Real World Interstitial Glucose Profiles of a Large Cohort of Physically Active Men and Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Handling and Statistical Analyses
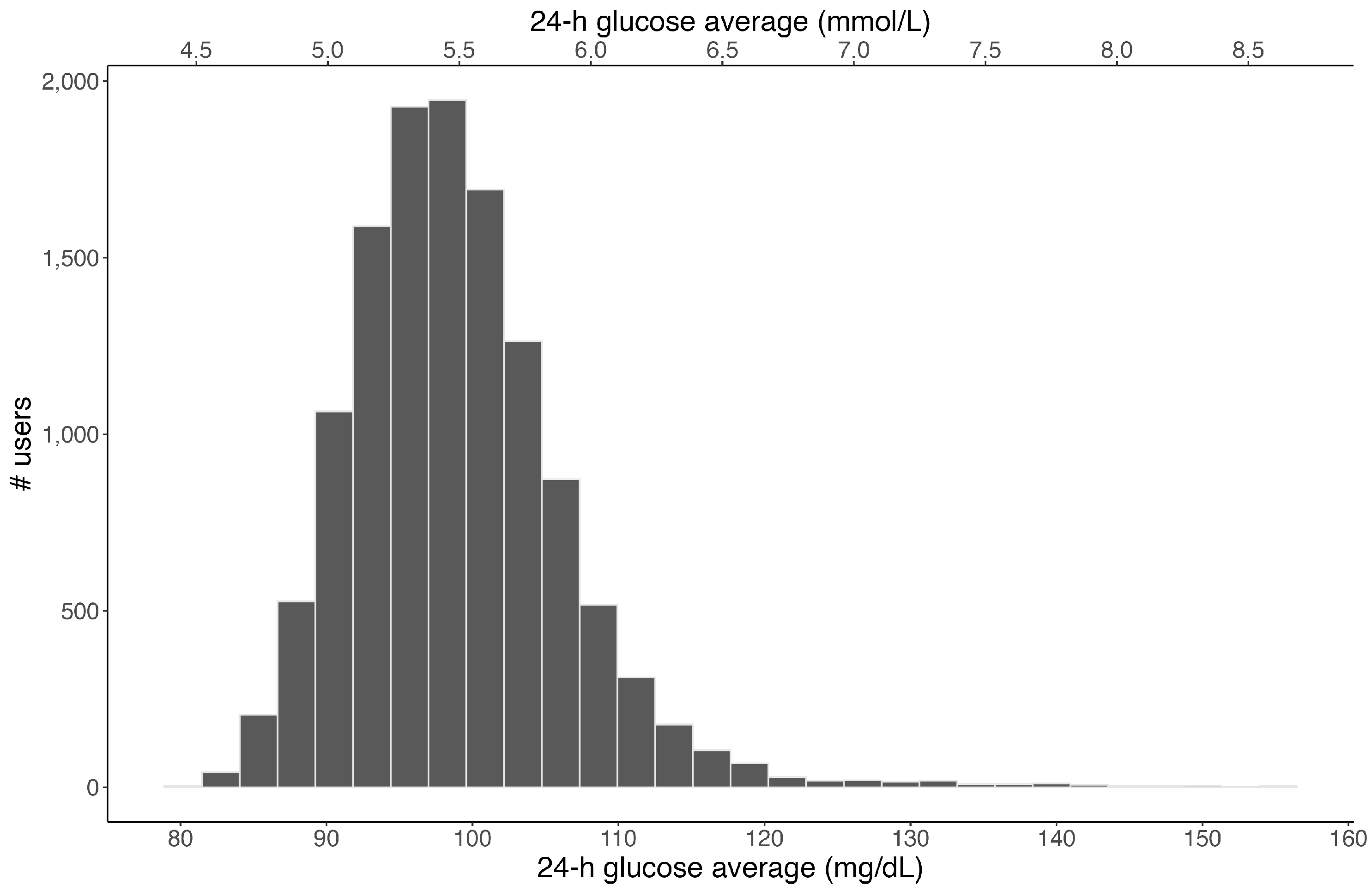
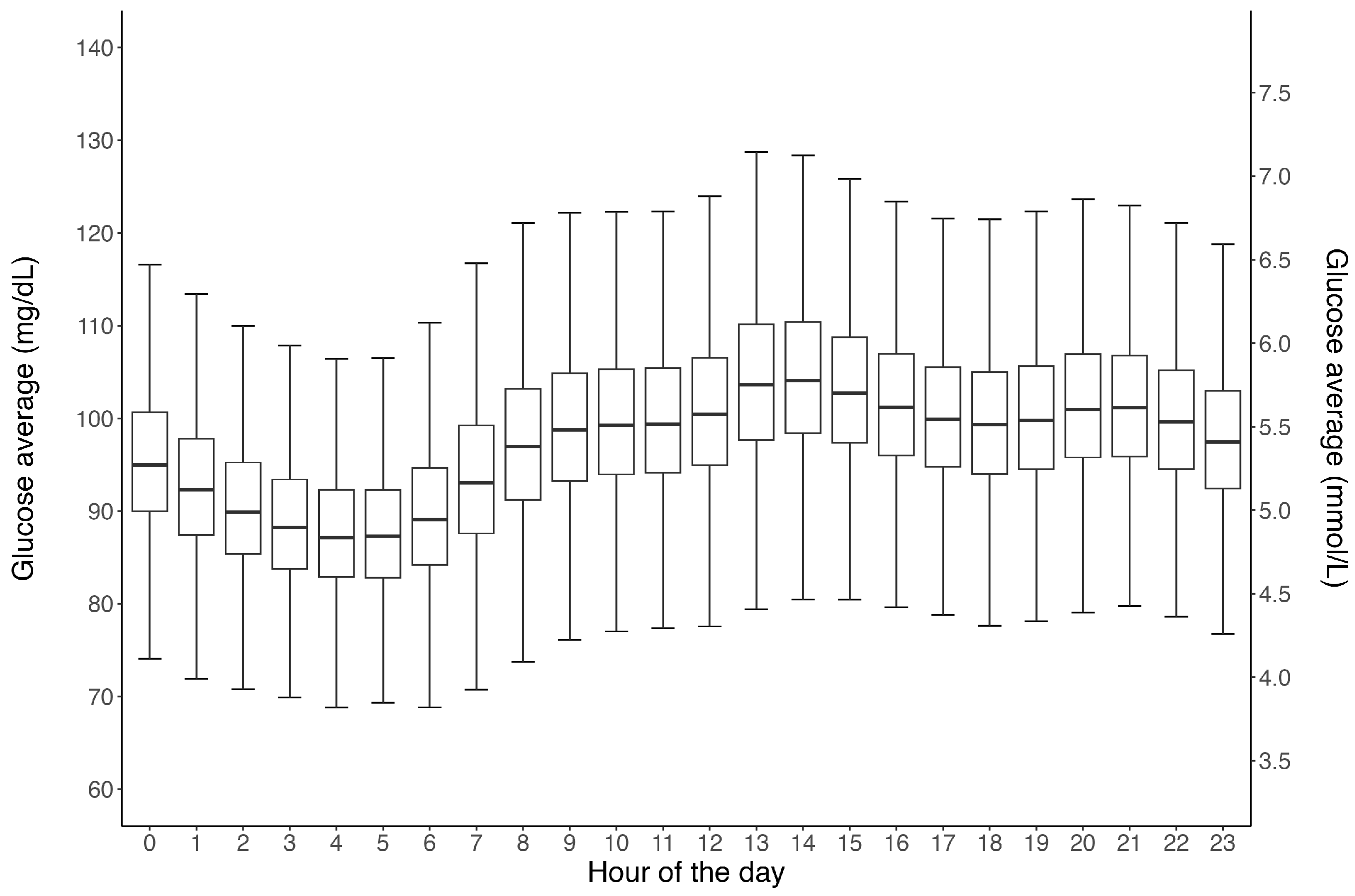
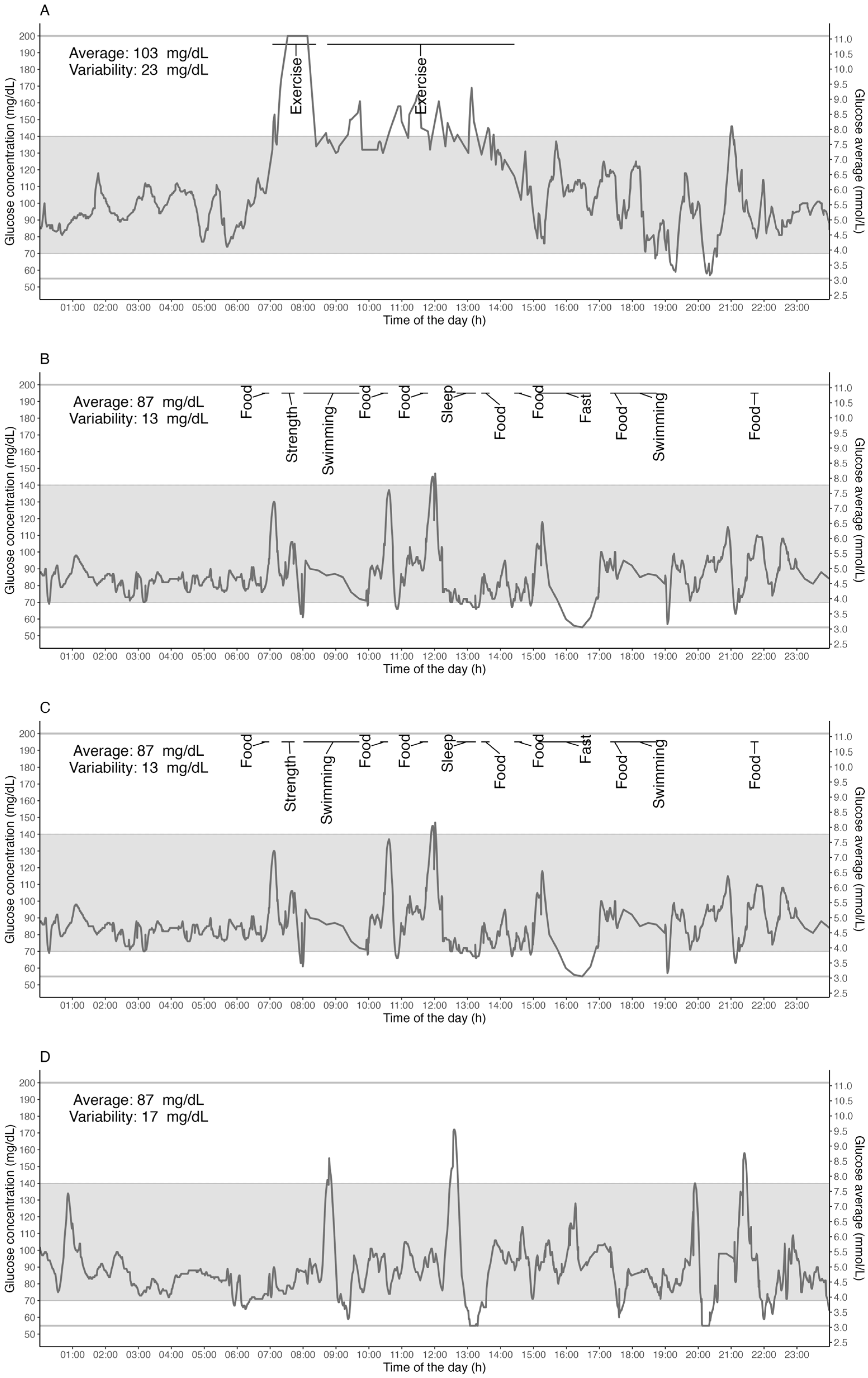
3. Results
3.1. Time below Range; TBR (<70 mg/dL)
3.2. Time above Range: TAR (>140 mg/dL)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brooks, G.A. The Precious Few Grams of Glucose During Exercise. Int. J. Mol. Sci. 2020, 21, 5733. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, D.H. Four Grams of Glucose. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E11–E21. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.A.; Gordon, B.; Derick, C.L. Some Changes in the Chemical Constituents of the Blood Following a Marathon Race. J. Am. Med. Assoc. 1924, 82, 1778. [Google Scholar] [CrossRef]
- Coyle, E.F.; Coggan, A.R. Effectiveness of Carbohydrate Feeding in Delaying Fatigue during Prolonged Exercise. Sports Med. 1984, 1, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International Society of Sports Nutrition Position Stand: Nutrient Timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef]
- Heikura, I.A.; Stellingwerff, T.; Mero, A.A.; Uusitalo, A.L.T.; Burke, L.M. A Mismatch Between Athlete Practice and Current Sports Nutrition Guidelines Among Elite Female and Male Middle- and Long-Distance Athletes. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 351–360. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Ahn, D.; Drincic, A. Continuous Glucose Monitoring: A Review of the Technology and Clinical Use. Diabetes Res. Clin. Pract. 2017, 133, 178–192. [Google Scholar] [CrossRef]
- Moser, O.; Riddell, M.C.; Eckstein, M.L.; Adolfsson, P.; Rabasa-Lhoret, R.; van den Boom, L.; Gillard, P.; Nørgaard, K.; Oliver, N.S.; Zaharieva, D.P.; et al. Glucose Management for Exercise Using Continuous Glucose Monitoring (CGM) and Intermittently Scanned CGM (IsCGM) Systems in Type 1 Diabetes: Position Statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) Endorsed by JDRF and Supported by the American Diabetes Association (ADA). Diabetologia 2020, 63, 2501–2520. [Google Scholar] [CrossRef]
