Respiratory Rate Monitoring via a Fibre Bragg Grating-Embedded Respirator Mask with a Wearable Miniature Interrogator
Abstract
1. Introduction
2. Materials and Methods
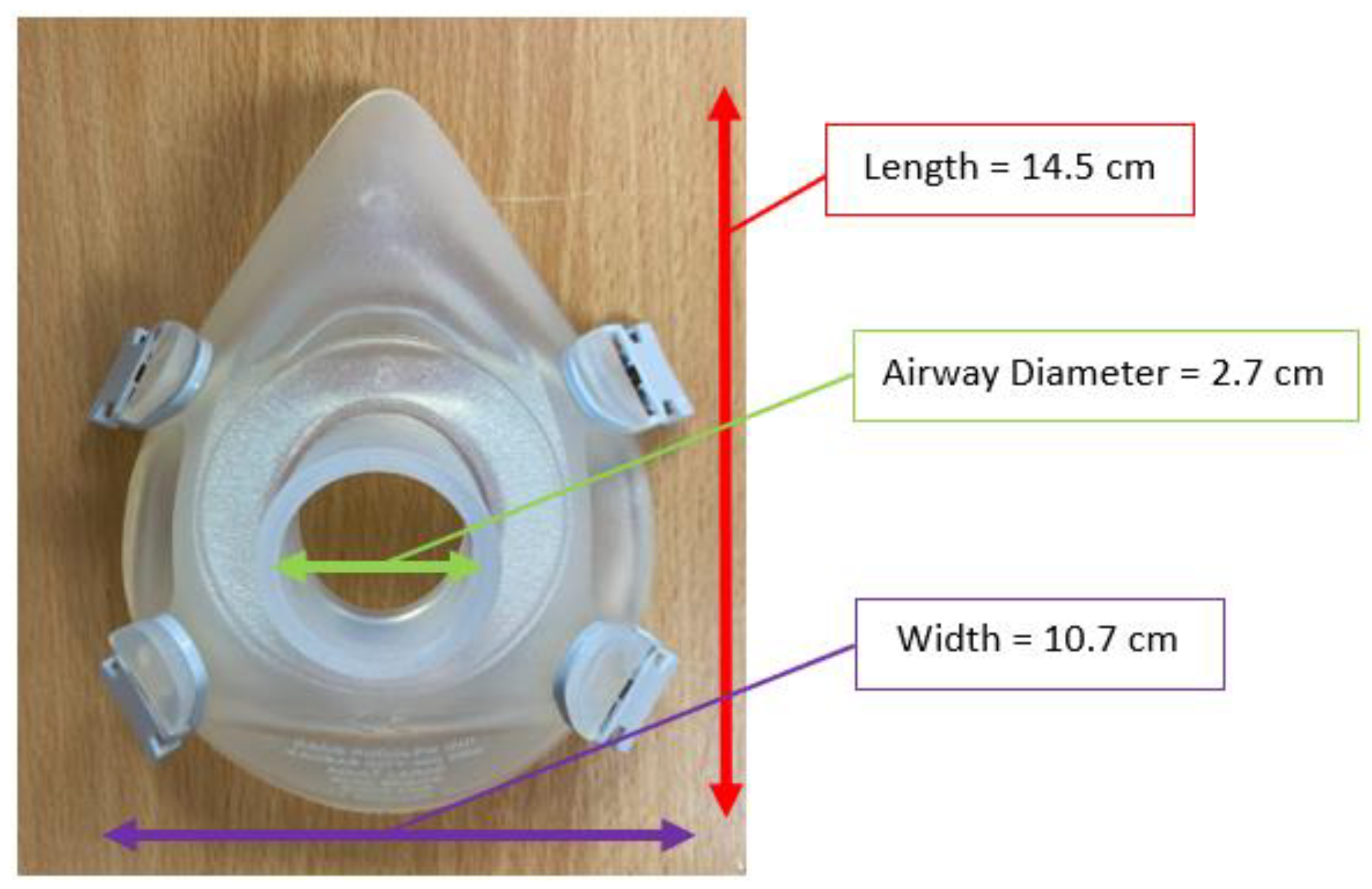
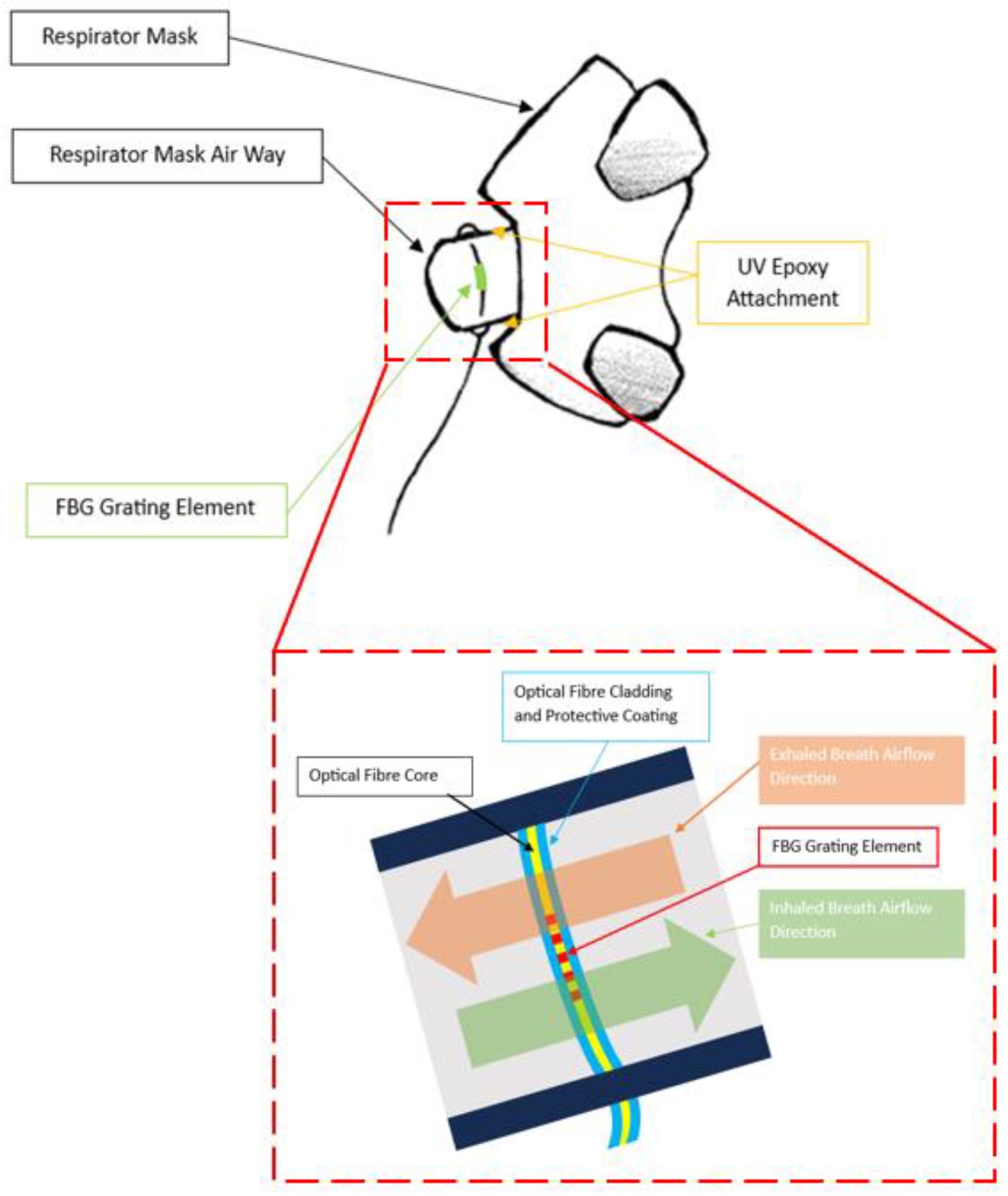
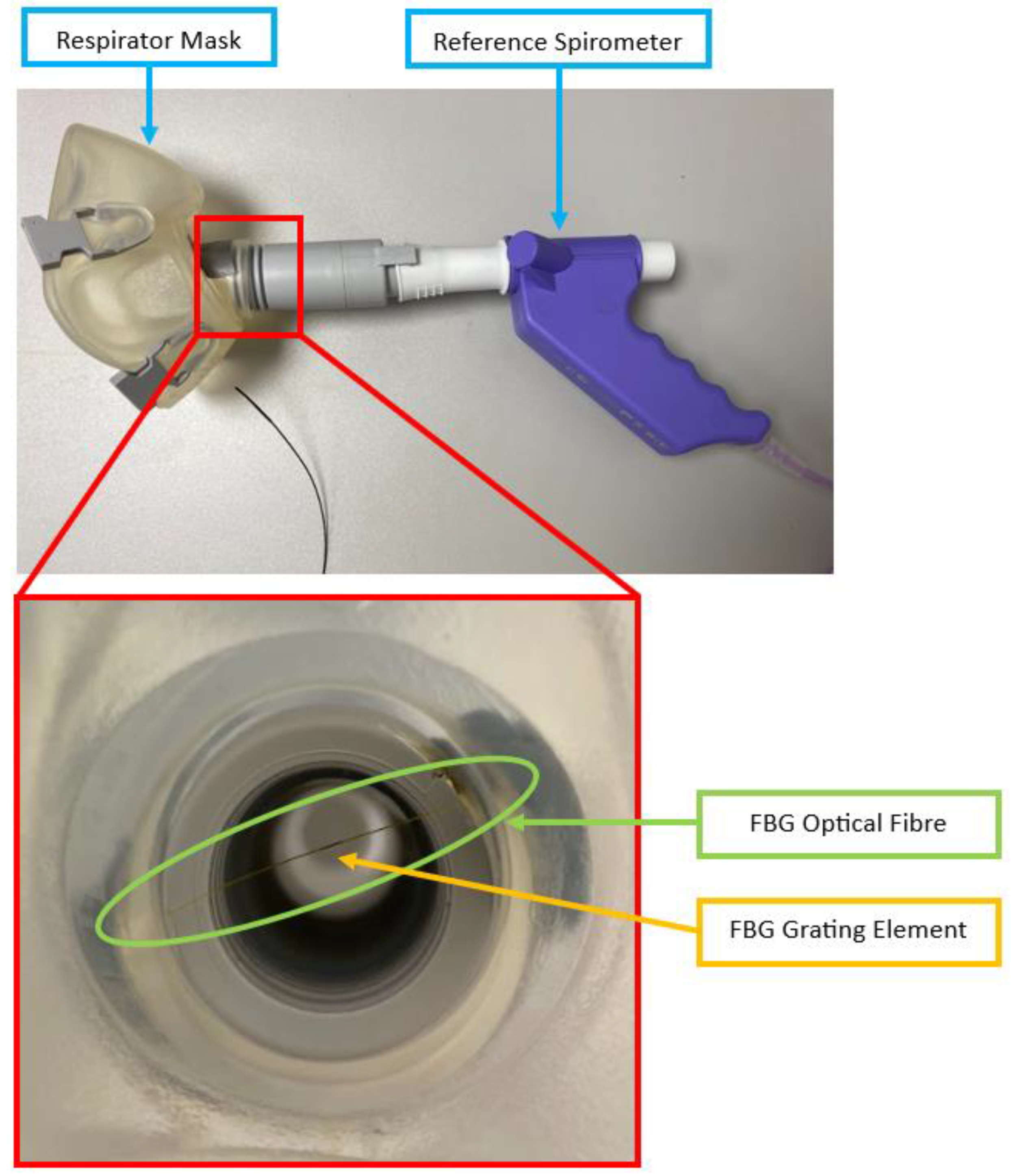
2.1. FBG Respirator Mask Design
2.2. Sensing Principle
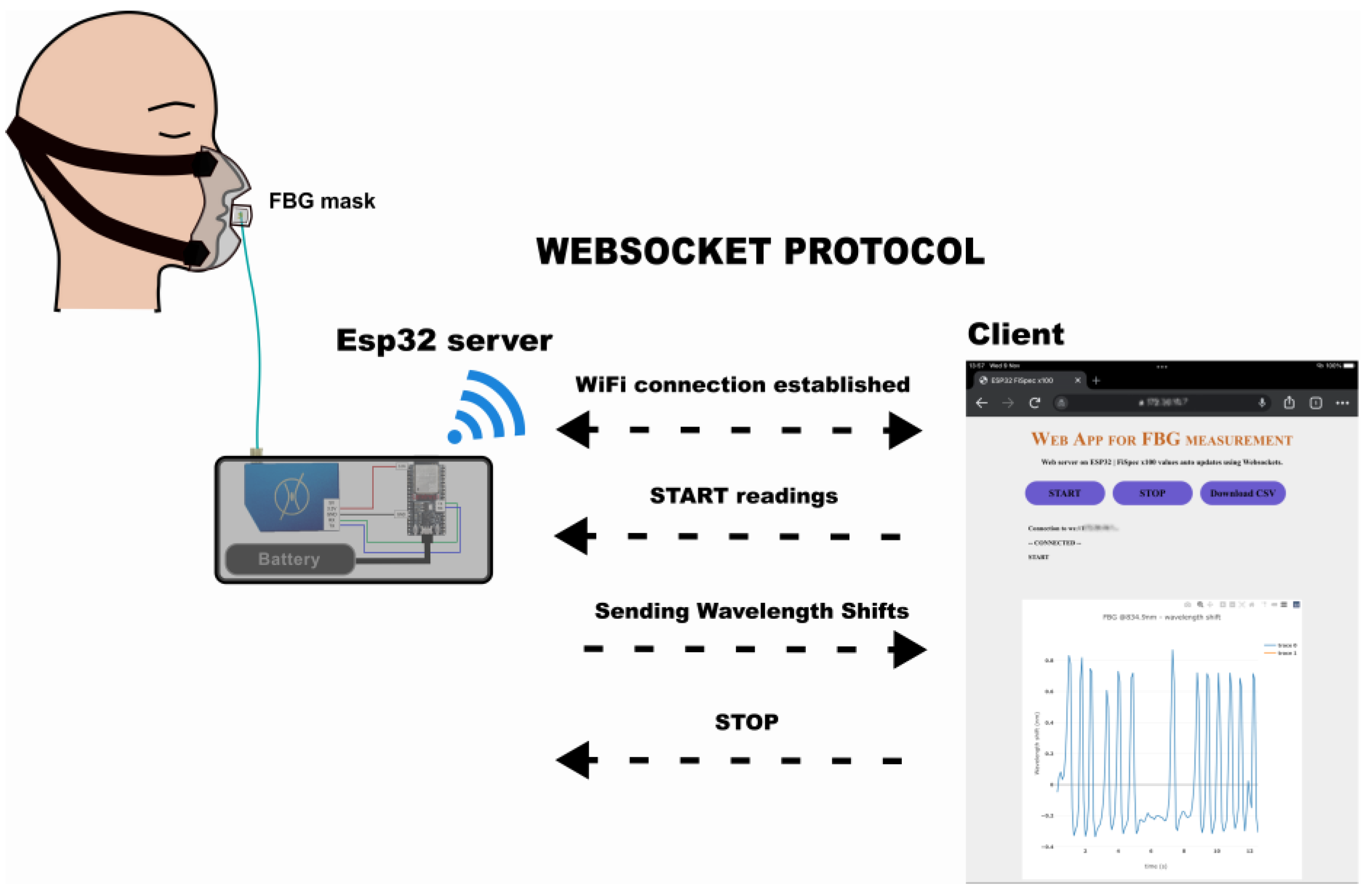
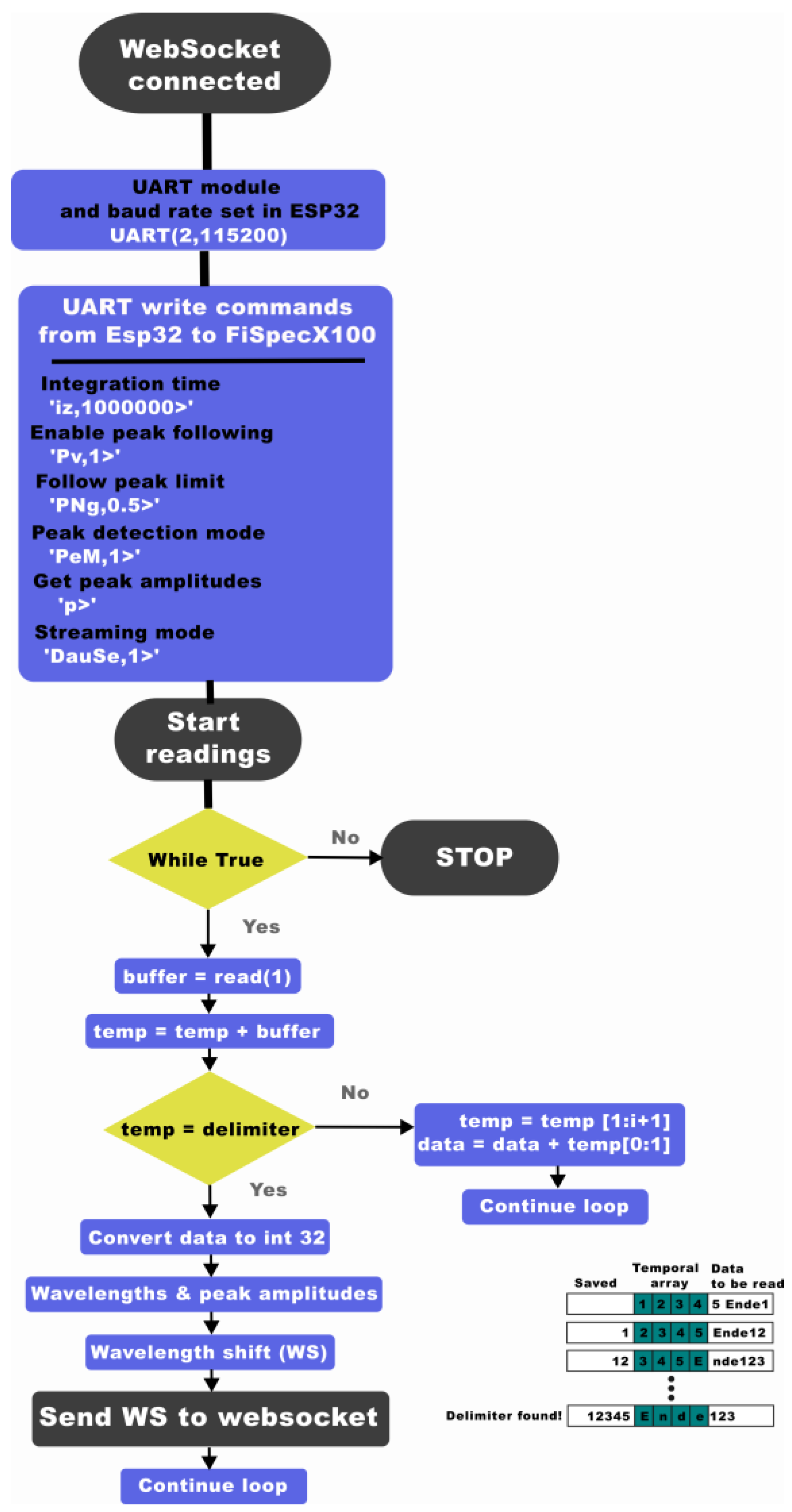
