A Signal-On Microelectrode Electrochemical Aptamer Sensor Based on AuNPs–MXene for Alpha-Fetoprotein Determination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Manufacture of Microelectrode
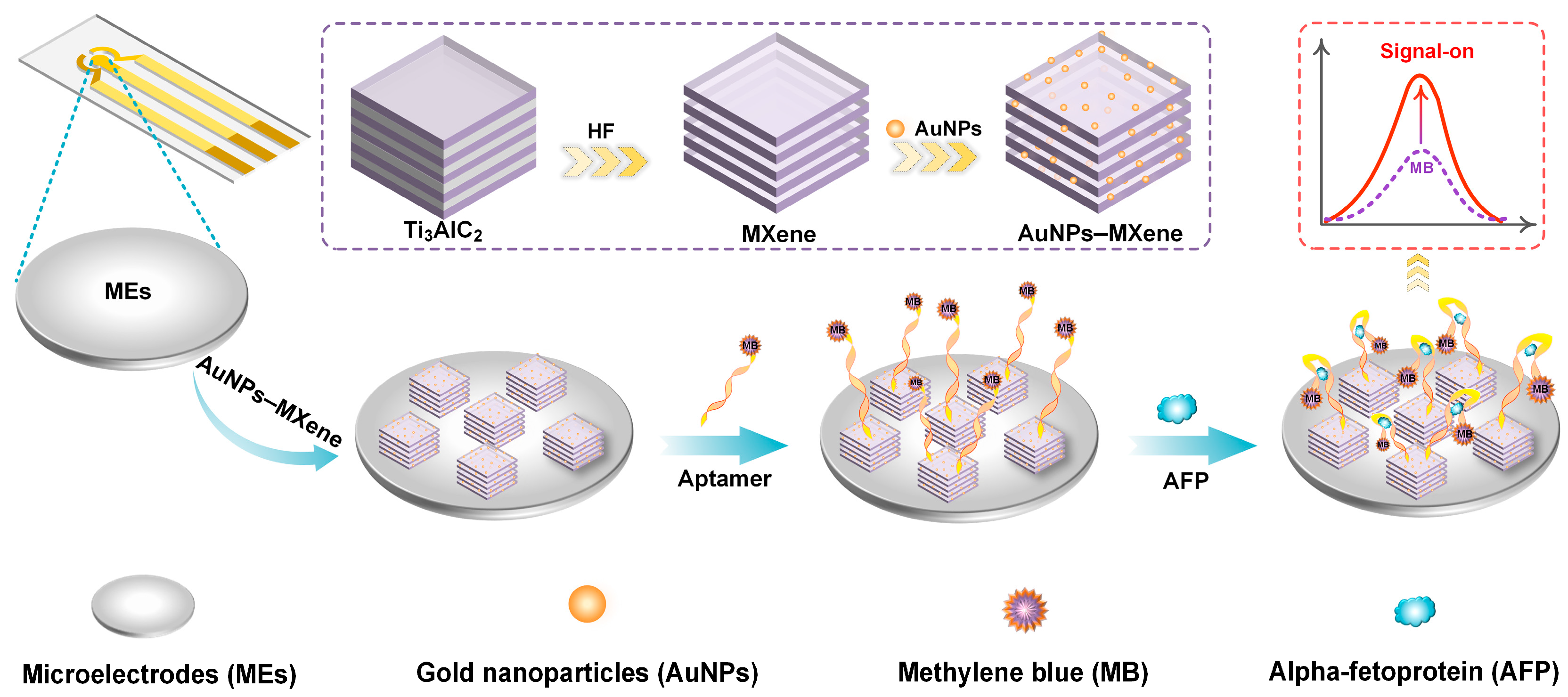
2.3. Preparation of MXene and AuNPs–MXene
2.4. Modification of the Electrode
2.5. Preparation of Serum Samples
3. Results and Discussion
3.1. Characterization of Ti3AlC2, MXene, and AuNPs–MXene
3.2. Electrochemical Behavior During Gradual Modification
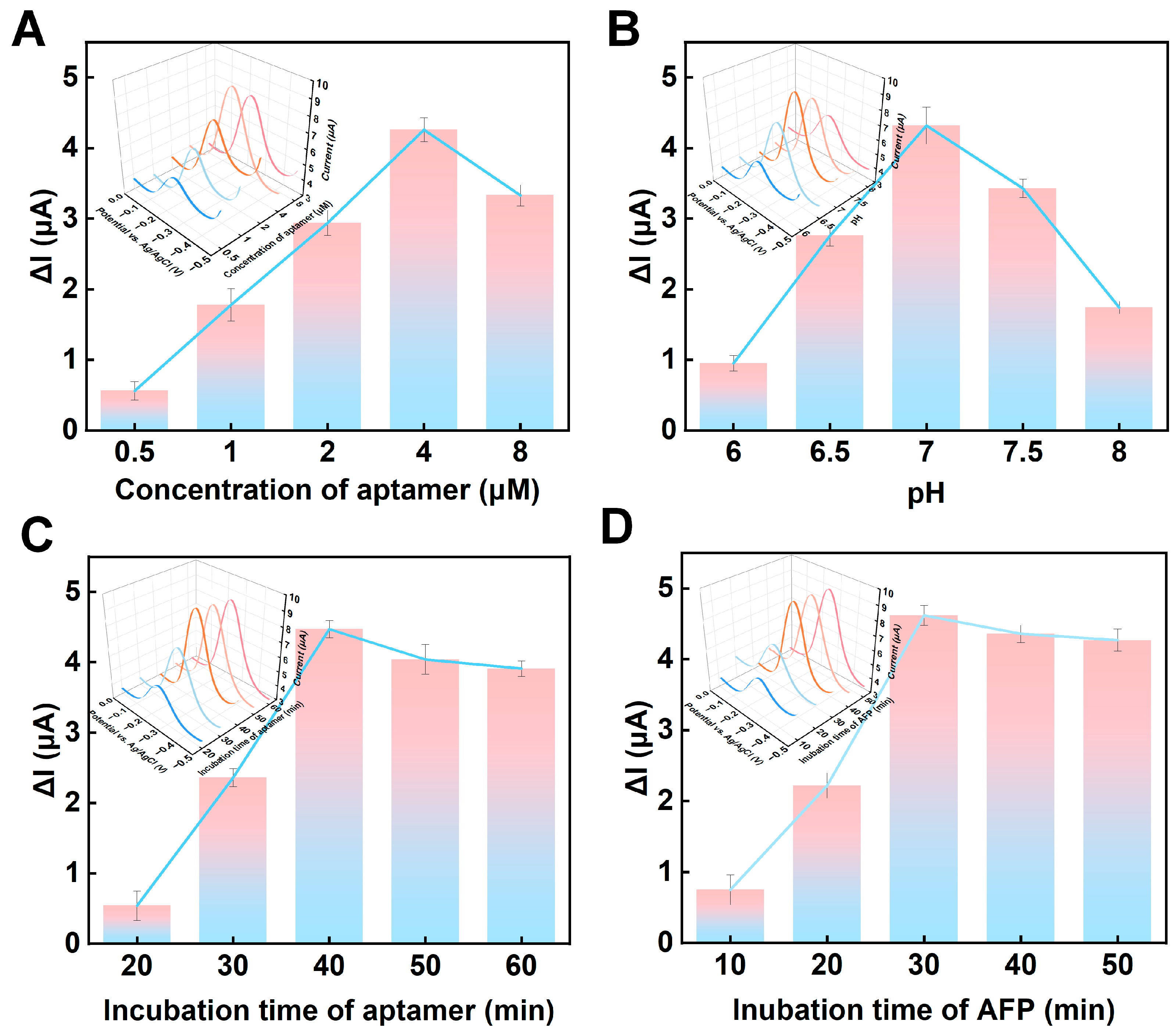
3.3. Optimization of Experimental Parameters
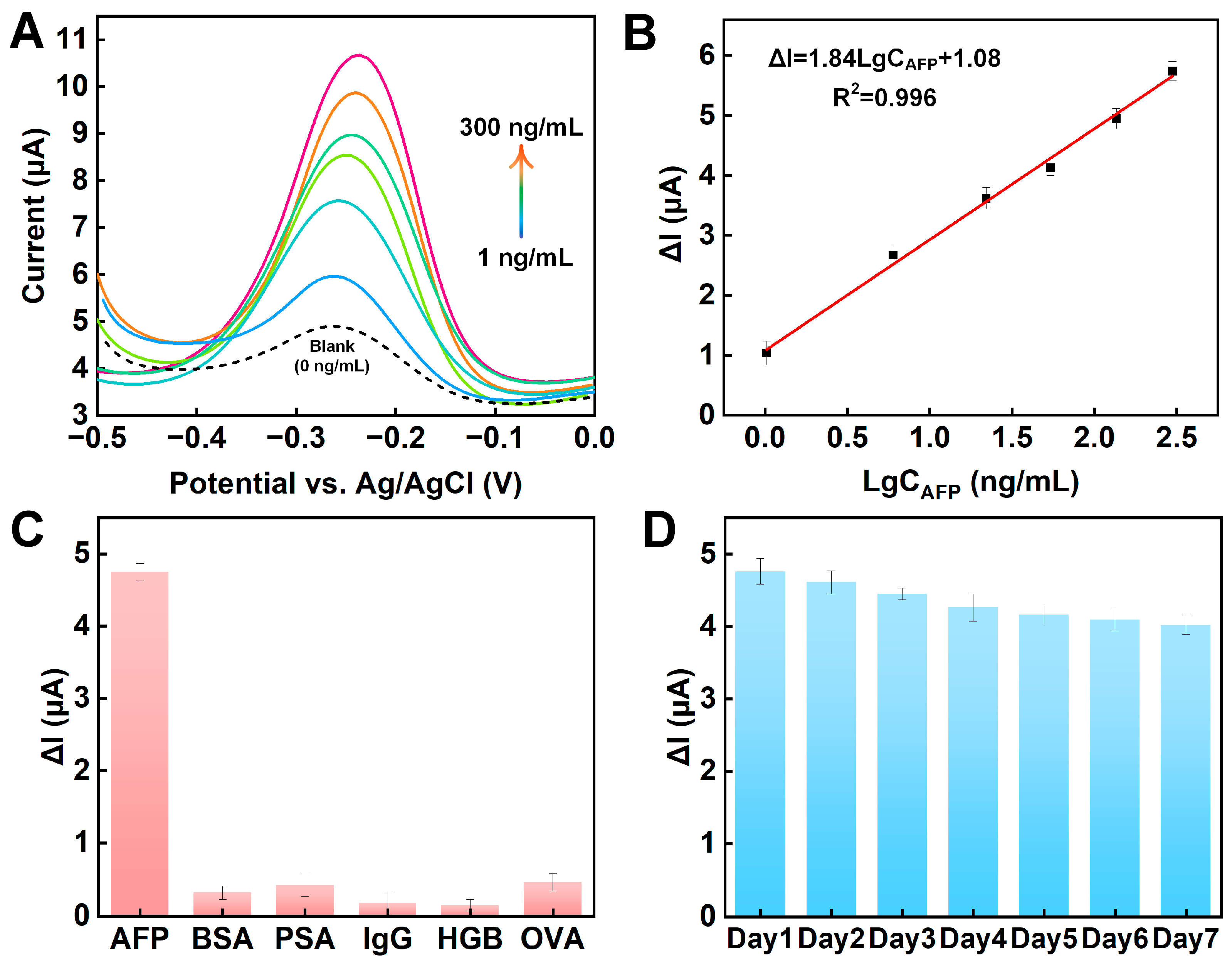
3.4. Analytical Performance
3.5. Selectivity, Reproducibility, and Stability
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammadinejad, A.; Kazemi Oskuee, R.; Eivazzadeh-Keihan, R.; Rezayi, M.; Baradaran, B.; Maleki, A.; Hashemzaei, M.; Mokhtarzadeh, A.; de la Guardia, M. Development of biosensors for detection of alpha-fetoprotein: As a major biomarker for hepatocellular carcinoma. TrAC Trends Anal. Chem. 2020, 130, 115961. [Google Scholar] [CrossRef]
- Rong, S.; Zou, L.; Li, Y.; Guan, Y.; Guan, H.; Zhang, Z.; Zhang, Y.; Gao, H.; Yu, H.; Zhao, F.; et al. An ultrasensitive disposable sandwich-configuration electrochemical immunosensor based on OMC@AuNPs composites and AuPt-MB for alpha-fetoprotein detection. Bioelectrochemistry 2021, 141, 107846. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, G.; Yang, X. Electrochemical immunosensor based on Fe3O4/MWCNTs-COOH/AuNPs nanocomposites for trace liver cancer marker alpha-fetoprotein detection. Talanta 2023, 259, 124492. [Google Scholar] [CrossRef]
- Niu, Y.; Yang, T.; Ma, S.; Peng, F.; Yi, M.; Wan, M.; Mao, C.; Shen, J. Label-free immunosensor based on hyperbranched polyester for specific detection of α-fetoprotein. Biosens. Bioelectron. 2017, 92, 1–7. [Google Scholar] [CrossRef]
