Noninvasive Early Detection of Systemic Inflammatory Response Syndrome of COVID-19 Inpatients Using a Piezoelectric Respiratory Rates Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. System Configuration of a 40-min Frequency Distribution of Respiratory Rates (M40FD-RR) SIRS Monitor
2.1.1. Hardware Composition
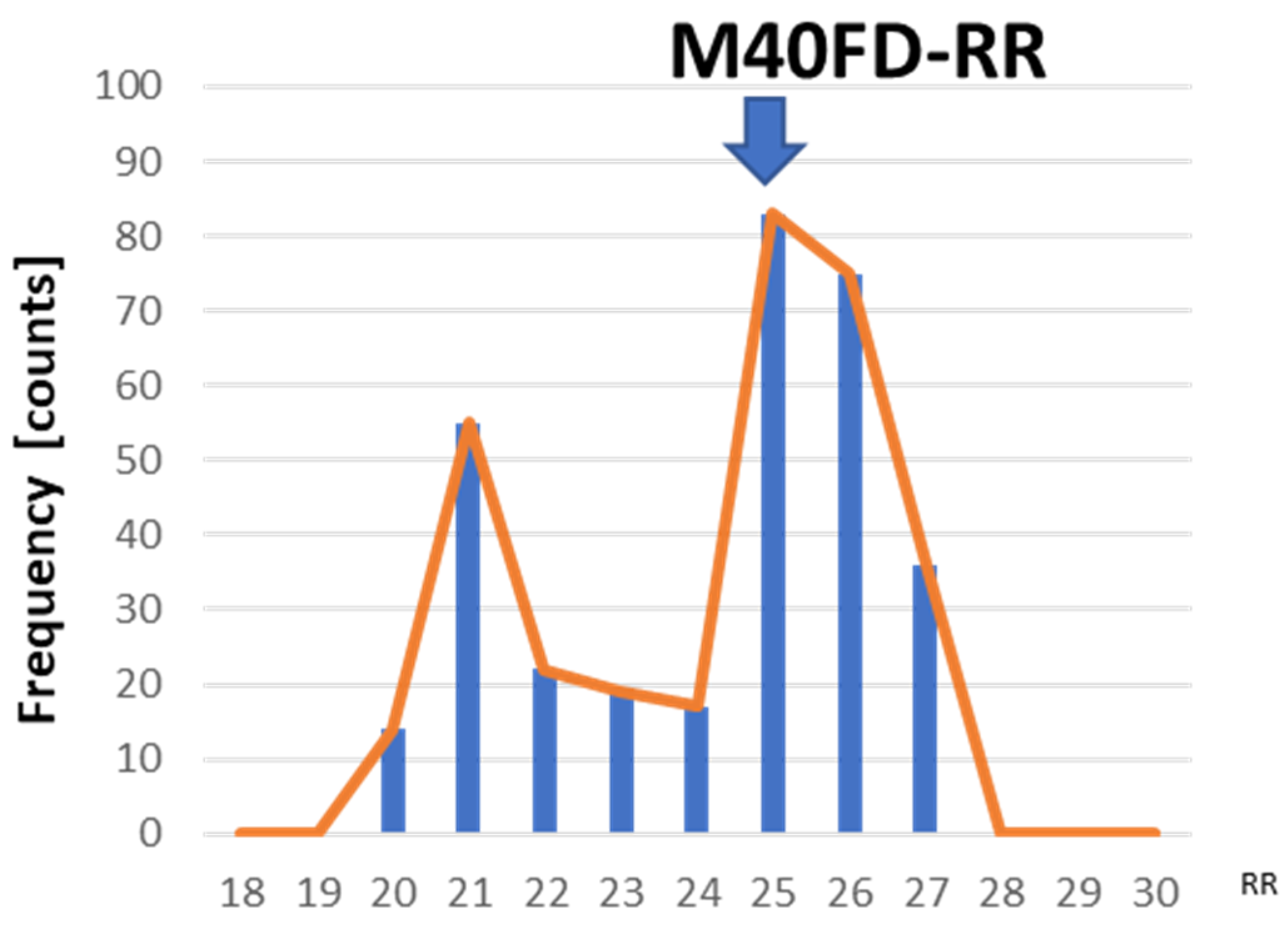
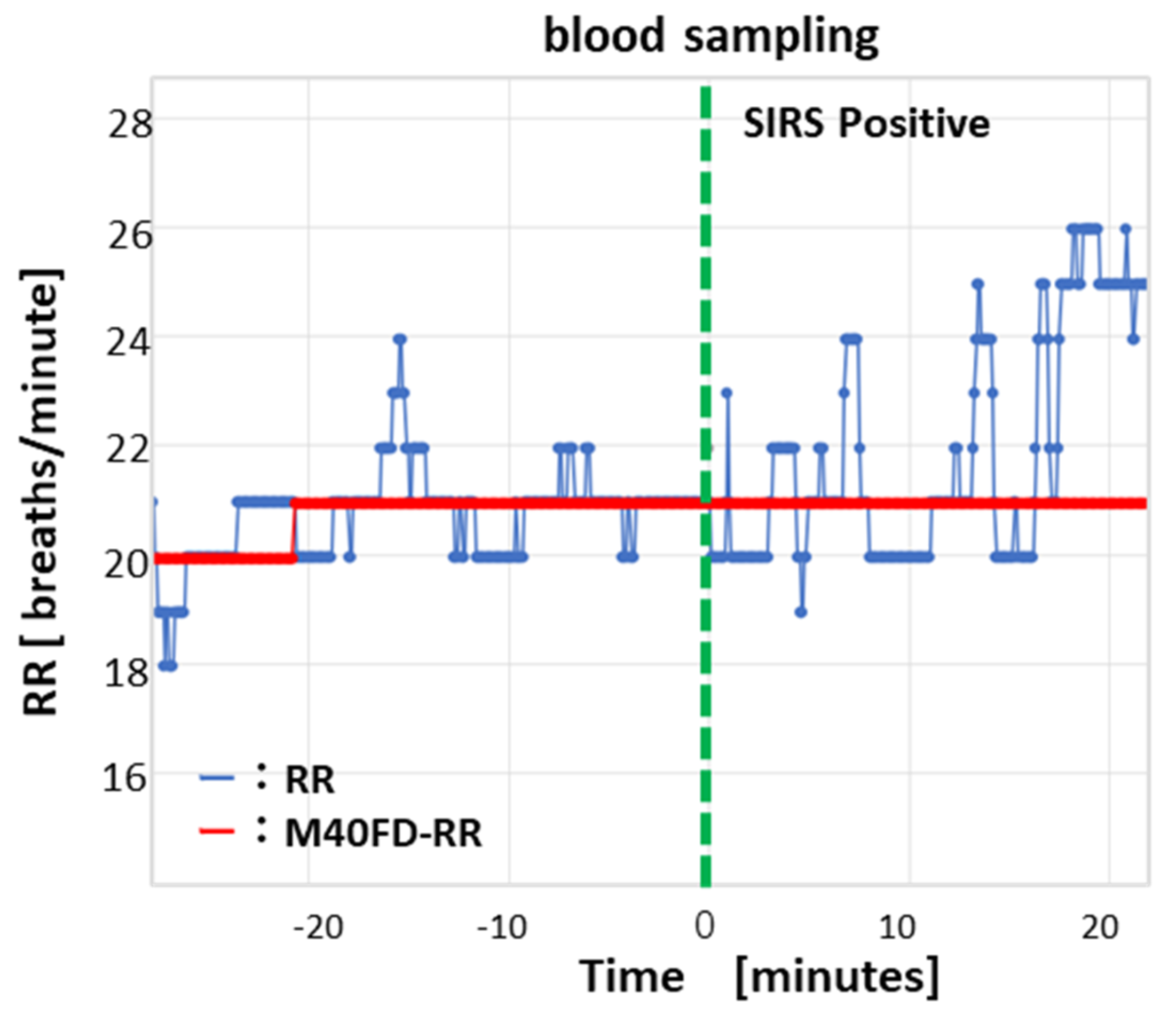
2.1.2. Data Processing Algorithm of the M40FD-RR SIRS Monitor
2.2. Clinical Testing Method
2.2.1. Patient Recruitment, Exclusion Criteria, and Nurse Records
2.2.2. Clinical Testing of the M40FD-RR SIRS Monitor
2.2.3. Sample Size and Outcome
3. Results
3.1. Bland–Altman Plots of M40FD-RR and Conventional RR
3.2. Classification Accuracy of the M40FD-RR SIRS Monitor
4. Discussion
4.1. Promotion of Early Testing for SIRS and Reduction in Patient Burden
4.2. Non-Contact HR Measurement to Reduce Patient Burden
4.3. Reduction in the White Coat Effect by M40FD-RR
4.4. Improvement of Accuracy Through the Addition of Prefrontal Cortex Temperature Measurement
4.5. Selecting the Best Sensor for RR
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gandhi, R.T.; Lynch, J.B.; Rio, C. Mild or Moderate COVID-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Arnaldez, F.I.; O’Day, S.J.; Drake, C.G.; Fox, B.A.; Fu, B.; Urba, W.J.; Montesarchio, V.; Weber, J.S.; Wei, H.; Wigginton, J.M.; et al. The Society for Immunotherapy of Cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response. J. Immunother. Cancer 2020, 8, e000930. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 1992, 20, 864–874. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Serafim, R.; Gomes, J.A.; Salluh, J.; Póvoa, P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome Criteria for the Diagnosis of Sepsis and Prediction of Mortality: A Systematic Review and Meta-Analysis. Chest 2018, 153, 646–655. [Google Scholar] [CrossRef]
- Dagdanpurev, S.; Abe, S.; Sun, G.; Nishimura, H.; Choimaa, L.; Hakozaki, Y.; Matsui, T. A novel machine-learning-based infection screening system via 2013–2017 seasonal influenza patients’ vital-signs as training datasets. J. Infect. 2019, 78, 409–421. [Google Scholar] [CrossRef]
