Multi-Sensor Soil Probe and Machine Learning Modeling for Predicting Soil Properties
Abstract
1. Introduction
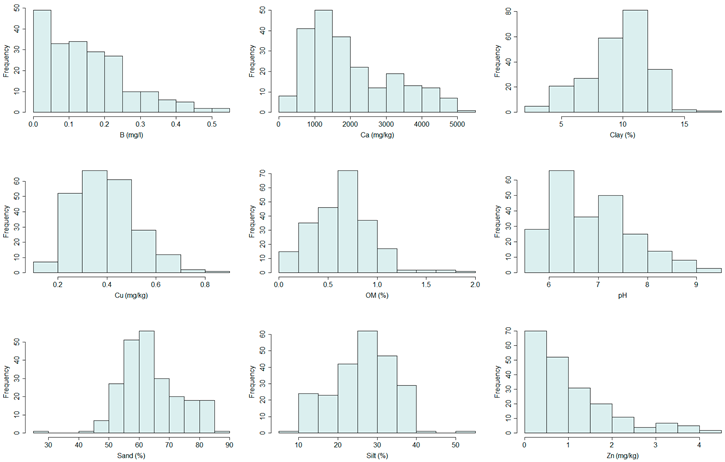
Study Area
2. Materials and Methods
2.1. Digital Soil Core System and Probe
2.1.1. Soil Data Collection
2.1.2. Crop Data Collection
2.2. Data Pre-Processing and Harmonization
2.3. Spectral Data Processing
2.4. Processing of Digital Soil Images
2.5. Processing of Audio Data
2.6. Processing of Other Sensor Data
2.7. Data Feature Selection
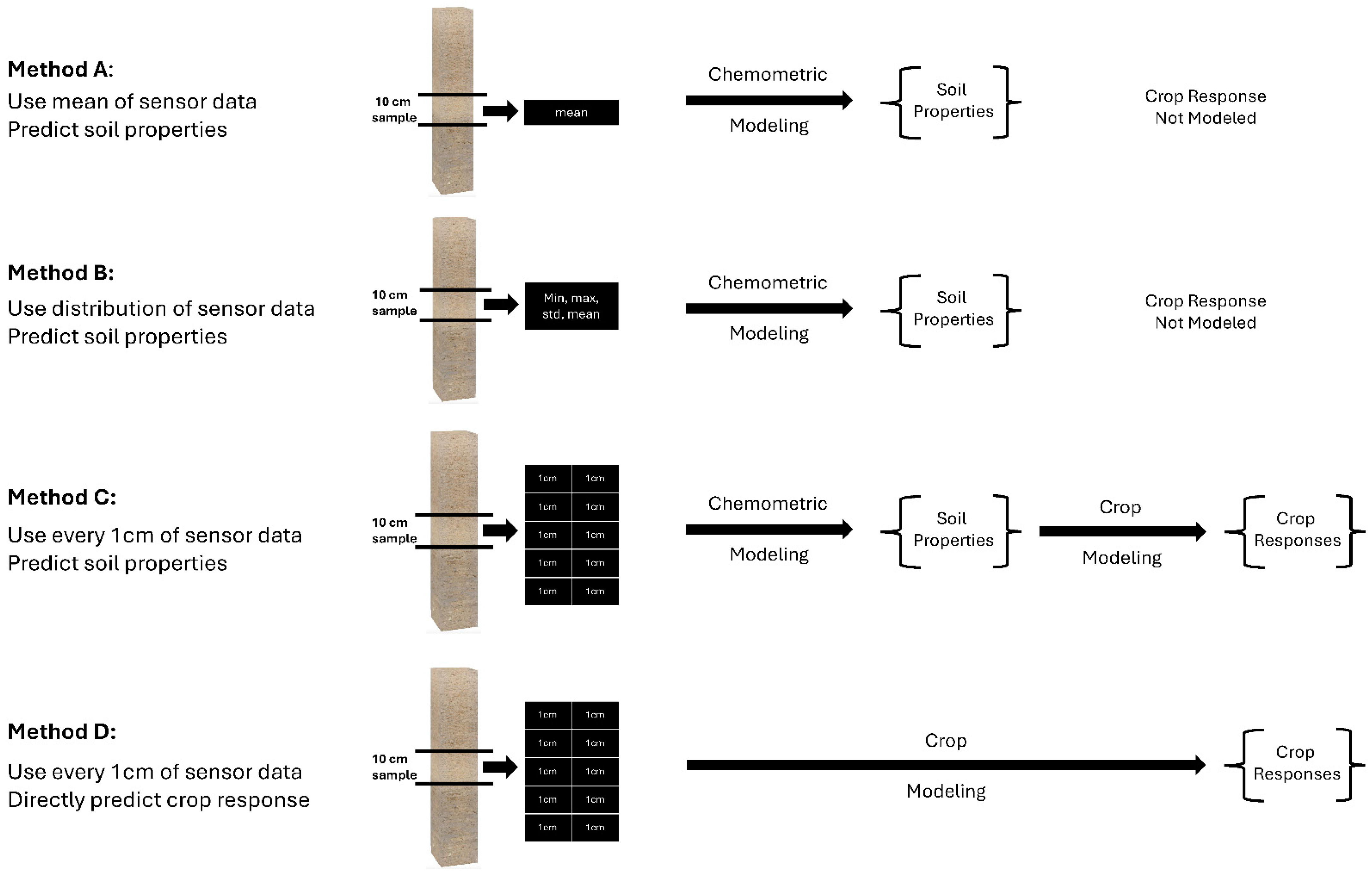
2.8. Comparison of Training Methods
2.9. Modeling Approach
3. Results
3.1. Feature Selections for Modeling
3.2. Predictive Accuracy of Soil Properties Modeling Methods
3.3. In Situ DSC System to Ex Situ Laboratory Properties to DVS Digital Crop Performance vs. DSC System to DVS Digital Crop Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