- Ishihara, K.; Uchiyama, N.; Kizaki, S.; Mori, E.; Nonaka, T.; Oneda, H. Application of Continuous Glucose Monitoring for Assessment of Individual Carbohydrate Requirement during Ultramarathon Race. Nutrients 2020, 12, 1121. [Google Scholar] [CrossRef]
- Burke, L.M. Nutritional Approaches to Counter Performance Constraints in High-level Sports Competition. Exp. Physiol. 2021, 106, 2304–2323. [Google Scholar] [CrossRef]
- Kovatchev, B.P.; Patek, S.D.; Ortiz, E.A.; Breton, M.D. Assessing Sensor Accuracy for Non-Adjunct Use of Continuous Glucose Monitoring. Diabetes Technol. Ther. 2015, 17, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Alexander, C.M.; Amiel, S.A.; Arreaza-Rubin, G.; Beck, R.W.; Bergenstal, R.M.; Buckingham, B.A.; Carroll, J.; Ceriello, A.; Chow, E.; et al. Continuous Glucose Monitoring and Metrics for Clinical Trials: An International Consensus Statement. Lancet Diabetes Endocrinol. 2023, 11, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.M.; Markey, J.C.; Ebert-May, D. The Other Half of the Story: Effect Size Analysis in Quantitative Research. CBE—Life Sci. Educ. 2013, 12, 345–351. [Google Scholar] [CrossRef] [PubMed]
- DuBose, S.N.; Li, Z.; Sherr, J.L.; Beck, R.W.; Tamborlane, W.V.; Shah, V.N. Effect of Exercise and Meals on Continuous Glucose Monitor Data in Healthy Individuals Without Diabetes. J. Diabetes Sci. Technol. 2021, 15, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Zahedani, A.D.; Veluvali, A.; McLaughlin, T.; Aghaeepour, N.; Hosseinian, A.; Agarwal, S.; Ruan, J.; Tripathi, S.; Woodward, M.; Hashemi, N.; et al. Digital Health Application Integrating Wearable Data and Behavioral Patterns Improves Metabolic Health. NPJ Digit. Med. 2023, 6, 216. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Perelman, D.; Breschi, A.; Limcaoco, P.; Kellogg, R.; McLaughlin, T.; Snyder, M. Glucotypes Reveal New Patterns of Glucose Dysregulation. PLoS Biol. 2018, 16, e2005143. [Google Scholar] [CrossRef]