- Matsui, T.; Kobayashi, T.; Hirano, M.; Kanda, M.; Sun, G.; Otake, Y.; Okada, M.; Watanabe, S.; Hakozaki, Y. A pneumonia screening system based on parasympathetic activity monitoring in non-contact way using compact radars beneath the bed mattress. J. Infect. 2020, 81, e142–e144. [Google Scholar] [CrossRef]
- Miller, D.J.; Capodilupo, J.V.; Lastella, M.; Sargent, C.; Roach, G.D.; Lee, V.H.; Capodilupo, E.R. Analyzing changes in respiratory rate to predict the risk of COVID-19 infection. PLoS ONE 2020, 15, e0243693. [Google Scholar] [CrossRef]
- Signore, A.M.; Rescio, G.; Flancioso, L.; Casino, F.; Leone, A. Aluminum nitride thin film piezoelectric pressure sensor for respiratory rate detection. Sensors 2024, 24, 2071. [Google Scholar] [CrossRef]
- Tanaka, H.; Yokose, M.; Takaki, S.; Mihara, T.; Saigusa, Y.; Goto, T. Measurement accuracy of a microwave doppler sensor beneath the mattress as a continuous respiratory rate monitor: A method comparison study. J. Clin. Monit. Comput. 2024, 38, 77–88. [Google Scholar] [CrossRef]
- Konica Minolta VS1. 2024. Available online: https://www.konicaminolta.jp/healthcare/products/vsmonitor/vs1/index.html (accessed on 27 May 2024).
- Clinical Management of COVID-19: Interim Guidance, 27 May 2020. Available online: https://iris.who.int/handle/10665/332196 (accessed on 25 September 2024).
- Japanese COVID-19 Criteria Before 8 May 2023. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000164708_00079.html (accessed on 27 May 2024).
- Granholm, A.; Pedersen, N.E.; Lippert, A.; Petersen, L.F.; Rasmussen, L.S. Respiratory rates measured by a standardised clinical approach, ward staff, and a wireless device. Acta Anaesthesiol. Scand. 2016, 60, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Flenady, T.; Dwyer, T.; Applegarth, J. Accurate respiratory rates count: So should you! Australas. Emerg. Nurs. J. 2017, 20, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef]
- Donavedian, A. Evaluating the Quality of Medical Care. Milbank Q. 2005, 83, 691–729. [Google Scholar] [CrossRef]
