Evaluation of Performance and Longevity of Ti-Cu Dry Electrodes: Degradation Analysis Using Anodic Stripping Voltammetry
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Ti-Cu Electrodes
2.2. Characterisation of the Ti-Cu Electrodes
2.3. Degradation of the Ti-Cu Electrodes
2.4. ASV Analysis
3. Results
3.1. Thin Film Characterisation
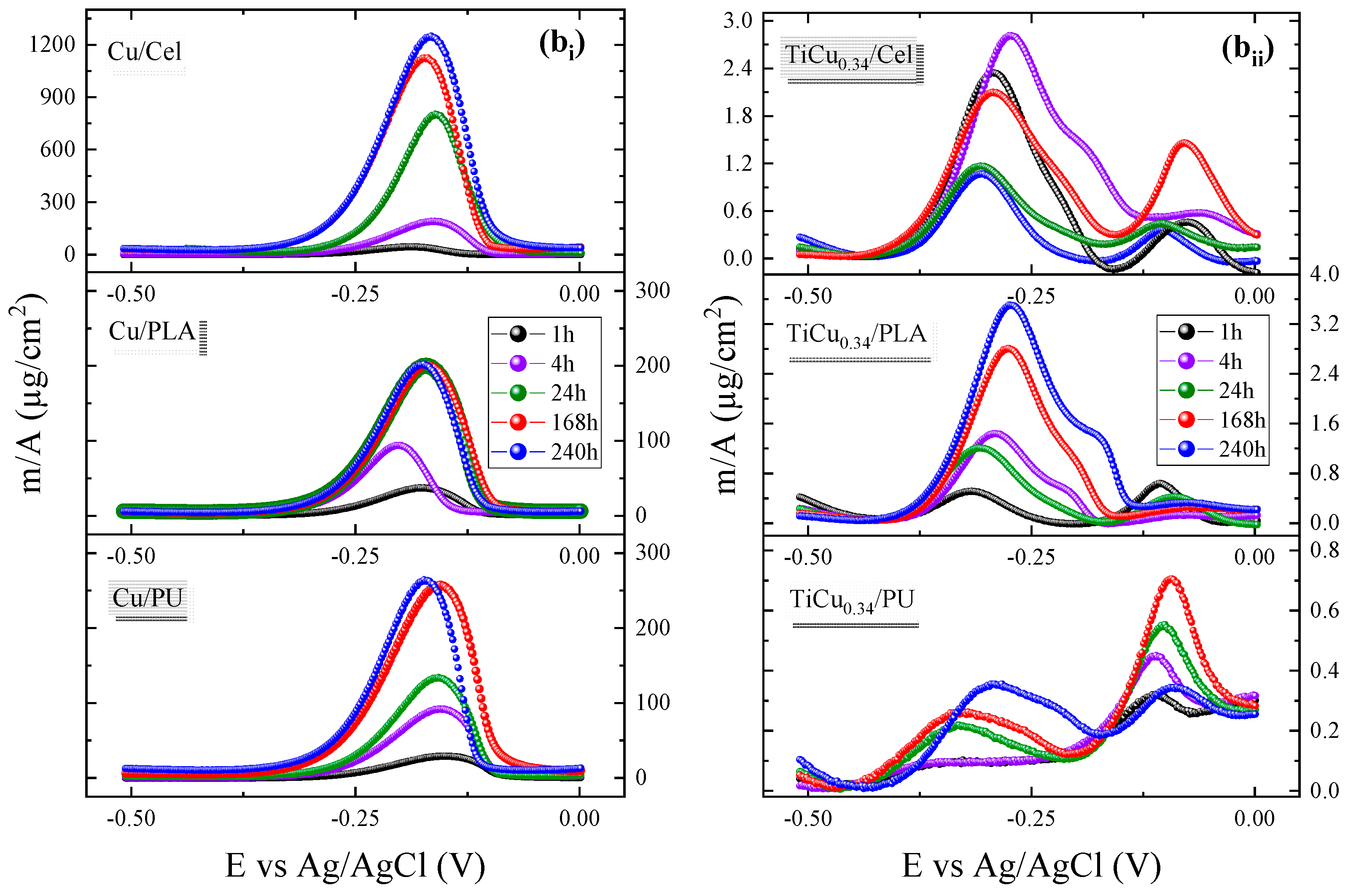
3.2. Voltammetry Analysis
3.2.1. ASV Optimisation
3.2.2. Determination of Cu(II) into Artificial Sweat
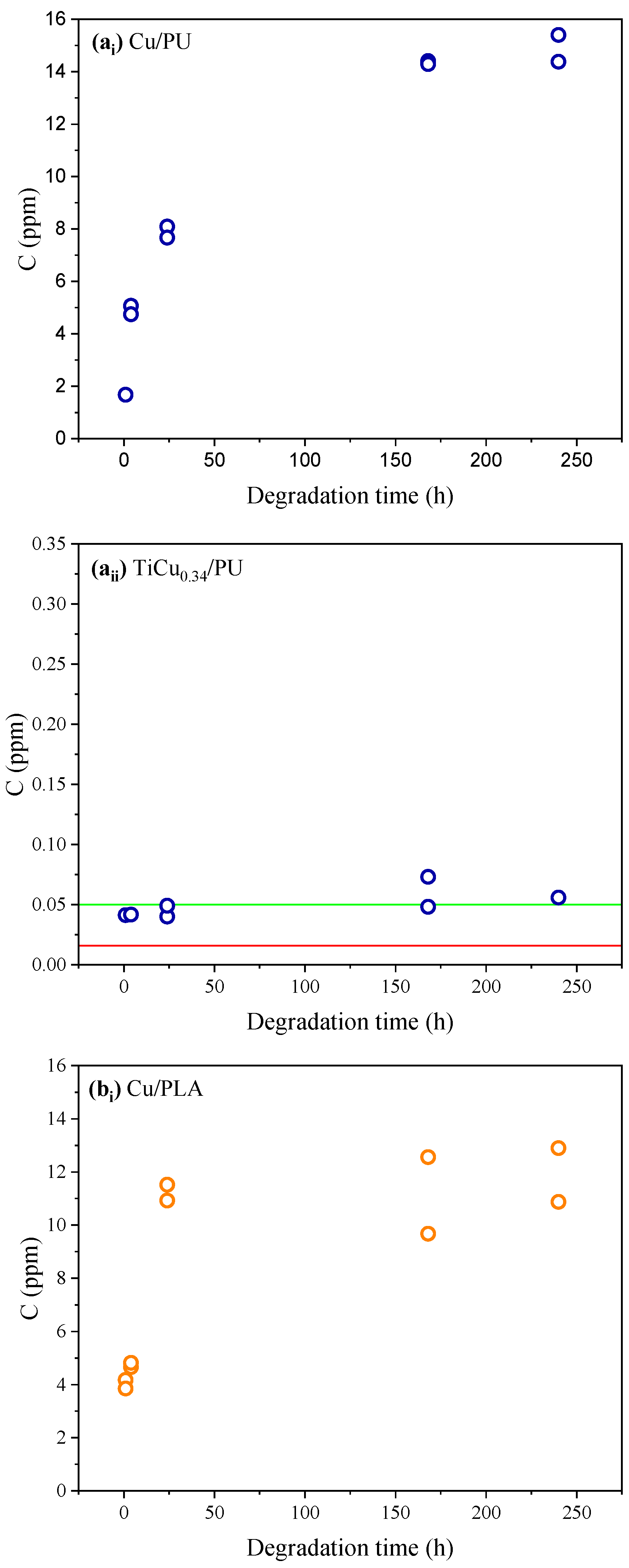
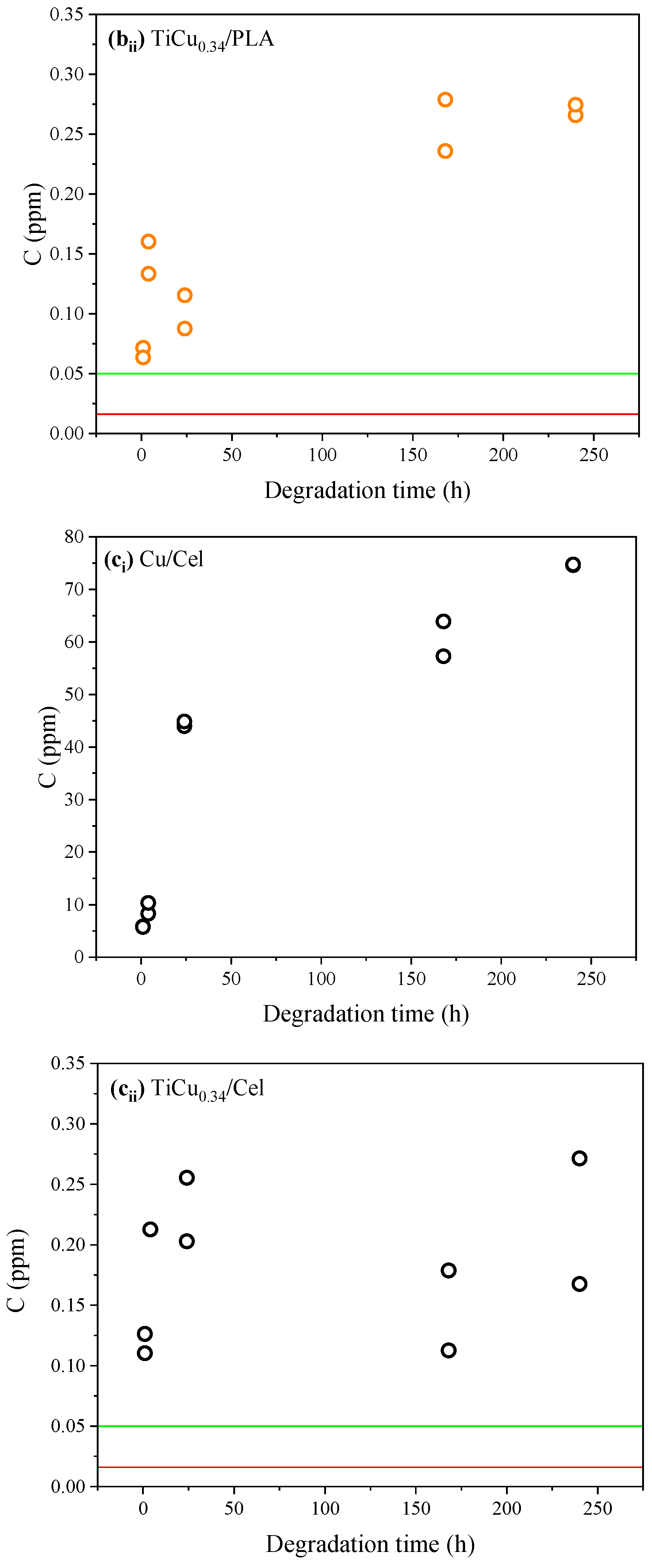
3.3. Analytical Assessment of Ti-Cu Electrodes in Artificial Sweat
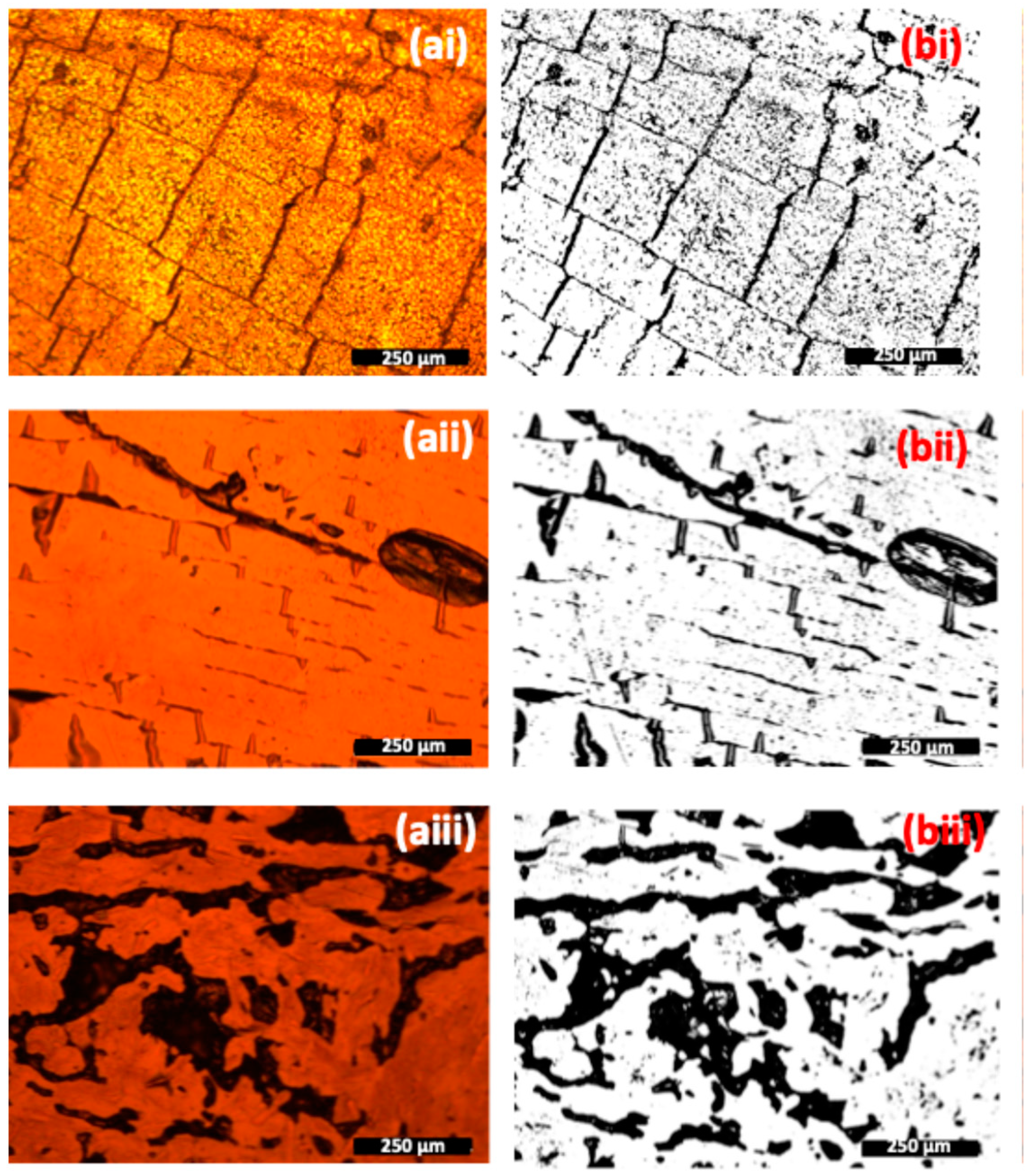
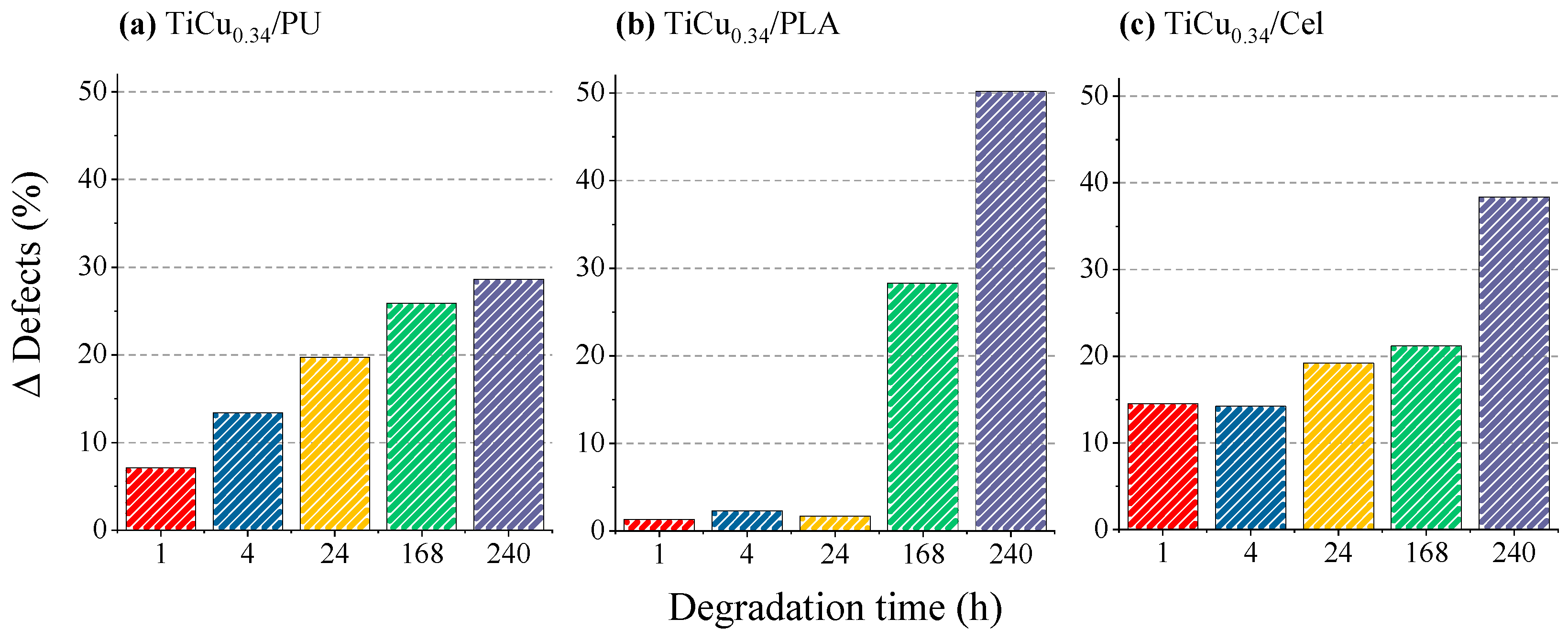
3.4. Optical Characterisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, C.; Veloso, H.; Hayes, M.; Cullinan, M.A.; Vaz, F. Nanostructured (Ti,Cu)N dry electrodes for advanced control of the neuromuscular activity. IEEE Sens. J. 2022, 23, 3629–3639. [Google Scholar] [CrossRef]
- Poon, C.C.; Liu, Q.; Gao, H.; Lin, W.-H.; Zhang, Y.-T. Wearable Intelligent Systems for E-Health. J. Comput. Sci. Eng. 2011, 5, 246–256. [Google Scholar] [CrossRef]
- Niu, X.; Gao, X.; Liu, Y.; Liu, H. Surface bioelectric dry Electrodes: A review. Measurement 2021, 183, 109774. [Google Scholar] [CrossRef]
- Kalasin, S.; Surareungchai, W. Challenges of Emerging Wearable Sensors for Remote Monitoring toward Telemedicine Healthcare. Anal. Chem. 2023, 95, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef]