2.3. Miniature Interrogator Instrumentation
2.4. Volunteer Study
2.4.1. Setup
2.4.2. Protocol
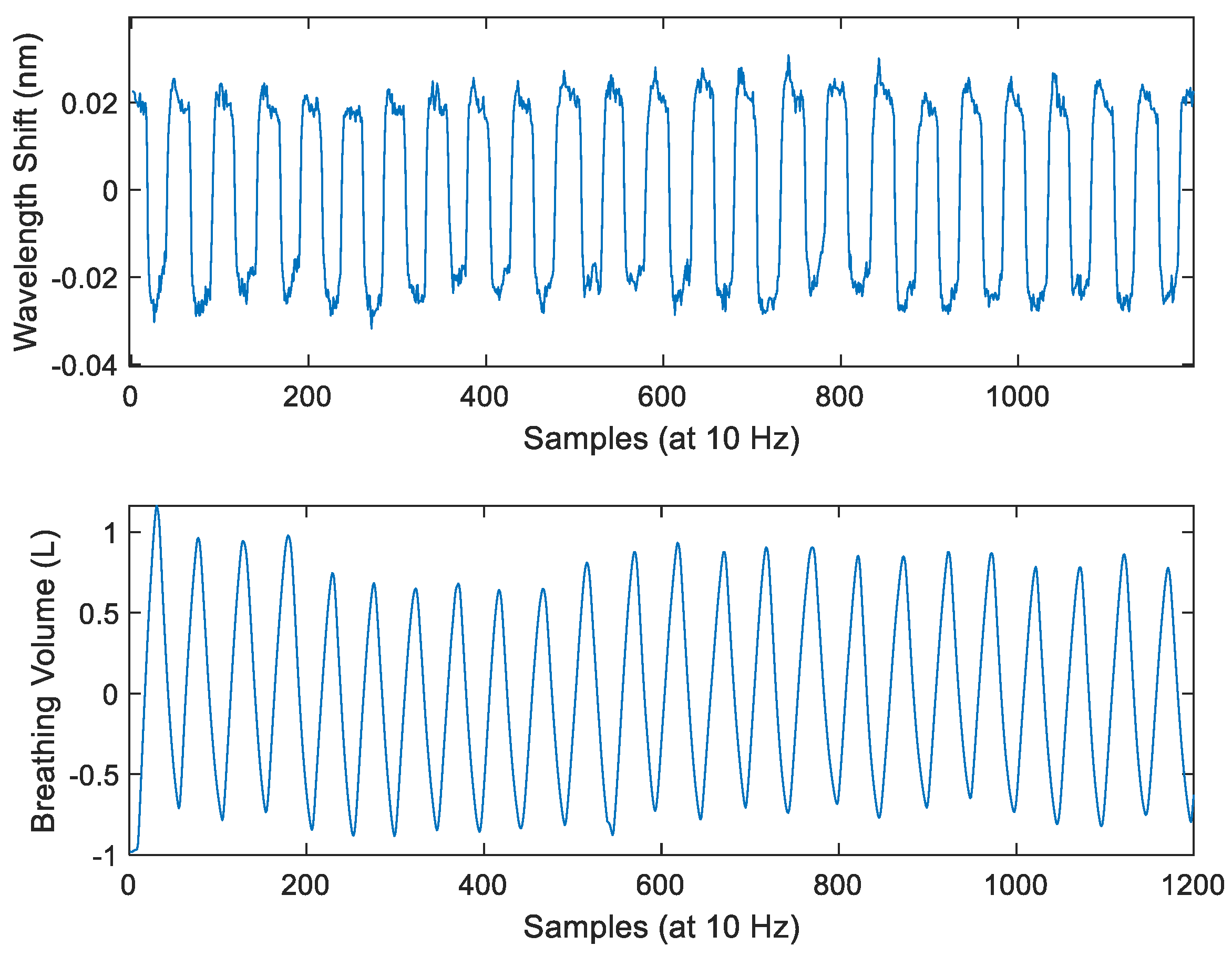
2.4.3. Data Processing and Interpretation
3. Results
4. Discussions
- (1)
- Effect of mask structures and filters—during mining and factory work, workers are expected to wear industrial-grade respirator masks fitted with filters (such as P100, Honeywell International, Charlotte, NC, USA) to protect them from airborne harmful substances. They also typically have separate valves for inhalation and exhalation (for example, PD-101 Full-Face Industrial Air Purifying Respirator, Parcil Safety, Evansville, IN, USA). In some cases, a dedicated electric-powered portable air purifying unit is also included to pump filtered air into the mask (for example, Versaflo TR-800, 3M, Maplewood, MN, USA). These features can introduce different air movements and turbulence patterns than the respirator mask currently used in the Mask System. Thus, this investigation will include studying the effect of filter addition to the respirator mask airway, different airway structures, and different FBG placements in these structures to find the placement protocol that produces the greatest ratio of FBG sensitivity to breath-induced temperature variation and the noise from air turbulence.
