperzusammensetzung, die Muskelkraft und die Muskelausdauer bei Patienten mit Typ-2 Diabetes. Phys. Ther. Sci. 2019, 33, 53–59. [Google Scholar]
- Tanaka, S.; Kamiya, K.; Matsue, Y.; Yonezawa, R.; Saito, H.; Hamazaki, N.; Matsuzawa, R.; Nozaki, K.; Yamashita, M.; Wakaume, K.; et al. Effects of electrical muscle stimulation on physical function in frail older patients with acute heart failure: A randomized controlled trial. Eur. J. Prev. Cardiol. 2022, 29, e286–e288. [Google Scholar] [CrossRef] [PubMed]
- Teschler, M.; Heimer, M.; Schmitz, B.; Kemmler, W.; Mooren, F.C. Four weeks of electromyostimulation improves muscle function and strength in sarcopenic patients: A three-arm parallel randomized trial. J. Cachexia Sarcopenia Muscle 2021, 12, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Teschler, M.; Weissenfels, A.; Bebenek, M.; Frohlich, M.; Kohl, M.; von Stengel, S.; Kemmler, W. Very high creatine kinase CK levels after WB_EMS. Are there implications for health. Int. J. Clin. Exp. Med. 2016, 9, 22841–22850. [Google Scholar]
- Tsurumi, T.; Tamura, Y.; Nakatani, Y.; Furuya, T.; Tamiya, H.; Terashima, M.; Tomoe, T.; Ueno, A.; Shimoyama, M.; Yasu, T. Neuromuscular Electrical Stimulation during Hemodialysis Suppresses Postprandial Hyperglycemia in Patients with End-Stage Diabetic Kidney Disease: A Crossover Controlled Trial. J. Clin. Med. 2022, 11, 6239. [Google Scholar] [CrossRef]
- Vaculikova, P.P.; Kotkova, M.A.; Struhar, I.; Balousova, D.N. Impact of Whole-Body Electromyostimulation and Resistance Training on Bone Mineral Density in women at risk for Osteopororosis. IJPESS 2022, 16, 69–79. [Google Scholar] [CrossRef]
- Vaculikova, P.P.; Kotkova, M.A.; Struhar, I.; Balousova, D.; Rozsypal, R. Impact of Whole-Body Electromyostimulation and Resistance Training on the Level of Functional Fitness in Elderly Women. Stud. Sport. 2023, 16, 115–126. [Google Scholar] [CrossRef]
- van Buuren, F.; Horstkotte, D.; Mellwig, K.; Fruend, A.; Bogunovic, N.; Dimitriadis, Z.; Vortherms, J.; Humphrey, R.; Niebauer, J. Electrical Myostimulation (EMS) Improves Glucose Metabolism and Oxygen Uptake in Type 2 Diabetes Mellitus Patients—Results from the EMS Study. Diabetes Technol. Ther. 2015, 17, 413–419. [Google Scholar] [CrossRef] [PubMed]
- van Buuren, F.; Mellwig, K.P.; Prinz, C.; Korber, B.; Frund, A.; Fritzsche, D.; Faber, L.; Kottmann, T.; Bogunovic, N.; Dahm, J.; et al. Electrical myostimulation improves left ventricular function and peak oxygen consumption in patients with chronic heart failure: Results from the exEMS study comparing different stimulation strategies. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2013, 102, 523–534. [Google Scholar] [CrossRef]
- von Stengel, S.; Bebenek, M.; Engelke, K.; Kemmler, W. Whole-Body Electromyostimulation to Fight Osteopenia in Elderly Females: The Randomized Controlled Training and Electrostimulation Trial (TEST-III). J. Osteoporos. 2015, 2015, 643520. [Google Scholar] [CrossRef]
- Weissenfels, A.; Teschler, M.; Willert, S.; Hettchen, M.; Frohlich, M.; Kleinoder, H.; Kohl, M.; von Stengel, S.; Kemmler, W. Effects of whole-body electromyostimulation on chronic nonspecific low back pain in adults: A randomized controlled study. J. Pain Res. 2018, 11, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Willert, S.; Weissenfels, A.; Kohl, M.; von Stengel, S.; Fröhlich, M.; Kleinöder, H.; Schöne, D.; Teschler, M.; Kemmler, W. Effects of Whole-Body Electromyostimulation (WB-EMS) on the energy-restriction-induced reduction of muscle mass during intended weight loss. Front. Physiol. 2019, 10, 1012. [Google Scholar] [CrossRef]
- Zink-Rückel, C.; Kohl, M.; von Stengel, S.; Kemmler, W. Once weekly whole-body electromyostimulation increase strength, stability and body composition in amateur golfers. A randomized controlled study. Int. J. Environ. Res. Public Health 2021, 18, 5628. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Asano, T.; Hamada, R.; Sato, S.; Miyasaka, J.; Murao, M.; Matsushita, M.; Kajimoto, T.; Otagaki, A.; Nankaku, M.; Kondo, T. Effects of early post-transplant belt electrode skeletal muscle electrical stimulation therapy on an allogeneic hematopoietic stem cell transplant recipient: A case study. Jpn. J. Tranplant 2022, 11, 206–210. [Google Scholar] [CrossRef]