- Matabuena, M.; Pazos-Couselo, M.; Alonso-Sampedro, M.; Fernández-Merino, C.; González-Quintela, A.; Gude, F. Reproducibility of Continuous Glucose Monitoring Results under Real-Life Conditions in an Adult Population: A Functional Data Analysis. Sci. Rep. 2023, 13, 13987. [Google Scholar] [CrossRef]
- D’Souza, N.C.; Kesibi, D.; Yeung, C.; Shakeri, D.; D’Souza, A.I.; Macpherson, A.K.; Riddell, M.C. The Impact of Sex, Body Mass Index, Age, Exercise Type and Exercise Duration on Interstitial Glucose Levels during Exercise. Sensors 2023, 23, 9059. [Google Scholar] [CrossRef]
- Sprague, J.E.; Arbeláez, A.M. Glucose Counterregulatory Responses to Hypoglycemia. Pediatr. Endocrinol. Rev. 2011, 9, 463–473; quiz 474–475. [Google Scholar]
- Costill, D.L.; Coyle, E.; Dalsky, G.; Evans, W.; Fink, W.; Hoopes, D. Effects of Elevated Plasma FFA and Insulin on Muscle Glycogen Usage during Exercise. J. Appl. Physiol. 1977, 43, 695–699. [Google Scholar] [CrossRef]
- Brouns, F.; Rehrer, N.; Saris, W.; Beckers, E.; Menheere, P.; Hoor, F. Effect of Carbohydrate Intake During Warming-up on the Regulation of Blood Glucose During Exercise. Int. J. Sports Med. 1989, 10, S68–S75. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.L.P.G.; Jeukendrup, A.E. Prevalence of Hypoglycemia Following Pre-Exercise Carbohydrate Ingestion Is Not Accompanied by Higher Insulin Sensitivity. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Jeukendrup, A.E. Effects of Pre-Exercise Ingestion of Carbohydrate on Glycaemic and Insulinaemic Responses during Subsequent Exercise at Differing Intensities. Eur. J. Appl. Physiol. 2003, 88, 466–471. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Killer, S.C. The Myths Surrounding Pre-Exercise Carbohydrate Feeding. Ann. Nutr. Metab. 