- (2)
- FBG pressure sensor—in the environment where the ambient temperature is high or varies greatly (such as during underground mining where workers can face temperatures up to 37 °C [48]), the temperature of the exhaled breath and surrounding air can be comparable, making it difficult to identify the peak in the signal with only the findpeaks function. A solution to this is to incorporate an FBG pressure sensor into the Mask System to detect pressure changes due to inhalation and exhalation [49]. If ambient air temperature and breath temperature are significantly different, then the temperature sensor is advantageous to the pressure sensor in detecting inhalation and exhalation during quiet breathing, where the pressure drop is low due to small airflow [50]. On the other hand, in the case of minor temperature differences (causing less wavelength shift than the Mask System’s noise shown in Figure 8a, pressure measurement data can be used to assist the breath detection algorithm. Thus, research to design the FBG air pressure sensor and placement within the mask for accurate breath detection is required.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brekke, I.J.; Puntervoll, L.H.; Pedersen, P.B.; Kellett, J.; Brabrand, M. The value of vital sign trends in predicting and monitoring clinical deterioration: A systematic review. PLoS ONE 2019, 14, e0210875. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, A.; Massaroni, C.; Schena, E.; Sacchetti, M. The Importance of Respiratory Rate Monitoring: From Healthcare to Sport and Exercise. Sensors 2020, 20, 6396. [Google Scholar] [CrossRef] [PubMed]
- Loring, S.H.; Garcia-Jacques, M.; Malhotra, A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J. Appl. Physiol. 2009, 107, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Mazza, J. Asthma. Allergy Asthma Clin. Immunol. 2011, 7 (Suppl. S1), S2. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Luo, Y.; Song, M.; Liu, Y.; Shu, T.; Liu, Y.; Pang, J.; Wang, J.; Wang, C. Pneumoconiosis: Current status and future prospects. Chin. Med. J. 2021, 134, 898–907. [Google Scholar] [CrossRef]
- Curone, D.; Secco, E.L.; Caldani, L.; Lanatà, A.; Paradiso, R.; Tognetti, A.; Magenes, G. Assessment of Sensing Fire-Fighters Uniforms for Physiological Parameters Measurement in Harsh Environment. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 501–511. [Google Scholar] [CrossRef]
- Aqueveque, P.; Gutierrez, C.; Rodriguez, F.S.; Pino, E.J.; Morales, A.S.; Wiechmann, E. Monitoring Physiological Variables of Mining Workers at High Altitude. IEEE Trans. Ind. Appl. 2017, 53, 2628–2634. [Google Scholar] [CrossRef]
- Que, C.; Kolmaga, C.; Durand, L.; Kelly, S.; Macklem, P.T. Phonospirometry for noninvasive measurement of ventilation: Methodology and preliminary results. J. Appl. Physiol. 2002, 93, 1515–1526. [Google Scholar] [CrossRef]
- Bates, A.; Ling, M.J.; Mann, J.; Arvind, D.K. Respiratory Rate and Flow Waveform Estimation from Tri-axial Accelerometer Data. In Proceedings of the 2010 International Conference on Body Sensor Networks, Singapore, 7–9 June 2010; pp. 144–150. [Google Scholar]
- Fekr, A.R.; Radecka, K.; Zilic, Z. Tidal volume variability and respiration rate estimation using a wearable accelerometer sensor. In Proceedings of the 4th International Conference on Wireless Mobile Communication and Healthcare—Transforming Healthcare Through Innovations in Mobile and Wireless Technologies (MOBIHEALTH), Athens, Greece, 3–5 November 2014; pp. 1–6. [Google Scholar]
- Aqueveque, P.; Gómez, B.; Monsalve, E.; Germany, E.; Ortega-Bastidas, P.; Dubo, S.; Pino, E.J. Simple Wireless Impedance Pneumography System for Unobtrusive Sensing of Respiration. Sensors 2020, 20, 5228. [Google Scholar] [CrossRef]
- Kwon, H.; Ikeda, K.; Kim, S.; Thiele, R.H. Non-contact thermography-based respiratory rate monitoring in a post-anesthetic care unit. J. Clin. Monit. Comput. 2021, 35, 1291–1297. [Google Scholar] [CrossRef]
- Hayward, N.; Shaban, M.; Badger, J.; Jones, I.; Wei, Y.; Spencer, D.; Isichei, S.; Knight, M.; Otto, J.; Rayat, G.; et al. A capaciflector provides continuous and accurate respiratory rate monitoring for patients at rest and during exercise. J. Clin. Monit. Comput. 2022, 36, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Dawood, J.; Muller, M.; Carlson, C.S. “Are you breathing?”—Design, build and testing of a low-cost, portable respiratory rate monitor. Curr. Dir. Biomed. Eng. 2022, 8, 109–112. [Google Scholar] [CrossRef]
- Berkebile, J.A.; Mabrouk, S.A.; Ganti, V.G.; Srivatsa, A.V.; Sanchez-Perez, J.A.; Inan, O.T. Towards Estimation of Tidal Volume and Respiratory Timings via Wearable-Patch-Based Impedance Pneumography in Ambulatory Settings. IEEE Trans. Biomed. Eng. 2022, 69, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Massaroni, C.; Lopes, D.S.; Presti, D.L.; Schena, E.; Silvestri, S. Contactless Monitoring of Breathing Patterns and Respiratory Rate at the Pit of the Neck: A Single Camera Approach. J. Sens. 2018, 11, 4567213. [Google Scholar] [CrossRef]
- Bhongade, A.; Gupta, R.; Gandhi, T.K.; Ap, P. A Portable Low-Cost Respiration Rate Measurement System for Sleep Apnea Detection. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–5. [Google Scholar]