- Shan, C.-W.; Chen, Z.; Han, G.-C.; Feng, X.-Z.; Kraatz, H.-B. Electrochemical immuno-biosensors for the detection of the tumor marker alpha-fetoprotein: A review. Talanta 2024, 271, 125638. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Deng, D.; Liu, R.; Lv, Y. Mass Spectrometric Assay of Alpha-Fetoprotein Isoforms for Accurate Serological Evaluation. Anal. Chem. 2020, 92, 4807–4813. [Google Scholar] [CrossRef]
- Liu, S.; Ma, Y.; Cui, M.; Luo, X. Enhanced electrochemical biosensing of alpha-fetoprotein based on three-dimensional macroporous conducting polymer polyaniline. Sens. Actuators B Chem. 2018, 255, 2568–2574. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, M.; Lai, W.; Zhang, M.; Ma, C.; Li, P.; Li, J.; Li, H.; Hong, C. Preparation of a pH-responsive controlled-release electrochemical immunosensor based on polydopamine encapsulation for ultrasensitive detection of alpha-fetoprotein. Microchim. Acta 2022, 189, 334. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Hu, S.; Cai, K.; Peng, C.; Luo, L.; Gu, Y.; Mei, Y. Electrochemical immunosensor based on PtNPs/MoS2@rGO composite for the detection of alpha-fetoprotein in human serum. Microchim. Acta 2024, 191, 662. [Google Scholar] [CrossRef]
- Zong, H.; Zhang, Y.; Liu, X.; Xu, Z.; Ye, J.; Lu, S.; Guo, X.; Yang, Z.; Zhang, X.; Chai, M.; et al. Recent trends in smartphone-based optical imaging biosensors for genetic testing: A review. View 2023, 4, 20220062. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Geng, X.; Tan, R.; Xu, S.; Sun, L. Electrochemical sensors for plant signaling molecules. Biosens. Bioelectron. 2025, 267, 116757. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Luo, F.; Wang, P.; Lin, Z. Miniaturized electrochemical sensors and their point-of-care applications. Chin. Chem. Lett. 2020, 31, 589–600. [Google Scholar] [CrossRef]