2010, 57, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Zignoli, A.; Fontana, F.Y.; Lipman, D.J.; Skroce, K.; Maturana, F.M.; Zisser, H.C. Association between Pre-Exercise Food Ingestion Timing and Reactive Hypoglycemia: Insights from a Large Database of Continuous Glucose Monitoring Data. Eur. J. Sport Sci. 2023, 23, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.N.; DuBose, S.N.; Li, Z.; Beck, R.W.; Peters, A.L.; Weinstock, R.S.; Kruger, D.; Tansey, M.; Sparling, D.; Woerner, S.; et al. Continuous Glucose Monitoring Profiles in Healthy Nondiabetic Participants: A Multicenter Prospective Study. J. Clin. Endocrinol. Metab. 2019, 104, 4356–4364. [Google Scholar] [CrossRef]
- Cox, G.R.; Snow, R.J.; Burke, L.M. Race-Day Carbohydrate Intakes of Elite Triathletes Contesting Olympic-Distance Triathlon Events. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 299–306. [Google Scholar] [CrossRef][Green Version]
- Frentsos, J.A.; Baer, J.T. Increased Energy and Nutrient Intake during Training and Competition Improves Elite Triathletes’ Endurance Performance. Int. J. Sport Nutr. 1997, 7, 61–71. [Google Scholar] [CrossRef]
- Reinhard, C.; Galloway, S.D.R. Carbohydrate Intake Practices and Determinants of Food Choices During Training in Recreational, Amateur, and Professional Endurance Athletes: A Survey Analysis. Front. Nutr. 2022, 9, 862396. [Google Scholar] [CrossRef]
- Adams, P. The Impact of Brief High-Intensity Exercise on Blood Glucose Levels. Diabetes Metab. Syndr. Obes. 2013, 2013, 113–122. [Google Scholar] [CrossRef]
- Gordon, B.A.; Taylor, C.J.; Church, J.E.; Cousins, S.D. A Comparison of the Gluco-Regulatory Responses to High-Intensity Interval Exercise and Resistance Exercise. Int. J. Environ. Res. Public Health 2021, 18, 287. [Google Scholar] [CrossRef]