- Al-Ayyad, M.; Abu Owida, H.; De Fazio, R.; Al-Naami, B.; Visconti, P. Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies. Electronics 2023, 12, 1520. [Google Scholar] [CrossRef]
- Lopes, C.; Fiedler, P.; Rodrigues, M.S.; Borges, J.; Bertollo, M.; Alves, E.; Barradas, N.P.; Comani, S.; Haueisen, J.; Vaz, F. Me-Doped Ti–Me Intermetallic Thin Films Used for Dry Biopotential Electrodes: A Comparative Case Study. Sensors 2021, 21, 8143. [Google Scholar] [CrossRef]
- Carvalho, D.; Marques, S.; Siqueira, G.; Ferreira, A.; Santos, J.; Geraldo, D.; Castro, C.R.; Machado, A.V.; Vaz, F.; Lopes, C. Enhancing the Longevity and Functionality of Ti-Ag Dry Electrodes for Remote Biomedical Applications: A Comprehensive Study. Sensors 2023, 23, 8321. [Google Scholar] [CrossRef]
- Lopes, C.; Rodrigues, M.S.; Ferreira, A.; Macedo, F.; Borsan, I.; Gabor, C.; Pop, M.-A.; Alves, E.; Barradas, N.P.; Munteanu, D.; et al. The influence of the nanostructure design on the optical, electrical and thermal properties of TiNx thin films prepared by reactive magnetron sputtering. Mater. Chem. Phys. 2023, 306, 127981. [Google Scholar] [CrossRef]
- Bersier, P.M.; Howell, J.; Bruntlett, C. Tutorial review. Advanced electroanalytical techniques versus atomic absorption spectrometry, inductively coupled plasma atomic emission spectrometry and inductively coupled plasma mass spectrometry in environmental analysis. Analyst 1994, 119, 219–232. [Google Scholar] [CrossRef]