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in Electrochemical Sensors: A Review. Electroanalysis 2021, 33, 1827–1851. [Google Scholar] [CrossRef]
- Teymourian, H.; Parrilla, M.; Sempionatto, J.R.; Montiel, N.F.; Barfidokht, A.; Van Echelpoel, R.; De Wael, K.; Wang, J. Wearable Electrochemical Sensors for the Monitoring and Screening of Drugs. ACS Sens. 2020, 5, 2679–2700. [Google Scholar] [CrossRef]
- Sankauskaite, A.; Pauliukaite, R.; Baltusnikaite-Guzaitiene, J.; Abraitiene, A. Smart textile with integrated wearable electrochemical sensors. Curr. Opin. Electrochem. 2023, 42, 101410. [Google Scholar] [CrossRef]
- Xue, Z.; Gai, Y.; Wu, Y.; Liu, Z.; Li, Z. Wearable mechanical and electrochemical sensors for real-time health monitoring. Commun. Mater. 2024, 5, 211. [Google Scholar] [CrossRef]
- Bariya, M.; Shahpar, Z.; Park, H.; Sun, J.; Jung, Y.; Gao, W.; Nyein, H.Y.Y.; Liaw, T.S.; Tai, L.-C.; Ngo, Q.P.; et al. Roll-to-Roll Gravure Printed Electrochemical Sensors for Wearable and Medical Devices. ACS Nano 2018, 12, 6978–6987. [Google Scholar] [CrossRef]
- Bujes-Garrido, J.; Arcos-Martínez, M.J. Development of a wearable electrochemical sensor for voltammetric determination of chloride ions. Sens. Actuators B Chem. 2017, 240, 224–228. [Google Scholar] [CrossRef]
- Dong, X.; Yan, X.; Li, M.; Liu, H.; Li, J.; Wang, L.; Wang, K.; Lu, X.; Wang, S.; He, B. Ultrasensitive detection of chloramphenicol using electrochemical aptamer sensor: A mini review. Electrochem. Commun. 2020, 120, 106835. [Google Scholar] [CrossRef]
- Meng, X.; Li, J.; Wu, Y.; Cao, X.; Zhang, Z. Rational design of hairpin aptamer using intrinsic disorder mechanism to enhance sensitivity of aptamer folding-based electrochemical sensor for tobramycin. Sens. Actuators B Chem. 2023, 394, 134354. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, C.; Figueroa-Miranda, G.; Offenhäusser, A.; Mayer, D. Amplification of aptamer sensor signals by four orders of magnitude via interdigitated organic electrochemical transistors. Biosens. Bioelectron. 2019, 144, 111668. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, Q. A reagentless electrochemical sensor for aflatoxin B1 with sensitive signal-on responses using aptamer with methylene blue label at specific internal thymine. Biosens. Bioelectron. 2020, 167, 112478. [Google Scholar] [CrossRef] [PubMed]
- Mahato, K.; Wang, J. Electrochemical sensors: From the bench to the skin. Sens. Actuators B Chem. 2021, 344, 130178. [Google Scholar] [CrossRef]
- Solangi, N.H.; Mubarak, N.M.; Karri, R.R.; Mazari, S.A.; Jatoi, A.S. Advanced growth of 2D MXene for electrochemical sensors. Environ. Res. 2023, 222, 115279. [Google Scholar] [CrossRef]
- Baumgarten, L.G.; Dreyer, J.P.; de Campos, C.E.M.; Germano, A.T.; Vitali, L.; Spinelli, A.; Santana, E.R.; Winiarski, J.P.; Vieira, I.C. Two-dimensional titanium carbide MXene embedded in exfoliated graphite nanoplatelets for voltammetric sensing of thiamethoxam in beekeeping products. Electrochim. Acta 2024, 494, 144423. [Google Scholar] [CrossRef]
- Tan, A.Y.S.; Awan, H.T.A.; Cheng, F.; Zhang, M.; Tan, M.T.T.; Manickam, S.; Khalid, M.; Muthoosamy, K. Recent advances in the use of MXenes for photoelectrochemical sensors. Chem. Eng. J. 2024, 482, 148774. [Google Scholar] [CrossRef]
- Mohanapriya, D.; Satija, J.; Senthilkumar, S.; Kumar Ponnusamy, V.; Thenmozhi, K. Design and engineering of 2D MXenes for point-of-care electrochemical detection of bioactive analytes and environmental pollutants. Coord. Chem. Rev. 2024, 507, 215746. [Google Scholar] [CrossRef]
- Huang, H.; Yang, W. MXene-Based Micro-Supercapacitors: Ink Rheology, Microelectrode Design and Integrated System. ACS Nano 2024, 18, 4651–4682. [Google Scholar] [CrossRef]