- Chakarova, N.; Dimova, R.; Grozeva, G.; Tankova, T. Assessment of Glucose Variability in Subjects with Prediabetes. Diabetes Res. Clin. Pract. 2019, 151, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Bolli, G.B.; De Feo, P.; De Cosmo, S.; Perriello, G.; Ventura, M.M.; Calcinaro, F.; Lolli, C.; Campbell, P.; Brunetti, P.; Gerich, J.E. Demonstration of a Dawn Phenomenon in Normal Human Volunteers. Diabetes 1984, 33, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Arslanian, S.; Ohki, Y.; Becker, D.J.; Drash, A.L. Demonstration of a Dawn Phenomenon in Normal Adolescents. Horm. Res. 1990, 34, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.I.; Lin, Q.X.; Gwynne, J.T.; Jacobs, S. Fasting Early Morning Rise in Peripheral Insulin: Evidence of the Dawn Phenomenon in Nondiabetes. Diabetes Care 1984, 7, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Ruiz, J.; Urbain, J.L.; Chen, X. Evidence for a Circadian Rhythm of Insulin Secretion. Am. J. Physiol.-Endocrinol. Metab. 1996, 271, E246–E252. [Google Scholar] [CrossRef]
- Van Cauter, E.; Polonsky, K.S.; Scheen, A.J. Roles of Circadian Rhythmicity and Sleep in Human Glucose Regulation. Endocr. Rev. 1997, 18, 716–738. [Google Scholar] [CrossRef]
- Phillips, N.E.; Collet, T.-H.; Naef, F. Uncovering Personalized Glucose Responses and Circadian Rhythms from Multiple Wearable Biosensors with Bayesian Dynamical Modeling. Cell Rep. Methods 2023, 3, 100545. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Nutrition for Endurance Sports: Marathon, Triathlon, and Road Cycling. J. Sports Sci. 2011, 29, S91–S99. [Google Scholar] [CrossRef]
- Zhou, J.; Li, H.; Ran, X.; Yang, W.; Li, Q.; Peng, Y.; Li, Y.; Gao, X.; Luan, X.; Wang, W.; et al. Reference Values for Continuous Glucose Monitoring in Chinese Subjects. Diabetes Care 2009, 32, 1188–1193. [Google Scholar] [CrossRef]
- Nuutila, P.; Knuuti, M.J.; Mäki, M.; Laine, H.; Ruotsalainen, U.; Teräs, M.; Haaparanta, M.; Solin, O.; Yki-Järvinen, H. Gender and Insulin Sensitivity in the Heart and in Skeletal Muscles: Studies Using Positron Emission Tomography. Diabetes 1995, 44, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.-M.; Kiens, B. Gender Differences in Skeletal Muscle Substrate Metabolism—Molecular Mechanisms and Insulin Sensitivity. Front. Endocrinol. 2014, 5, 195. [Google Scholar] [CrossRef] [PubMed]