- Hwang, H.; Lee, K.; Lee, E. A Real-Time Remote Respiration Measurement Method with Improved Robustness Based on a CNN Model. Appl. Sci. 2022, 12, 11603. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Hossain, S.N.; Jin, C.; Singh, A.K.; Nguyen, M.T.D.; Deverell, L.; Nguyen, V.; Gates, F.S.; Fernandez, I.G.; Melencio, M.V.; et al. An investigation into the effectiveness of using acoustic touch to assist people who are blind. PLoS ONE 2023, 18, e0290431. [Google Scholar] [CrossRef]
- Shafiq, G.; Veluvolu, K.C. Multimodal chest surface motion data for respiratory and cardiovascular monitoring applications. Sci. Data 2017, 4, 170052. [Google Scholar] [CrossRef]
- Hamouda, S.; Amneenah, N. Electromagnetic Interference Impacts on Electronic Systems and Regulations. Int. J. Adv. Multidiscip. Res. Stud. 2024, 4, 124–127. [Google Scholar]
- Yan, Y.; Cao, W. The mining method of anti-electromagnetic interference for electronic equipment in coal mine by considering network communication technology. Microelectron. J. 2021, 109, 104987. [Google Scholar] [CrossRef]
- McIntyre, W.F.; Michael, K.A.; Baranchuk, A. Electromagnetic interference induced by magnetic resonance imaging. Can. J. Cardiol. 2010, 26, e64. [Google Scholar] [CrossRef]
- Zhou, C.; Reyes, M.; Girman, M. Electromagnetic Interference (EMI) In Underground Coal Mines: A Literature Review and Practical Considerations. Min. Metall. Explor. 2022, 39, 421–431. [Google Scholar] [CrossRef]
- Zhuang, Y.; Han, T.; Yang, Q.; O’Malley, R.; Kumar, A.; Gerald, R.E.; Huang, J. A Fibre-Optic Sensor-Embedded and Machine Learning Assisted Smart Helmet for Multi-Variable Blunt Force Impact Sensing in Real Time. Biosensors 2022, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.J.; Jang, K.W.; Seo, J.K.; Heo, J.Y.; Moon, J.S.; Jun, J.H.; Park, J.Y.; Lee, B.S. Development of optical fibre-based respiration sensor for noninvasive respiratory monitoring. Opt. Rev. 2011, 18, 132–138. [Google Scholar] [CrossRef]
- Dziuda, L.; Krej, M.; Skibniewski, F.W. Fibre Bragg Grating Strain Sensor Incorporated to Monitor Patient Vital Signs During MRI. IEEE Sens. J. 2013, 13, 4986–4991. [Google Scholar] [CrossRef]
- Elsarnagawy, T. A Simultaneous and Validated Wearable FBG Heartbeat and Respiration Rate Monitoring System. Sens. Lett. 2015, 13, 48–51. [Google Scholar] [CrossRef]
- Presti, D.L.; Massaroni, C.; Schena, P.S.E.; Formica, D.; Caponero, M.A.; Tomaso, G.D. Smart textile based on FBG sensors for breath-by-breath respiratory monitoring: Tests on women. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–6. [Google Scholar]
- Sinha, R.; Hernandez, F.U.; He, C.; Korposh, S.; Correia, R.; Norris, A.M.; Liu, C.; Hayes-Gill, B.R.; Morgan, S.P. Development and validation of a novel fibre-optic respiratory rate sensor (FiRRS) integrated in oxygen delivery devices. J. Phys. D Appl. Phys. 2021, 54, 124002. [Google Scholar] [CrossRef]
- Di Tocco, J.; Presti, D.L.; Zaltieri, M.; D’Alesio, G.; Filosa, M.; Massari, L.; Aliperta, A.; Rienzo, M.D.; Carrozza, M.C.; Ferrarin, M.; et al. A Wearable System Based on Flexible Sensors for Unobtrusive Respiratory Monitoring in Occupational Settings. IEEE Sens. J. 2021, 21, 14369–14378. [Google Scholar] [CrossRef]
- Tavares, C.; Leitão, C.; Presti, D.L.; Domingues, M.F.; Alberto, N.; Silva, H.; Antunes, P. Respiratory and heart rate monitoring using an FBG 3D-printed wearable system. Biomed. Opt. Express 2022, 13, 2299–2311. [Google Scholar] [CrossRef]
- He, C.; Wang, Q.; Qu, Z. Research Progress of Electromagnetic Shielding Materials. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 032027. [Google Scholar] [CrossRef]
- Vegfors, M.; Ugnell, H.; Hök, B.; Öberg, Å.; Lennmarken, C. Experimental evaluation of two new sensors for respiratory rate monitoring. Physiol. Meas. 1993, 14, 171–181. [Google Scholar] [CrossRef]
- Roudjane, M.; Bellemare-Rousseau, S.; Khalil, M.; Gorgutsa, S.; Miled, A.; Messaddeq, Y. A Portable Wireless Communication Platform Based on a Multi-Material Fibre Sensor for Real-Time Breath Detection. Sensors 2018, 18, 973. [Google Scholar] [CrossRef] [PubMed]
- Othonos, A.; Kalli, K.; Pureur, D.; Mugnier, A. Fibre Bragg Gratings. In Wavelength Filters in Fibre Optics; Springer: Berlin/Heidelberg, Germany, 2006; pp. 189–269. [Google Scholar]
- Li, Z.; Dong, B.; Chen, E.; Li, Y.; Zhao, W.; Wang, Y.; Gao, C. High sensitivity FBG humidity sensor coated with graphene and polyimide films. Opt. Fibre Technol. 2021, 66, 102635. [Google Scholar] [CrossRef]
- Mihailov, S.J. Fibre Bragg Grating Sensors for Harsh Environments. Sensors 2012, 12, 1898–1918. [Google Scholar] [CrossRef] [PubMed]
- Tahir, B.A.; Ali, J.; Rahman, R.A. Fabrication of fibre grating by phase mask and its sensing application. J. Optoelectron. Adv. Mater. 2006, 8, 1604–1609. [Google Scholar]
- Hill, B.; Annesley, S.H. Monitoring respiratory rate in adults. Br. J. Community Nurs. 2020, 29, 12–16. [Google Scholar] [CrossRef]
- Popov, T.A.; Dunev, S.; Kralimarkova, T.Z.; Kraeva, S.; DuBuske, L.M. Evaluation of a simple, potentially individual device for exhaled breath temperature measurement. Respir. Med. 2007, 101, 2044–2050. [Google Scholar] [CrossRef]
- Svensson, H.; Nilsson, D.; Bjermer, L.; Tufvesson, E. Exhaled Breath Temperature Increases after Exercise in Asthmatics and Controls Subject. Respiration 2012, 84, 283–290. [Google Scholar] [CrossRef]