- Michibata, A.; Haraguchi, M.; Murakawa, Y.; Ishikawa, H. Electrical stimulation and virtual reality-guided balance training for managing paraplegia and trunk dysfunction due to spinal cord infarction. BMJ Case Rep. 2022, 15, e244091. [Google Scholar] [CrossRef] [PubMed]
- Recker, R.R. Early postmenopausal bone loss and what to do about it. Ann. N. Y. Acad. Sci. 2011, 1240, E26–E30. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Wagner, H.; Lühmann, D.; Muche-Borowski, C.; Schäfer, I.; Dubben, H.; Hansen, H.; Thiesemann, R.; von Renteln-Kruse, W.; Hofmann, W. Multimorbidity S3 Guideline AWMF Register No. 053-047 DEGAM Guideline No. 20. Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin. 2017. Available online: www.degam-leitlinien.de (accessed on 11 October 2023).
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Shojaa, M.; Steele, J.; Berger, J.; Fröhlich, M.; Schoene, D.; von Stengel, S.; Kleinöder, H.M. Efficacy of Whole-Body Electromyostimulation (WB-EMS) on body composition and muscle strength in non-athletic adults. A systematic review and meta-analysis. Front. Physiol. 2021, 12, 640657. [Google Scholar] [CrossRef]
- Berger, J.; Fröhlich, M.; Kemmler, W. WB-EMS Market Development—Perspectives and Threats. Int. J. Environ. Res. Public Health 2022, 19, 14211. [Google Scholar] [CrossRef]
- BMU. Regulation on Protection against Harmful Effects of Non-Ionizing Radiation in Human Applications (NiSV) [Verordnung zum Schutz vor schädlichen Wirkungen nichtionisierender Strahlung bei der Anwendung am Menschen (NiSV)]; Bundesministerium-für-Umwelt-Naturschutz-und-nukleare-Sicherheit: Bonn, Germany, 2019. [Google Scholar]
- BMU. Requirements for the Acquisition of Expertise for Applications of non-Ionizing Radiation Sources on Humans [Anforderungen an den Erwerb der Fachkunde für Anwendungen nichtionisierender Strahlungsquellen am Menschen]; Bundesministerium-für-Umwelt-Naturschutz-und-nukleare-Sicherheit: Bonn, Germany, 2020. [Google Scholar]
- Galicia Ernst, I.; Torbahn, G.; Schwingshackl, L.; Knuttel, H.; Kob, R.; Kemmler, W.; Sieber, C.C.; Batsis, J.A.; Villareal, D.T.; Stroebele-Benschop, N.; et al. Outcomes addressed in randomized controlled lifestyle intervention trials in community-dwelling older people with (sarcopenic) obesity-An evidence map. Obes. Rev. 2022, 23, e13497. [Google Scholar] [CrossRef]
- Gotz, M.; Heiss, R.; von Stengel, S.; Roemer, F.; Berger, J.; Nagel, A.; Uder, M.; Kemmler, W. Spatial Distribution of Muscular Effects of Acute Whole-Body Electromyostimulation at the Mid-Thigh and Lower Leg-A Pilot Study Applying Magnetic Resonance Imaging. Sensors 2022, 22, 10017. [Google Scholar] [CrossRef]
- Numata, H.; Nakase, J.; Inaki, A.; Mochizuki, T.; Oshima, T.; Takata, Y.; Kinuya, S.; Tsuchiya, H. Effects of the belt electrode skeletal muscle electrical stimulation system on lower extremity skeletal muscle activity: Evaluation using positron emission tomography. J. Orthop. Sci. 2016, 21, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Stollberger, C.; Finsterer, J. Side effects of and contraindications for whole-body electro-myo-stimulation: A viewpoint. BMJ Open Sport Exerc. Med. 2019, 5, e000619. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Fröhlich, M.; Ludwig, O.; Eifler, C.; von Stengel, S.; Willert, S.; Teschler, M.; Weissenfels, A.; Kleinoder, H.; Micke, F.; et al. Position statement and updated international guideline for safe and effective whole-body electromyostimulation training-the need for common sense in WB-EMS application. Front. Physiol. 2023, 14, 1174103. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).