- Harvey, D. Modern Analytical Chemistry, 1st ed.; Bensink, J.L., Ed.; McGraw-Hill: Boston, MA, USA, 2000. [Google Scholar]
- Meucci, V.; Battaglia, F.; Marchetti, V.; Gori, E.; Intorre, L. Rapid and simultaneous electrochemical method to measure copper and lead in canine liver biopsy. MethodsX 2020, 7, 101154. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Analytical Electrochemistry, 2nd ed.; Wiley-VCH Verlag GmbH: New York, NY, USA, 2000. [Google Scholar]
- Borrill, A.J.; Reily, N.E.; Macpherson, J.V. Addressing the practicalities of anodic stripping voltammetry for heavy metal detection: A tutorial review. Analyst 2019, 144, 6834–6849. [Google Scholar] [CrossRef]
- Wygant, B.R.; Lambert, T.N. Thin Film Electrodes for Anodic Stripping Voltammetry: A Mini-Review. Front. Chem. 2022, 9, 809535. [Google Scholar] [CrossRef]
- Pungjunun, K.; Chaiyo, S.; Jantrahong, I.; Nantaphol, S.; Siangproh, W.; Chailapakul, O. Anodic stripping voltammetric determination of total arsenic using a gold nanoparticle-modified boron-doped diamond electrode on a paper-based device. Microchim. Acta 2018, 185, 324. [Google Scholar] [CrossRef]
- Planková, A.; Jampílek, J.; Švorc, L.; Hanko, M.; Mikuš, P. Anodic stripping voltammetry: Affordable and reliable alternative to inductively coupled plasma-based analytical methods. Monatshefte Für Chem.-Chem. Mon. 2018, 149, 913–920. [Google Scholar] [CrossRef]