- Anghel, M.; Iacobescu, F. The Influence of Temperature and CO2 in Exhaled Breath. In Proceedings of the 16th International Congress of Metrology, Paris, France, 7–10 October 2013; p. 10012. [Google Scholar]
- Pleil, J.D.; Wallace, M.A.G.; Davis, M.D.; Matty, C.M. The physics of human breathing: Flow, timing, volume, and pressure parameters for normal, on-demand, and ventilator respiration. J. Breath. Res. 2021, 15, 042002. [Google Scholar] [CrossRef]
- Henricson, J.; Glasin, J.; Rindebratt, S.; Wilhelms, D. Respiratory rate monitoring in healthy volunteers by central photoplethysmography compared to capnography. J. Biophotonics 2022, 15, e202100270. [Google Scholar] [CrossRef]
- Troyee, T.G.; Gani, M.M.; Hasan, M. Design and Implementation of Low-Cost Respiratory Rate Measurement Device. Arab. J. Sci. Eng. 2022, 49, 6959–6969. [Google Scholar] [CrossRef]
- Goldfine, C.E.; Oshim, M.F.T.; Chapman, B.P.; Ganesan, D.; Rahman, T.; Carreiro, S.P. Contactless Monitoring System Versus Gold Standard for Respiratory Rate Monitoring in Emergency Department Patients: Pilot Comparison Study. JMIR Form. Res. 2024, 8, e44717. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, S.; Cheng, J.; Li, S.; Li, M. An Underground Air-Route Temperature Prediction Model for Ultra-Deep Coal Mines. Minerals 2015, 5, 527–545. [Google Scholar] [CrossRef]
- Pant, S.; Umesh, S.; Asokan, S. Fibre Bragg Grating Respiratory Measurement Device. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–5. [Google Scholar]
- Monjezi, M.; Jamaati, H. The effects of face mask specifications on work of breathing and particle filtration efficiency. Med. Eng. Phys. 2021, 98, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, Y.; Xu, S.; Liu, H.; Li, X. Fibre Bragg Grating-Based Smart Garment for Monitoring Human Body Temperature. Sensors 2022, 22, 4252. [Google Scholar] [CrossRef]
- Ghafoori, M.; Clevenger, C.; Abdallah, M.; Rens, K. Heart rate modeling and prediction of construction workers based on physical activity using deep learning. Autom. Constr. 2023, 155, 105077. [Google Scholar] [CrossRef]
- Nasirzadeh, F.; Karmakar, C.; Habib, A.; Neelangal, K.B.; Mir, M.; Lee, S.; Arneld, T. Continuous monitoring of body temperature for objective detection of health and safety risks in construction sites: An analysis of the accuracy and comfort of off-the-shelf wearable sensors. Heliyon 2024, 10, e26947. [Google Scholar] [CrossRef]
- Carmo, J.P.; da Silva, A.M.F.; Rocha, R.P.; Correia, J.H. Application of Fibre Bragg Gratings to Wearable Garments. IEEE Sens. J. 2012, 12, 261–266. [Google Scholar] [CrossRef]
- Diaz, C.A.R.; Leal-Junior, A.G.; Avellar, L.M.; Antunes, P.F.C.; Pontes, M.J.; Marques, C.A.; Frizera, A.; Ribeiro, M.R.N. Perrogator: A Portable Energy-Efficient Interrogator for Dynamic Monitoring of Wavelength-Based Sensors in Wearable Applications. Sensors 2019, 19, 2962. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Y.; Zhao, Q.; Li, Y.; Li, H.; Qu, J.; Zhang, D. A Miniaturized, Low-Cost and Portable Fibre Bragg Grating Interrogation System for Remote Monitoring. Optik 2021, 248, 168054. [Google Scholar]
- Li, H.; Yang, H.; Li, E.; Liu, Z.; Wei, K. Wearable sensors in intelligent clothing for measuring human body temperature based on optical fibre Bragg grating. Opt. Express 2012, 20, 11740–11752. [Google Scholar] [CrossRef]




| Author, Reference, and Year | Operation Principle | n | Portable | Performance/Error | Limitations |
|---|---|---|---|---|---|
| Bates et al., [9], 2010 | Accelerometer (chest wall angular displacement) | 1 | No | RMSE: 0.38 BPM (3 BPM peak error) | Prone to respiration-irrelevant motion artefacts |
| Curone et al., [6], 2012 | Piezoelectric sensor strap (voltage variation due to respiration-induced stress) | 6 | No | BA: bias range = −5 to 0 BPM, max upper LOA = 11, min lower LOA = −17 | |
| Fekr et al., [10], 2018 | Accelerometer (chest wall 3D movement) | 8 | No | Mean CC: 0.85 BPM, SD not given | |
| Massaroni et al., [16], 2018 | RGB video analysis | 12 | No | BA: −0.03 BPM bias (−3.51 to 3.46 BPM LOA) | Prone to interference due to camera obstructions and respiration-irrelevant motion artefacts |
| Aqueveque et al., [11], 2020 | Thoracic impedance plethysmography (four electrodes, two on each hemithorax) | 15 | No | BA: right thorax (−0.18 BPM bias, −2.96 to 2.6 LOA), left thorax (−0.52 BPM bias, −3.08 to 2.07 LOA) | Prone to inaccuracies due to motion artefacts and breathing movement pattern variation |
| Kwon et al., [12], 2020 | Thermography imaging (respiration-induced temperature variation near nostril) | 101 | No | BA: −0.15 BPM bias (2.65 to −2.92 LOA) | Prone to interference due to camera obstructions and other movements |
| Hayward et al., [13], 2022 | Capaciflector (chest wall motion) | 61 | No | BA: static (bias range = 0.95 to 0.85 BPM, min lower LOA = −2.30, max upper LOA = 3.90); exercise (bias range = 0.31 to 0.32 BPM, min lower LOA = −3.51, max upper LOA = 3.36) | Prone to cross-talks due to moisture buildup in the sensing element due to skin humidity and sweating |
| Dawood et al., [14], 2022 | Thermistor mask (respiration-induced temperature variation near nostril) | 5 | Yes | MPE: 5.6% (SD not reported) | Prone to EM-induced self-heating |