- Patil, A.M.; Jadhav, A.A.; Chodankar, N.R.; Avatare, A.T.; Hong, J.; Dhas, S.D.; Patil, U.M.; Jun, S.C. Recent progress of MXene synthesis, properties, microelectrode fabrication techniques for microsupercapacitors and microbatteries energy storage devices and integration: A comprehensive review. Coord. Chem. Rev. 2024, 517, 216020. [Google Scholar] [CrossRef]
- Shankar, S.; Chen, Y.; Averbeck, S.; Hendricks, Q.; Murphy, B.; Ferleger, B.; Driscoll, N.; Shekhirev, M.; Takano, H.; Richardson, A.; et al. Transparent MXene Microelectrode Arrays for Multimodal Mapping of Neural Dynamics. Adv. Healthc. Mater. 2024, 2402576. [Google Scholar] [CrossRef]
- Gurusamy, L.; Karuppasamy, L.; Anandan, S.; Barton, S.C.; Chuang, Y.-H.; Liu, C.-H.; Wu, J.J. Review of oxygen-vacancies nanomaterials for non-enzymatic electrochemical sensors application. Coord. Chem. Rev. 2023, 484, 215102. [Google Scholar] [CrossRef]
- Iftikhar, T.; Asif, M.; Aziz, A.; Ashraf, G.; Jun, S.; Li, G.; Liu, H. Topical advances in nanomaterials based electrochemical sensors for resorcinol detection. Trends Environ. Anal. Chem. 2021, 31, e00138. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, D.; Gu, C.; Qiu, Y.; Wan, H.; Wang, P. 3D microgroove electrical impedance sensing to examine 3D cell cultures for antineoplastic drug assessment. Microsyst. Nanoeng. 2020, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Su, X.; Wang, H.; Wang, C.; Zhou, X.; Zou, X.; Zhang, W. Programmable dual-electric-field immunosensor using MXene-Au-based competitive signal probe for natural parathion-methyl detection. Biosens. Bioelectron. 2022, 214, 114546. [Google Scholar] [CrossRef]