- Southampton Electrochemistry Group. Instrumental Methods in Electrochemistry, Repr. em Ellis Horwood Series in Physical Chemistry; Horwood: Chichester, UK, 2011. [Google Scholar]
- Brainina, K.Z.; Malakhova, N.A.; Stojko, N.Y. Stripping voltammetry in environmental and food analysis. Fresenius J. Anal. Chem. 2000, 368, 307–325. [Google Scholar] [CrossRef]
- Bakirhan, N.K.; Uslu, B.; Ozkan, S.A. Sensitive and Selective Assay of Antimicrobials on Nanostructured Materials by Electrochemical Techniques. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 55–83. [Google Scholar] [CrossRef]
- Wang, T.; Yue, W. Carbon Nanotubes Heavy Metal Detection with Stripping Voltammetry: A Review Paper. Electroanalysis 2017, 29, 2178–2189. [Google Scholar] [CrossRef]
- Bobrowski, A.; Krlicka, A. Anodic Stripping Voltammetric Determination of Copper Traces in Carbonate Minerals and Fly Ash Extracts Using a Screen-Printed Electrode Modified In Situ with Antimony Film. Insights Anal. Electrochem. 2015, 1, 1–6. [Google Scholar] [CrossRef][Green Version]
- Stankovic, D.; Roglic, G.; Andjelkovic, I.; Skrivanj, S.; Mutic, J.; Manojlovic, D. SW Anodic Stripping Voltammetry Determination of Copper with New Tape of Modified GC Electrode. Anal. Bioanal. Chem. 2011, 3, 556–564. [Google Scholar]
