The Presence/Absence of an Awake-State Dominant EEG Rhythm in Delirious Patients Is Related to Different Symptoms of Delirium Evaluated by the Intensive Care Delirium Screening Checklist (ICDSC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. EEG Measurements
2.3. Statistical Analysis
3. Results
3.1. EEG Patterns Regarding the Dominant Rhythms
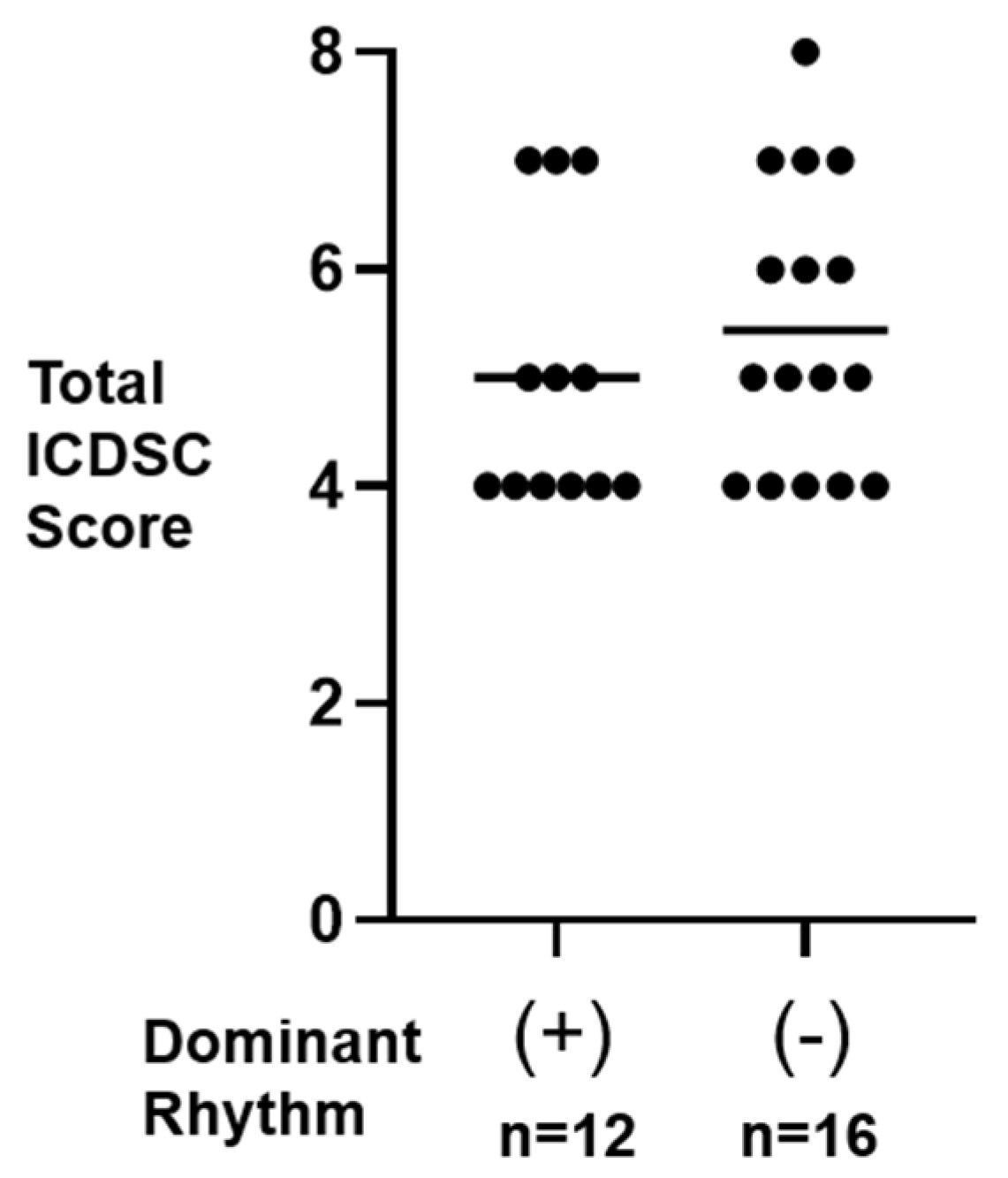
3.2. Total ICDSC Score in Dominant (+) and Dominant (−) Participants
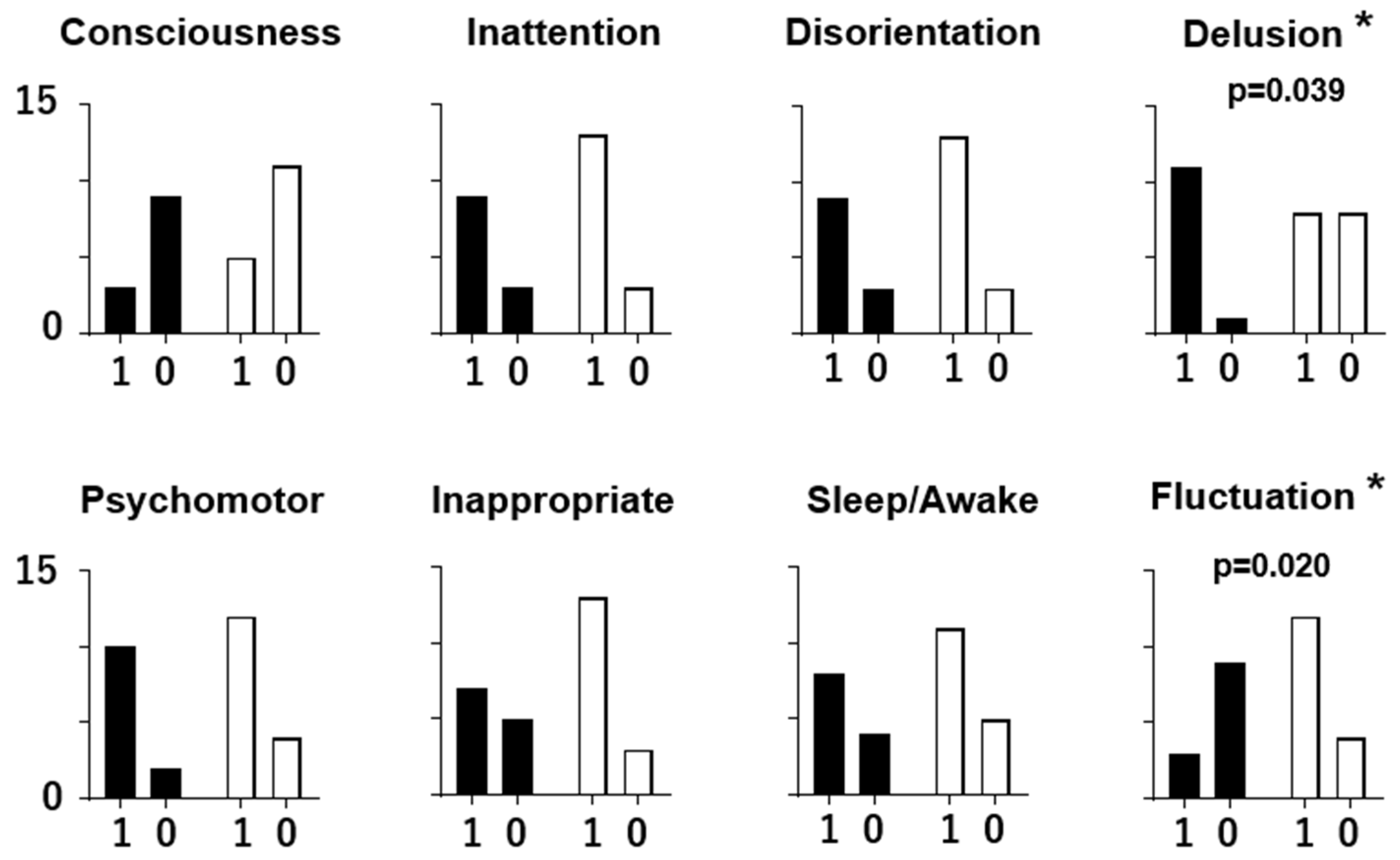
3.3. Individual ICDSC Scores and EEG Patterns
4. Discussion
4.1. EEG Characteristics in Delirious Participants
4.2. Two Types of Delirium with Distinct Symptoms and EEG Patterns
4.3. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moraga, A.V.; Rodriguez-Pascual, C. Accurate Diagnosis of Delirium in Elderly Patients. Curr. Opin. Psychiatry 2007, 20, 262–267. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Inouye, S.K.; van Dyck, C.H.; Alessi, C.A.; Balkin, S.; Siegal, A.P.; Horwitz, R.I. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann. Intern. Med. 1990, 113, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Sessler, C.N.; Gosnell, M.S.; Grap, M.J.; Brophy, G.M.; O’Neal, P.V.; Keane, K.A.; Tesoro, E.P.; Elswick, R.K. The Richmond Agitation Sedation Scale: Validity and reliability in adult intensive care unit patients. Am. J. Respir. Crit. Care. Med. 2002, 166, 1338–1344. [Google Scholar] [CrossRef]
- Bergeron, N.; Dubois, M.J.; Dumont, M.; Dial, S.; Skrobik, Y. Intensive Care Delirium Screening Checklist: Evaluation of a new screening tool. Intensive Care Med. 2001, 27, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Ormseth, C.H.; LaHue, S.C.; Oldham, M.A.; Josephson, S.A.; Whitaker, E.; Douglas, V.C. Predisposing and Precipitating Factors Associated with Delirium: A Systematic Review. JAMA Netw. Open 2023, 6, e2249950. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, S.T.; Lavan, J.N. Clinical significance of delirium subtypes in older people. Age Ageing 1999, 28, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Boettger, S.; Breitbart, W. Phenomenology of the subtypes of delirium: Phenomenological differences between hyperactive and hypoactive delirium. Palliat. Support. Care 2011, 9, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Hayhurst, C.J.; Marra, A.; Han, J.H.; Patel, M.B.; Brummel, N.E.; Thompson, J.L.; Jackson, J.C.; Chandrasekhar, R.; Ely, E.W.; Pandharipande, P.P.; et al. Association of Hypoactive and Hyperactive Delirium with Cognitive Function After Critical Illness. Crit. Care Med. 2020, 48, e480–e488. [Google Scholar] [CrossRef] [PubMed]
- Koponen, H.; Partanen, J.; Pääkkönen, A.; Mattila, E.; Riekkinen, P.J. EEG spectral analysis in delirium. J. Neurol. Neurosurg. Psychiatry 1989, 52, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.P. Utility of electroencephalography in delirium: Past view and current practices. Int. Paychogeriatr. 1991, 3, 211–229. [Google Scholar] [CrossRef]
- Jacobson, S.; Jerrier, H. EEG in delirium. Semin. Clin. Neuropsychiatry 2000, 5, 86–92. [Google Scholar] [PubMed]
- Plaschke, K.; Hill, H.; Engelhardt, R.; Thomas, C.; von Haken, R.; Scholz, M.; Kopitz, J.; Bardenheuer, H.J.; Weisbrod, M.; Weigand, M.A. EEG changes and serum anticholinergic activity measured in patients with delirium in the intensive care unit. Anaesthesia 2007, 62, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Hestermann, U.; Walther, S.; Pfueller, U.; Hack, M.; Oster, P.; Mundt, C.; Weisbrod, M. Prolonged activation EEG differentiates dementia with and without delirium in frail elderly patients. J. Neurol. Neurosurg. Psychiatry 2008, 79, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Palanca, B.J.A.; Wildes, T.S.; Ju, Y.S.; Ching, S.; Avidan, M.S. Electroencephalography and delirium in the postoperative period. Br. J. Anaesth. 2017, 119, 294–307. [Google Scholar] [CrossRef] [PubMed]
- van der Kooi, A.W.; Zaal, I.J.; Klijn, F.A.; Koek, H.L.; Meijer, R.C.; Leijten, F.S.; Slooter, A.J. Delirium detection using EEG: What and how to measure. Chest 2015, 147, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, G.; Chan, A.C.; Sparr, N.A.; Zarei, K.; Gaul, L.N.; Heinzman, J.T.; Robles, J.; Yuki, K.; Chronis, T.J.; Ando, T.; et al. Delirium detection by a novel bispectral electroencephalography device in general hospital. Psychiatry Clin. Neurosci. 2018, 72, 856–863. [Google Scholar] [CrossRef]