- Bharti, A.; Singh, S.; Munthala, D.; Roy, S.; Pojprapai, S.; Suksaweang, S.; Sain, S.; Roy, S.S.; Mohamed, J.J.; Avasthi, D.K.; et al. Development of a nucleic acid-based screen printed electrochemical biosensor using Ti3C2Tx-MXene for the detection of SARS-CoV-2. Microchem. J. 2023, 195, 109521. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, D.; Xie, M.; Liu, X.; Wang, Y.; Hu, X.; Wang, S. Electrochemical sensor for effective detection of methyl parathion applying multidimensional MXene/CNHs/PPy nanocomposite to synergistically immobilize acetylcholinesterase. Food Chem. 2024, 460, 140432. [Google Scholar] [CrossRef]
- Han, S.; Zou, M.; Pu, X.; Lu, Y.; Tian, Y.; Li, H.; Liu, Y.; Wu, F.; Huang, N.; Shen, M.; et al. Smart MXene-based bioelectronic devices as wearable health monitor for sensing human physiological signals. View 2023, 4, 20230005. [Google Scholar] [CrossRef]
- Wang, H.; Cai, L.; Wang, Y.; Liu, C.; Fang, G.; Wang, S. Covalent molecularly imprinted electrochemical sensor modulated by borate ester bonds for hygromycin B detection based on the synergistic signal amplification of Cu-MOF and MXene. Food Chem. 2022, 383, 132382. [Google Scholar] [CrossRef]
- Mavis Xhakaza, N.; Chokkareddy, R.; Redhi, G.G. Ionic liquid based electrochemical sensor for the detection of efavirenz. J. Mol. Liq. 2022, 368, 120444. [Google Scholar] [CrossRef]
- Upan, J.; Youngvises, N.; Tuantranont, A.; Karuwan, C.; Banet, P.; Aubert, P.-H.; Jakmunee, J. A simple label-free electrochemical sensor for sensitive detection of alpha-fetoprotein based on specific aptamer immobilized platinum nanoparticles/carboxylated-graphene oxide. Sci. Rep. 2021, 11, 13969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wu, D.; Ma, H.; Pang, X.; Fan, D.; Wei, Q.; Du, B. Ultrasensitive Label-free Electrochemical Immunosensor based on Multifunctionalized Graphene Nanocomposites for the Detection of Alpha Fetoprotein. Sci. Rep. 2017, 7, 42361. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, S.; Wang, Z.; Xue, Y.; Dong, C.; Zeng, J.; Huang, Y.; Liang, J.; Zhou, Z. Label-free electrochemical aptasensor for detection of alpha-fetoprotein based on AFP-aptamer and thionin/reduced graphene oxide/gold nanoparticles. Anal. Biochem. 