- Lieu, N.T.; Trung, P.Q.; Trang, L.T.T.; Giang, L.T. Simultaneous effect of pH, deposition time, deposition potential, and step potential on the stripping peak current of copper on platinum nanoflowers modified glassy carbon electrode (PtNFs/GCE) using response surface methodology. Vietnam. J. Chem. 2020, 58, 302–308. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Fiedler, P.; Küchler, N.; Domingues, R.P.; Lopes, C.; Borges, J.; Haueisen, J.; Vaz, F. Dry Electrodes for Surface Electromyography Based on Architectured Titanium Thin Films. Materials 2020, 13, 2135. [Google Scholar] [CrossRef] [PubMed]
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Barradas, N.; Alves, E.; Jeynes, C.; Tosaki, M. Accurate simulation of backscattering spectra in the presence of sharp resonances. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2006, 247, 381–389. [Google Scholar] [CrossRef]
- Barradas, N.; Jeynes, C. Advanced physics and algorithms in the IBA DataFurnace. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 1875–1879. [Google Scholar] [CrossRef]
- Proença, M.; Borges, J.; Rodrigues, M.S.; Meira, D.I.; Sampaio, P.; Dias, J.P.; Pedrosa, P.; Martin, N.; Bundaleski, N.; Teodoro, O.M.; et al. Nanocomposite thin films based on Au-Ag nanoparticles embedded in a CuO matrix for localized surface plasmon resonance sensing. Appl. Surf. Sci. 2019, 484, 152–168. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Hou, X.; Qiu, J.; Zhang, E.; Hu, J. Enhanced mechanical, bio-corrosion, and antibacterial properties of Ti-Cu alloy by forming a gradient nanostructured surface layer. Surf. Coat. Technol. 