- Fleischmann, R.; Tränkner, S.; Bathe-Peters, R.; Rönnefarth, M.; Schmidt, S.; Schreiber, S.J.; Brandt, S.A. Quantitative EEG Analyses in Delirium: Confirmatory Results from a Large Retrospective Case-Control Study. Clin. EEG Neurosci. 2018, 1, 1550059418767584. [Google Scholar] [CrossRef]
- Numan, T.; van den Boogaard, M.; Kamper, A.M.; Rood, P.J.T.; Peelen, L.M.; Slooter, A.J.C. Delirium Detection Using Relative Delta Power Based on 1-minute Single-Channel EEG: A Multicentre Study. Br. J. Anaesth. 2019, 122, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.; Crouch, B.; Webster, N.; Platt, B. Delirium Screening in the Intensive Care Unit Using Emerging QEEG Techniques: A Pilot Study. AIMS Neurosci. 2020, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Bong, S.H.; Lee, J.H.; Choi, T.Y.; Yoon, S.Y.; Kim, J.W. The Usefulness of Quantitative Electroencephalography in Diagnosis and Severity Evaluation of Delirium: A Retrospective Study. Psychiatry Investig. 2023, 20, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Nadler, J.W.; Preud’homme, X.A.; Fang, E.; Daughtry, R.L.; Chapman, J.B.; Attarian, D.; Wellman, S.; Krystal, A.D. Pilot prospective study of post-surgery sleep and EEG predictors of post-operative delirium. Clin. Neurophysiol. 2017, 128, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Schüßler, J.; Ostertag, J.; Georgii, M.-T.; Fleischmann, A.; Schneider, G.; Pilge, S.; Kreuzer, M. Preoperative characterization of baseline EEG recordings for risk stratification of post-anesthesia care unit delirium. J. Clin. Anesth. 2023, 86, 111058. [Google Scholar] [CrossRef] [PubMed]
- Barnwell, L.H. A Clinical Trial of a Novel Electroencephalography Device as a Predictor of Postoperative Delirium. AANA J. 2023, 91, 125–129. [Google Scholar]
- Kinoshita, H.; Saito, J.; Kushikata, T.; Oyama, T.; Takekawa, D.; Hashiba, E.; Sawa, T.; Hirota, K. The Perioperative Frontal Relative Ratio of the Alpha Power of Electroencephalography for Predicting Postoperative Delirium After Highly Invasive Surgery: A Prospective Observational Study. Anesth. Analg. 2023, 137, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Reilly, E.L. EEG Recording and Operation of the Apparatus. In Electroencephalography: Basic Principles. Clinical Applications, and Related Fields, 5th ed.; Niedermeyer, E., Lopes Da Sylva, F., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2005; pp. 139–159. [Google Scholar]