2018, 547, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Jothi, L.; Jaganathan, S.K.; Nageswaran, G. An electrodeposited Au nanoparticle/porous graphene nanoribbon composite for electrochemical detection of alpha-fetoprotein. Mater. Chem. Phys. 2020, 242, 122514. [Google Scholar] [CrossRef]
- Xu, T.; Chi, B.; Wu, F.; Ma, S.; Zhan, S.; Yi, M.; Xu, H.; Mao, C. A sensitive label-free immunosensor for detection α-Fetoprotein in whole blood based on anticoagulating magnetic nanoparticles. Biosens. Bioelectron. 2017, 95, 87–93. [Google Scholar] [CrossRef]



| Species | Original Level | Added | Found | Recovery | |
|---|---|---|---|---|---|
| Human serum | Sample 1 | 1.25 | 0.1 | 1.37 | 101.5% |
| Sample 2 | 1.25 | 1 | 2.22 | 98.7% | |
| Sample 3 | 1.25 | 10 | 11.65 | 103.6% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Chen, J.; Wu, S.; Qiu, Y.; Pan, Y. A Signal-On Microelectrode Electrochemical Aptamer Sensor Based on AuNPs–MXene for Alpha-Fetoprotein Determination. Sensors 2024, 24, 7878. https://doi.org/10.3390/s24247878
Su X, Chen J, Wu S, Qiu Y, Pan Y. A Signal-On Microelectrode Electrochemical Aptamer Sensor Based on AuNPs–MXene for Alpha-Fetoprotein Determination. Sensors. 2024; 24(24):7878. https://doi.org/10.3390/s24247878
Chicago/Turabian StyleSu, Xiaoyu, Junbiao Chen, Shanshan Wu, Yong Qiu, and Yuxiang Pan. 2024. "A Signal-On Microelectrode Electrochemical Aptamer Sensor Based on AuNPs–MXene for Alpha-Fetoprotein Determination" Sensors 24, no. 24: 7878. https://doi.org/10.3390/s24247878
APA StyleSu, X., Chen, J., Wu, S., Qiu, Y., & Pan, Y. (2024). A Signal-On Microelectrode Electrochemical Aptamer Sensor Based on AuNPs–MXene for Alpha-Fetoprotein Determination. Sensors, 24(24), 7878. https://doi.org/10.3390/s24247878