- Landen, S.; Hiam, D.; Voisin, S.; Jacques, M.; Lamon, S.; Eynon, N. Physiological and Molecular Sex Differences in Human Skeletal Muscle in Response to Exercise Training. J. Physiol. 2023, 601, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Dasgupta, D.; Roy, S. Blood Glucose Levels at Two Different Phases of Menstrual Cycle: A Study on a Group of Bengali-Speaking Hindu Ethnic Populations of West Bengal, India. Orient. Anthropol. Bi-Annu. Int. J. Sci. Man 2019, 19, 55–63. [Google Scholar] [CrossRef]


| Gender | |
| Female | 23% (n = 2872) |
| Male | 77% (n = 9632) |
| Age | |
| <20 years | 2% (n = 210) |
| 20–40 years | 54% (n = 6704) |
| 40–60 years | 41% (n = 5179) |
| >60 years | 3% (n = 411) |
| BMI | |
| Underweight (<18.5) | 2% (n = 299) |
| Normal (18.5–25) | 65% (n = 8135) |
| Overweight (25–30) | 22% (n = 2720) |
| Obesity (>30) | 11% (n = 1350) |
| Fitness level | |
| Not Reported | 9% (n = 1118) |
| Beginner (1–2 workouts/week) | 15% (n = 1819) |
| Intermediate (3–4 workouts/week) | 33% (n = 4147) |
| Advanced (4–5 workouts/week) | 31% (n = 3914) |
| Expert (>5 workouts/week) | 12% (n = 1506) |
| Primary sport | |
| Other | 10% (n = 1272) |
| Not Reported | 5% (n = 588) |
| Triathlon | 24% (n = 3033) |
| Cycling | 27% (n = 3342) |
| Running | 19% (n = 2339) |
| Swimming | 1% (n = 146) |
| Crossfit | 4% (n = 516) |
| Obstacle Course Racing | 1% (n = 66) |
| Gym | 9% (n = 1097) |
| Football (Soccer) | 1% (n = 105) |
| Meals | Exercise | Sleep | 24 h | |
|---|---|---|---|---|
| Age | ||||
| <20 years | 106 ± 12 (94–120) | 112 ± 13 (98–128) | 92 ± 10 (82–104) | 99 ± 8 (90–108) |
| 20–40 years | 106 ± 10 (93–119) | 107 ± 13 (92–123) | 91 ± 10 (79–102) | 98 ± 7 (90–106) |
| 40–60 years | 108 ± 12 (95–121) | 106 ± 13 (92–122) | 93 ± 11 (81–105) | 100 ± 8 (91–109) |
| >60 years | 113 ± 14 (98–132) | 108 ± 15 (90–125) | 96 ± 13 (85–109) | 104 ± 12 (93–118) |
| BMI | ||||