- Brent, J.R. Cost-Benefit Analysis versus Cost-Effectiveness Analysis from a Societal Perspective in Healthcare. Int. J. Environ. Res. Public Health. 2023, 20, 4637. [Google Scholar] [CrossRef]
- Saikevicius, L.; Raudonis, V.; Dervinis, G.; Baranauskas, V. Non-Contact Vision-Based Techniques of Vital Sign Monitoring: Systematic Review. Sensors 2024, 24, 3963. [Google Scholar] [CrossRef]
- Addison, S.A.; Cohen, C.; Borg, R.U.; Antunes, A.; Montgomery, D. Accurate and continuous respiratory rate using touchless monitoring technology. Respir. Med. 2023, 220, 107463. [Google Scholar] [CrossRef]
- Katoh, M.; Kanazawa, T.; Abe, Y.; Sun, G.; Matsui, T. Development of a non-contact 15-23cond paediatric respiratory rate monitor using microwave radar and its clinical application. Acta Paediatr. 2023, 112, 493–495. [Google Scholar] [CrossRef]
- Ali, M.; Elsayed, A.; Mendez, A.; Savaria, Y.; Sawan, M. Contact and Remote Breathing Rate Monitoring Techniques: A Review. IEEE Sens. J. 2021, 21, 14569–14586. [Google Scholar] [CrossRef]
- Asai, M.; Inasawa, A.; Matsui, T. Theoretical prediction of temperature difference between prefrontal cortex and forehead skin for fever screening. Therm. Sci. Eng. Prog. 2023, 37, 101595. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, Y.; Duan, Z.; Wu, Y.; Yuan, Z.; Zhang, M.; Tai, H. Ion gradient induced self-powered flexible pressure sensor. Chem. Eng. J. 2024, 490, 151660. [Google Scholar] [CrossRef]
- Dai, J.; Xie, G.; Chen, C.; Liu, Y.; Tai, H.; Jiang, Y.; Su, Y. Hierarchical piezoelectric composite film for self-powered moisture detection and wearable biomonitoring. Appl. Phys. Lett. 2024, 124, 053701. [Google Scholar] [CrossRef]



| Consenting patients * | 30 |
| Excluded patients (%) | 1 (3.3) |
| Patients with 1-day hospital stay (%) ** | 1 (3.3) |
| Number of participants (%) | 29 (96.7) |
| Number of Participants | N = 29 |
|---|---|
| Mean age (year) [interquartile range (IQR)] | 58.2 [15–90] |
| Sex, number (%) | |
| Male | 17 (58.6) |
| Female | 12 (41.4) |
| Mean hospitalization (days) [IQR] | 9.0 [4–20] |
| COVID-19 severity, number (%) | |
| Severe | 2 (6.9) |
| Moderate | 12 (41.4) |
| Mild | 14 (48.3) |