2023, 465, 129609. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Watch-Cases and Accessories—Gold Alloy Coverings—Part 2: Determination of Fineness, Thickness, Corrosion Resistance, and Adhesion; International Organization for Standardization (ISO): Geneva, Switzerland, 2015. [Google Scholar]
- Ma, D.; Jing, P.; Gong, Y.; Wu, B.; Deng, Q.; Li, Y.; Chen, C.; Leng, Y.; Huang, N. Structure and stress of Cu films prepared by high power pulsed magnetron sputtering. Vacuum 2019, 160, 226–232. [Google Scholar] [CrossRef]
- Le, M.-T.; Sohn, Y.-U.; Lim, J.-W.; Choi, G.-S. Effect of Sputtering Power on the Nucleation and Growth of Cu Films Deposited by Magnetron Sputtering. Mater. Trans. 2010, 51, 116–120. [Google Scholar] [CrossRef]
- Zhao, D.; Letz, S.; Jank, M.; März, M. Adhesion strength of ductile thin film determined by cross-sectional nanoindentation. Int. J. Mech. Sci. 2024, 270, 109103. [Google Scholar] [CrossRef]
- Lassnig, A.; Putz, B.; Hirn, S.; Többens, D.; Mitterer, C.; Cordill, M. Adhesion evaluation of thin films to dielectrics in multilayer stacks: A comparison of four-point bending and stressed overlayer technique. Mater. Des. 2021, 200, 109451. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Chang, T.-K.; Lee, Y.-S. Nanoindentating Mechanical Responses and Interfacial Adhesion Strength of Electrochemically Deposited Copper Film. J. Electrochem. Soc. 2005, 152, C657–C663. [Google Scholar] [CrossRef]