   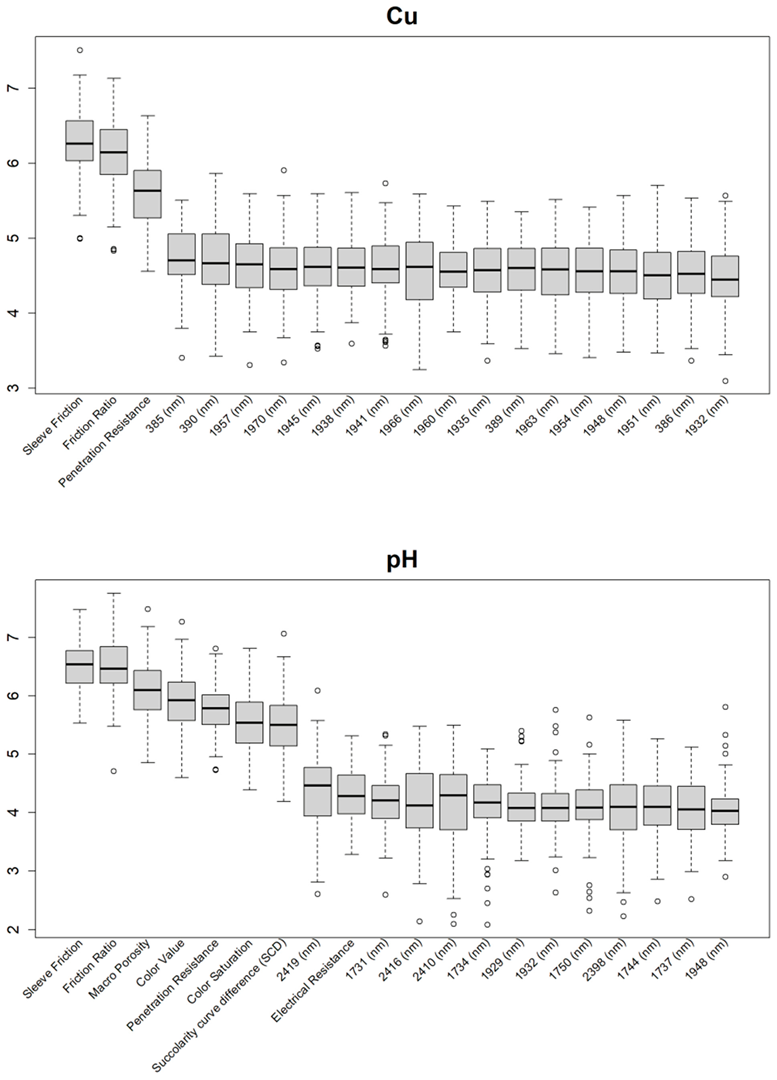 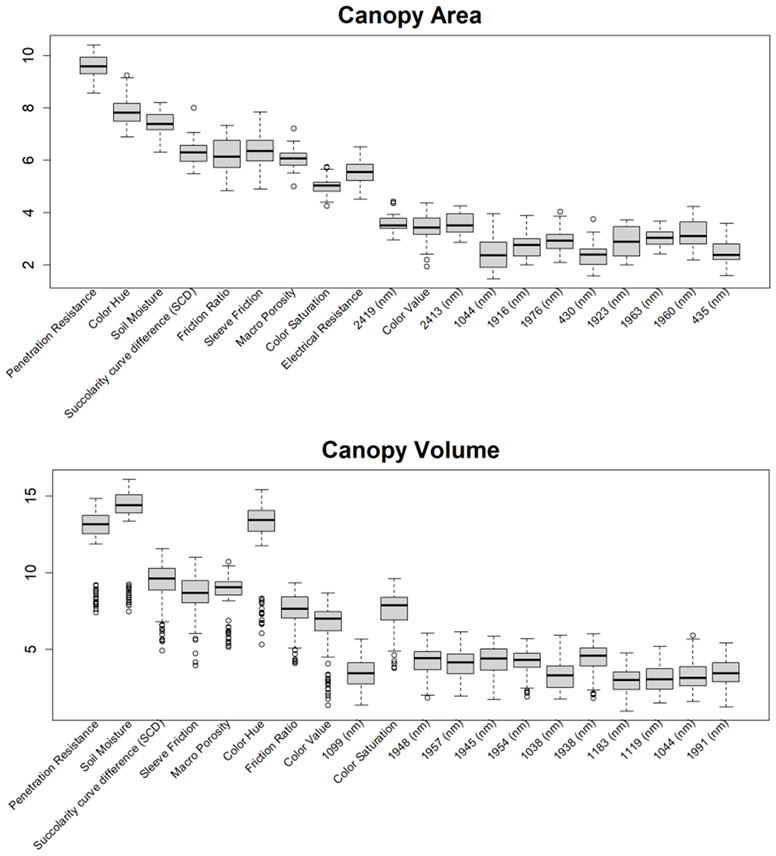 |
Appendix B
 |
Appendix C
| Methods | A | B | C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Field | Metrics | SSR 35-1 | St-15 | KG-18-19 | Mean | SSR 35-1 | St-15 | KG-18-19 | Mean | SSR 35-1 | St-15 | KG-18-19 | Mean |
| OM | R2 | 0.48 | 0.54 | 0.64 | 0.55 | 0.48 | 0.68 | 0.69 | 0.62 | 0.73 | 0.69 | 0.76 | 0.73 |
| RMSE | 0.4 | 0.24 | 0.18 | 0.27 | 0.4 | 0.2 | 0.17 | 0.26 | 0.25 | 0.2 | 0.15 | 0.20 | |
| bias | −0.03 | 0 | 0 | −0.01 | −0.03 | 0.01 | 0 | −0.01 | −0.01 | 0 | 0 | 0.00 | |
| RPIQ | 1.28 | 1.17 | 1.72 | 1.39 | 1.28 | 2.12 | 2.06 | 1.82 | 1.91 | 1.95 | 2.47 | 2.11 | |
| Sand | R2 | 0.41 | 0.5 | 0.55 | 0.49 | 0.41 | 0.66 | 0.62 | 0.56 | 0.57 | 0.59 | 0.73 | 0.63 |