- Shinba, T.; Nedachi, N.; Harakawa, S. Alterations in heart rate variability and electroencephalogram during 20-minute extremely low frequency electric field treatment in healthy men during the eyes-open condition. IEEJ Trans. Electr. Electron. Eng. 2023, 18, 38–44. [Google Scholar] [CrossRef]
- Ely, E.W.; Truman, B.; Manzi, D.J.; Sigl, J.C.; Shintani, A.; Bernard, G.R. Consciousness monitoring in ventilated patients: Bispectral EEG monitors arousal not delirium. Intensive Care Med. 2004, 30, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- van Montfort, S.J.T.; van Dellen, E.; Wattel, L.L.; Kant, I.M.J.; Numan, T.; Stam, C.J.; Slooter, A.J.C. Predisposition for Delirium and EEG Characteristics. Clin. Neurophysiol. 2020, 131, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- van Dellen, E.; van der Kooi, A.W.; Numan, T.; Koek, H.L.; Klijn, F.A.; Buijsrogge, M.P.; Stam, C.J.; Slooter, A.J. Decreased functional connectivity and disturbed directionality of information flow in the electroencephalography of intensive care unit patients with delirium after cardiac surgery. Anesthesiology 2014, 121, 328–335. [Google Scholar] [CrossRef]
- Tanabe, S.; Mohanty, R.; Lindroth, H.; Casey, C.; Ballweg, T.; Farahbakhsh, Z.; Krause, B.; Prabhakaran, V.; Banks, M.I.; Sanders, R.D. Cohort Study into the Neural Correlates of Postoperative Delirium: The Role of Connectivity and Slow-Wave Activity. Br. J. Anaesth. 2020, 125, 55–66. [Google Scholar] [CrossRef]
- van Montfort, S.J.T.; van Dellen, E.; Stam, C.J.; Ahmad, A.H.; Mentink, L.J.; Kraan, C.W.; Zalesky, A.; Slooter, A.J.C. Brain Network Disintegration as a Final Common Pathway for Delirium: A Systematic Review and Qualitative Meta-Analysis. Neuroimage Clin. 2019, 23, 101809. [Google Scholar] [CrossRef]
- Urdanibia-Centelles, O.; Nielsen, R.M.; Rostrup, E.; Vedel-Larsen, E.; Thomsen, K.; Nikolic, M.; Johnsen, B.; Møller, K.; Lauritzen, M.; Benedek, K. Automatic continuous EEG signal analysis for diagnosis of delirium in patients with sepsis. Clin. Neurophysiol. 2021, 132, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Miyazaki, T.; Nakajima, S.; Okamoto, S.; Moriyama, S.; Niinomi, K.; Takayama, K.; Kato, J.; Nakamura, I.; Hirano, Y.; et al. The Association between Electroencephalography with Auditory Steady-State Response and Postoperative Delirium. J. Pers. Med. 2022, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Sims, R.; Gottlieb, G. Differential Diagnosis of Dementia, Delirium and Depression. Implications for Drug Therapy. Drugs Aging 1994, 5, 431–445. [Google Scholar] [CrossRef]