- Lim, J.D.; Lee, P.M.; Chen, Z. Understanding the bonding mechanisms of directly sputtered copper thin film on an alumina substrate. Thin Solid Films 2017, 634, 6–14. [Google Scholar] [CrossRef]
- Volinsky, A.A.; Tymiak, N.I.; Kriese, M.D.; Gerberich, W.W.; Hutchinson, J.W. Quantitative Modeling and Measurement of Copper Thin Film Adhesion. MRS Proc. 1998, 539, 277. [Google Scholar] [CrossRef]
- Lopes, C.; Gabor, C.; Cristea, D.; Costa, R.; Domingues, R.; Rodrigues, M.; Borges, J.; Alves, E.; Barradas, N.; Munteanu, D.; et al. Evolution of the mechanical properties of Ti-based intermetallic thin films doped with different metals to be used as biomedical devices. Appl. Surf. Sci. 2019, 505, 144617. [Google Scholar] [CrossRef]
- Tang, J.-F.; Huang, P.-Y.; Lin, J.-H.; Liu, T.-W.; Yang, F.-C.; Chang, C.-L. Microstructure and Antimicrobial Properties of Zr-Cu-Ti Thin-Film Metallic Glass Deposited Using High-Power Impulse Magnetron Sputtering. Materials 2022, 15, 2461. [Google Scholar] [CrossRef]
- Senkov, O.; Miracle, D. Effect of the atomic size distribution on glass forming ability of amorphous metallic alloys. Mater. Res. Bull. 2001, 36, 2183–2198. [Google Scholar] [CrossRef]
- Aqua-Calc. n.d. Available online: https://www.aqua-calc.com/page/density-table/substance/titanium (accessed on 26 July 2024).