| RMSE | 8.37 | 9.06 | 6.47 | 7.97 | 8.37 c | 7.47 | 6.15 | 7.33 | 7.01 | 8.41 | 5.22 | 6.88 | |
| bias | 0.14 | 0.3 | −0.13 | 0.10 | 0.14 | 0.21 | −0.08 | 0.09 | 0.24 | −0.37 | −0.21 | −0.11 | |
| RPIQ | 1.46 | 1.25 | 1.47 | 1.39 | 1.46 | 2.5 | 1.9 | 1.95 | 1.95 | 2.45 | 2.37 | 2.26 | |
| Clay | R2 | 0.43 | 0.68 | 0.6 | 0.57 | 0.39 | 0.69 | 0.66 | 0.58 | 0.58 | 0.72 | 0.73 | 0.68 |
| RMSE | 3.47 | 3.49 | 1.61 | 2.86 | 3.59 | 3.41 | 1.48 | 2.83 | 3.08 | 3.26 | 1.36 | 2.57 | |
| bias | −0.04 | 0.03 | 0.01 | 0.00 | −0.04 | −0.24 | 0.02 | −0.09 | −0.19 | −0.06 | 0.01 | −0.08 | |
| RPIQ | 1.2 | 2.07 | 1.57 | 1.61 | 0.98 | 1.83 | 1.96 | 1.59 | 1.28 | 2.26 | 2.5 | 2.01 | |
| Silt | R2 | 0.59 | 0.49 | 0.48 | 0.52 | 0.54 | 0.55 | 0.55 | 0.55 | 0.61 | 0.6 | 0.69 | 0.63 |
| RMSE | 6.16 | 6.62 | 5.7 | 6.16 | 6.64 | 6.07 | 5.49 | 6.07 | 6.12 | 5.85 | 4.53 | 5.50 | |