- Downing, L.J.; Caprio, T.V.; Lyness, J.M. Geriatric Psychiatry Review: Differential Diagnosis and Treatment of the 3 D’s—Delirium, Dementia, and Depression. Curr. Psychiatry Rep. 2013, 15, 365. [Google Scholar] [CrossRef] [PubMed]

| Eyes | Delta | Theta | Alpha | Beta | |
|---|---|---|---|---|---|
| Dominant (+) | Open | 200.5 ± 399.6 | 50.0 ± 48.2 | 34.7 ± 18.9 a | 50.8 ± 52.1 |
| Closed | 92.3 ± 68.5 | 64.3 ± 63.4 b | 36.7 ± 27.0 b | 39.1 ± 45.6 | |
| Dominant (−) | Open | 134.9 ± 134.6 | 29.9 ± 34.2 | 18.1 ± 20.0 | 20.4 ± 24.4 |
| Closed | 103.5 ± 92.8 | 27.0 ± 33.8 | 16.6 ± 17.9 | 17.1 ± 20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinba, T.; Fujita, Y.; Ogawa, Y.; Shinba, Y.; Shinba, S. The Presence/Absence of an Awake-State Dominant EEG Rhythm in Delirious Patients Is Related to Different Symptoms of Delirium Evaluated by the Intensive Care Delirium Screening Checklist (ICDSC). Sensors 2024, 24, 8097. https://doi.org/10.3390/s24248097
Shinba T, Fujita Y, Ogawa Y, Shinba Y, Shinba S. The Presence/Absence of an Awake-State Dominant EEG Rhythm in Delirious Patients Is Related to Different Symptoms of Delirium Evaluated by the Intensive Care Delirium Screening Checklist (ICDSC). Sensors. 2024; 24(24):8097. https://doi.org/10.3390/s24248097
Chicago/Turabian StyleShinba, Toshikazu, Yusuke Fujita, Yusuke Ogawa, Yujiro Shinba, and Shuntaro Shinba. 2024. "The Presence/Absence of an Awake-State Dominant EEG Rhythm in Delirious Patients Is Related to Different Symptoms of Delirium Evaluated by the Intensive Care Delirium Screening Checklist (ICDSC)" Sensors 24, no. 24: 8097. https://doi.org/10.3390/s24248097
APA StyleShinba, T., Fujita, Y., Ogawa, Y., Shinba, Y., & Shinba, S. (2024). The Presence/Absence of an Awake-State Dominant EEG Rhythm in Delirious Patients Is Related to Different Symptoms of Delirium Evaluated by the Intensive Care Delirium Screening Checklist (ICDSC). Sensors, 24(24), 8097. https://doi.org/10.3390/s24248097