- Rajan, S.T.; Arockiarajan, A. Thin film metallic glasses for bioimplants and surgical tools: A review. J. Alloys Compd. 2021, 876, 159939. [Google Scholar] [CrossRef]
- Apreutesei, M.; Steyer, P.; Billard, A.; Joly-Pottuz, L.; Esnouf, C. Zr–Cu thin film metallic glasses: An assessment of the thermal stability and phases’ transformation mechanisms. J. Alloys Compd. 2015, 619, 284–292. [Google Scholar] [CrossRef]
- Jain, M.; Sharma, A.; Pajor, K.; Wieczerzak, K.; della Ventura, N.M.; Maeder, X.; Kruzic, J.J.; Gludovatz, B.; Michler, J. Mechanical properties and thermal stability of thin film metallic glass compared to bulk metallic glass from ambient to elevated temperatures. J. Alloys Compd. 2023, 960, 170728. [Google Scholar] [CrossRef]
- Agra-Gutiérrez, C.; Hardcastle, J.L.; Ball, J.C.; Compton, R.G. Anodic stripping voltammetry of copper at insonated glassy carbon-based electrodes: Application to the determination of copper in beer. Analyst 1999, 124, 1053–1057. [Google Scholar] [CrossRef]
- Abdeen, D.H.; El Hachach, M.; Koc, M.; Atieh, M.A. A Review on the Corrosion Behaviour of Nanocoatings on Metallic Substrates. Materials 2019, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Fovet, Y.; Gal, J.-Y.; Toumelin-Chemla, F. Influence of pH and fluoride concentration on titanium passivating layer: Stability of titanium dioxide. Talanta 2001, 53, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Porcayo-Calderon, J.; Rodríguez-Díaz, R.A.; Porcayo-Palafox, E.; Martinez-Gomez, L. Corrosion Performance of Cu-Based Coins in Artificial Sweat. J. Chem. 2016, 2016, 9542942. [Google Scholar] [CrossRef]
- Zhou, P.; Ogle, K. The Corrosion of Copper and Copper Alloys. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 478–489. [Google Scholar] [CrossRef]
- Hostýnek, J.J. Corrosion Chemistry of Copper: Formation of Potentially Skin-Diffusible Compounds. Exog. Dermatol. 2004, 3, 263–269. [Google Scholar] [CrossRef]





| Atmosphere | Exposure Time (min) | Power (W) | Working Pressure (Pa) | |
|---|---|---|---|---|
| PU | Ar | 5 | 50 | |
| PLA | Ar + N2 | 15 | 100 | |
| Cellulose | O2 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, D.; Rodrigues, A.M.; Santos, J.; Geraldo, D.; Ferreira, A.; Correa, M.A.; Alves, E.; Barradas, N.P.; Lopes, C.; Vaz, F. Evaluation of Performance and Longevity of Ti-Cu Dry Electrodes: Degradation Analysis Using Anodic Stripping Voltammetry. Sensors 2024, 24, 7477. https://doi.org/10.3390/s24237477
Carvalho D, Rodrigues AM, Santos J, Geraldo D, Ferreira A, Correa MA, Alves E, Barradas NP, Lopes C, Vaz F. Evaluation of Performance and Longevity of Ti-Cu Dry Electrodes: Degradation Analysis Using Anodic Stripping Voltammetry. Sensors. 2024; 24(23):7477. https://doi.org/10.3390/s24237477
Chicago/Turabian StyleCarvalho, Daniel, Ana Margarida Rodrigues, João Santos, Dulce Geraldo, Armando Ferreira, Marcio Assolin Correa, Eduardo Alves, Nuno Pessoa Barradas, Claudia Lopes, and Filipe Vaz. 2024. "Evaluation of Performance and Longevity of Ti-Cu Dry Electrodes: Degradation Analysis Using Anodic Stripping Voltammetry" Sensors 24, no. 23: 7477. https://doi.org/10.3390/s24237477
APA StyleCarvalho, D., Rodrigues, A. M., Santos, J., Geraldo, D., Ferreira, A., Correa, M. A., Alves, E., Barradas, N. P., Lopes, C., & Vaz, F. (2024). Evaluation of Performance and Longevity of Ti-Cu Dry Electrodes: Degradation Analysis Using Anodic Stripping Voltammetry. Sensors, 24(23), 7477. https://doi.org/10.3390/s24237477