| Bhongade et al., [17], 2023 | Inertial measurement unit (three units placed on chest, abdomen, and lateral left rib) | 3 | Yes | BREA: 97.50 ± 1.17% (static seated); 96.84 ± 1.76% (seated working); and 95.17 ± 2.66% (static supine) | Prone to respiration-irrelevant motion artefacts |
| Author, Reference, and Year | Operation Principle | n | Portable | Performance/Error | Limitations |
|---|---|---|---|---|---|
| Vegfors et al., [34], 1993 | Boundary reflection | 10 | No | MPE: spontaneous rate (0.1%, 1.91% SD), fixed rate (0%, 0.67% SD) | Prone to respiration-irrelevant motion artefacts |
| Yoo et al., [26], 2011 | Polycrystalline optical fibre, thermochromic microcapsule pigment (respiration-induced temperature variation near nostril) | 1 | No | No comparison with reference devices reported | Prone to respiration-irrelevant motion artefacts, discomfort due to placement requirements (directly on skin between nose and upper lip via adhesive) |
| Dziuda et al., [27], 2013 | FBG-embedded plexiglass bed (torso motion) | 3 | No | BA: 0.00 BPM bias (–0.42 to 0.43 LOA) | Applicable only for static supine position |
| Elsarnagawy, [28], 2015 | FBG-embedded strap (horizontal chest strain) | 10 | No | Exact match with manual counting | Prone to respiration-irrelevant motion artefacts, placement-induced discomfort (directly over mammilla) |
| Presti et al., [29], 2018 | FBG array (body strain) | 8 | No | PE: 0.22% mean (0.12% SD) | Prone to respiration-irrelevant motion artefacts |
| Sinha et al., [30], 2021 | FBG (respiration-induced temperature variation near nostril) | 18 | No | MPA: 88.1% (2.9% SD) | Interpretation algorithm undermines breathing frequency variation during measurements |
| Di Tocco et al., [31], 2021 | FBG (horizontal chest and abdomen strain) | 10 | No | MAPE: 2.34% (1.88% SD) | Prone to respiration-irrelevant motion artefacts |
| Tavares et al., [32], 2022 | FBG (horizontal chest strain) | 3 | No | Exact match with reference RR | |
| Roudjane et al., [35], 2018 | Hollow-core silica-based fibre array bending (horizontal chest strain) | 4 | Yes | No comparison with reference devices reported | Prone to respiration-irrelevant motion artefacts |
| Volunteer ID | Mask System Breath Count (First, Second, Third, Fourth, Fifth Experiment Repetitions) | Reference Spirometer Breath Count (First, Second, Third, Fourth, Fifth Experiment Repetitions) |
|---|---|---|
| 18131 | 24, 25, 21, 23, 23 | 24, 25, 21, 23, 23 |
| 20609 | 37, 38, 39, 38, 38 | 37, 38, 39, 38, 38 |
| 56464 | 37, 37, 35, 34, 31 | 37, 37, 35, 34, 31 |
| 57384 | 33, 33, 28, 28, 27 | 33, 33, 28, 28, 27 |
| 63350 | 19, 23, 22, 23, 20 | 19, 23, 22, 23, 20 |
| 65714 | 31, 31, 29, 31, 31 | 31, 31, 29, 31, 31 |
| 66409 | 20, 24, 19, 20, 22 | 20, 24, 19, 20, 22 |
| 77711 | 20, 20, 20, 20, 19 | 20, 20, 20, 20, 19 |
| 89277 | 36, 33, 40, 37, 41 | 36, 33, 40, 37, 41 |
| 95543 | 25, 33, 34, 35, 34 | 25, 33, 34, 35, 34 |
| System | No. of Channels | Max No. of FBG per Channel | Sampling Rate (hz) | Wavelength Range (nm) | Resolution (nm) | Dimension (mm × mm × mm) | Weight (g) | Power Consumption (W) | Battery-Powered | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mask System | 1 | 30 | 10 | 808–880 | 0.1 | 155 × 86 × 65 | 374 | 1.2 | Yes | |
| Commercial | SmartScan (Smartfibres, Bracknell, UK) | 4 | 16 | 2500 | 1528–1568 | 0.1 | 140 × 115 × 85 | 900 | 8.5 | No |
| LCM-2708 (Fibrestrike, Twinsburg, OH, USA) | 8 | 8 | 19,200 | 1515–1585 | 1 | 170 × 99 × 361 | 4536 | NR | Yes | |
| FSI S16PC (Femto Sensing, Atlanta, GA, USA) | 16 | 30 | 1 | 1510–1590 | 1 | 235 × 202 × 120 | 3500 | 18 | No | |
| Hyperion Si255 (Luna Innovations, Roanoke, VA, USA) | 16 | * | 5000 | 1460–1620 | NR | 307 × 274 × 69 | 4900 | 40 | No | |
| Novel | Carmo et. al. (2012) [54] | 1 | 16 | 36 | 1529–1561 | 1 | NR * | 250 | 1 | NR |
| Diaz et. al. (2019) [55] | 1 | * | 213 | 1522–1567 | 3.82 | 145 × 85 × 45 | 300 | 3 | Yes | |
| Tian et. al. (2021) [56] | 4 | * | 4000 | 1510–1595 | 0.1 | NR | NR | 6 | NR | |
| Li et. al. (2012) [57] | 1 | 3 | 500 | 1540–1560 | 0.08 | NR * | 10 | NR | Yes | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limweshasin, N.; Castro, I.A.; Korposh, S.; Morgan, S.P.; Hayes-Gill, B.R.; Faghy, M.A.; Correia, R. Respiratory Rate Monitoring via a Fibre Bragg Grating-Embedded Respirator Mask with a Wearable Miniature Interrogator. Sensors 2024, 24, 7476. https://doi.org/10.3390/s24237476
Limweshasin N, Castro IA, Korposh S, Morgan SP, Hayes-Gill BR, Faghy MA, Correia R. Respiratory Rate Monitoring via a Fibre Bragg Grating-Embedded Respirator Mask with a Wearable Miniature Interrogator. Sensors. 2024; 24(23):7476. https://doi.org/10.3390/s24237476
Chicago/Turabian StyleLimweshasin, Nat, Itzel Avila Castro, Serhiy Korposh, Stephen P. Morgan, Barrie R. Hayes-Gill, Mark A. Faghy, and Ricardo Correia. 2024. "Respiratory Rate Monitoring via a Fibre Bragg Grating-Embedded Respirator Mask with a Wearable Miniature Interrogator" Sensors 24, no. 23: 7476. https://doi.org/10.3390/s24237476
APA StyleLimweshasin, N., Castro, I. A., Korposh, S., Morgan, S. P., Hayes-Gill, B. R., Faghy, M. A., & Correia, R. (2024). Respiratory Rate Monitoring via a Fibre Bragg Grating-Embedded Respirator Mask with a Wearable Miniature Interrogator. Sensors, 24(23), 7476. https://doi.org/10.3390/s24237476