| Underweight (<18.5) | 106 ± 10 (95–121) | 106 ± 12 (92–120) | 91 ± 10 (80–104) | 98 ± 7 (89–107) |
| Normal (18.5–25) | 107 ± 11 (95–120) | 107 ± 13 (93–123) | 91 ± 10 (80–104) | 99 ± 7 (91–107) |
| Overweight (25–30) | 106 ± 11 (93–120) | 105 ± 13 (90–121) | 92 ± 11 (81–105) | 99 ± 8 (90–108) |
| Obesity (>30) | 106 ± 13 (92–121) | 104 ± 14 (89–122) | 91 ± 13 (78–103) | 99 ± 9 (90–109) |
| Fitness level | ||||
| Beginner | 106 ± 13 (92–120) | 102 ± 14 (87–118) | 91 ± 12 (79–106) | 99 ± 9 (89–108) |
| Intermediate | 106 ± 11 (94–120) | 105 ± 13 (91–121) | 91 ± 11 (79–103) | 98 ± 7 (90–107) |
| Advanced | 108 ± 10 (95–120) | 107 ± 12 (94–122) | 92 ± 10 (82–104) | 99 ± 7 (91–108) |
| Expert | 108 ± 10 (96–121) | 112 ± 12 (98–128) | 93 ± 10 (82–104) | 100 ± 7 (92–109) |
| Not Reported | 107 ± 12 (93–121) | 107 ± 15 (90–125) | 92 ± 12 (80–105) | 99 ± 9 (90–110) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skroce, K.; Zignoli, A.; Fontana, F.Y.; Maturana, F.M.; Lipman, D.; Tryfonos, A.; Riddell, M.C.; Zisser, H.C. Real World Interstitial Glucose Profiles of a Large Cohort of Physically Active Men and Women. Sensors 2024, 24, 744. https://doi.org/10.3390/s24030744
Skroce K, Zignoli A, Fontana FY, Maturana FM, Lipman D, Tryfonos A, Riddell MC, Zisser HC. Real World Interstitial Glucose Profiles of a Large Cohort of Physically Active Men and Women. Sensors. 2024; 24(3):744. https://doi.org/10.3390/s24030744
Chicago/Turabian StyleSkroce, Kristina, Andrea Zignoli, Federico Y. Fontana, Felipe M. Maturana, David Lipman, Andrea Tryfonos, Michael C. Riddell, and Howard C. Zisser. 2024. "Real World Interstitial Glucose Profiles of a Large Cohort of Physically Active Men and Women" Sensors 24, no. 3: 744. https://doi.org/10.3390/s24030744
APA StyleSkroce, K., Zignoli, A., Fontana, F. Y., Maturana, F. M., Lipman, D., Tryfonos, A., Riddell, M. C., & Zisser, H. C. (2024). Real World Interstitial Glucose Profiles of a Large Cohort of Physically Active Men and Women. Sensors, 24(3), 744. https://doi.org/10.3390/s24030744