| No symptoms | 1 (3.4) |
| Comorbidity, number (%) | |
| Pneumonia | 16 (55.2) |
| Chronic disease, number (%) | |
| Hypertension | 11 (37.9) |
| Dyslipidemia | 4 (13.8) |
| Diabetes | 3 (10.3) |
| Cardiac arrest | 3 (10.3) |
| Fatty liver | 2 (6.9) |
| Reflux esophagitis | 2 (6.9) |
| Constipation | 2 (6.9) |
| Bronchial asthma | 2 (6.9) |
| Hyponatremia | 1 (3.4) |
| Hypokalemia | 1 (3.4) |
| Primary biliary cirrhosis | 1 (3.4) |
| Hypothyroidism | 1 (3.4) |
| Chronic liver disease | 1 (3.4) |
| Hypercholesteremia | 1 (3.4) |
| Hyperuricemia | 1 (3.4) |
| Mitral valve replacement surgery | 1 (3.4) |
| Atrial fibrillation | 1 (3.4) |
| Gastric ulcer | 1 (3.4) |
| Obesity | 1 (3.4) |
| Liver failure | 1 (3.4) |
| Aortic valve stenosis | 1 (3.4) |
| Obstructive pulmonary disease | 1 (3.4) |
| Thrombocytopenia | 1 (3.4) |
| Leukopenia | 1 (3.4) |
| Insomnia | 1 (3.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, T.; Matsui, T.; Sugita, I.; Tateda, N.; Sato, S.; Hashimoto, K.; Suda, M. Noninvasive Early Detection of Systemic Inflammatory Response Syndrome of COVID-19 Inpatients Using a Piezoelectric Respiratory Rates Sensor. Sensors 2024, 24, 7100. https://doi.org/10.3390/s24227100
Kobayashi T, Matsui T, Sugita I, Tateda N, Sato S, Hashimoto K, Suda M. Noninvasive Early Detection of Systemic Inflammatory Response Syndrome of COVID-19 Inpatients Using a Piezoelectric Respiratory Rates Sensor. Sensors. 2024; 24(22):7100. https://doi.org/10.3390/s24227100
Chicago/Turabian StyleKobayashi, Tsuyoshi, Takemi Matsui, Isamu Sugita, Norihiro Tateda, Shohei Sato, Kenichi Hashimoto, and Masei Suda. 2024. "Noninvasive Early Detection of Systemic Inflammatory Response Syndrome of COVID-19 Inpatients Using a Piezoelectric Respiratory Rates Sensor" Sensors 24, no. 22: 7100. https://doi.org/10.3390/s24227100
APA StyleKobayashi, T., Matsui, T., Sugita, I., Tateda, N., Sato, S., Hashimoto, K., & Suda, M. (2024). Noninvasive Early Detection of Systemic Inflammatory Response Syndrome of COVID-19 Inpatients Using a Piezoelectric Respiratory Rates Sensor. Sensors, 24(22), 7100. https://doi.org/10.3390/s24227100