| bias | −0.13 | 0.21 | 0.08 | 0.05 | −0.03 | −0.16 | 0.07 | −0.04 | 0.1 | 0.17 | 0.18 | 0.15 | |
| RPIQ | 1.85 | 1.7 | 1.14 | 1.56 | 1.75 | 1.39 | 1.65 | 1.60 | 2.11 | 1.98 | 2.29 | 2.13 | |
| B | R2 | 0.67 | 0.62 | 0.25 | 0.51 | 0.49 | 0.53 | 0.35 | 0.46 | 0.81 | 0.7 | 0.49 | 0.67 |
| RMSE | 0.16 | 1.24 | 0.13 | 0.51 | 0.22 | 1.4 | 0.12 | 0.58 | 0.12 | 1.09 | 0.11 | 0.44 | |
| bias | 0 | 0.07 | 0 | 0.02 | 0.01 | 0 | 0 | 0.00 | 0 | 0 | 0 | 0.00 | |
| RPIQ | 2.25 | 1.29 | 0.8 | 1.45 | 1.63 | 1.42 | 1.27 | 1.44 | 2.82 | 1.59 | 1.54 | 1.98 | |
| Ca | R2 | 0.42 | 0.47 | 0.54 | 0.48 | 0.32 | 0.5 | 0.55 | 0.46 | 0.72 | 0.64 | 0.65 | 0.67 |
| RMSE | 738.46 | 875.83 | 838.52 | 817.60 | 776.29 | 857.52 | 832.42 | 822.08 | 483.76 | 705.47 | 764.21 | 651.15 | |
| bias | −27.78 | −21.28 | −7.15 | −18.74 | 11.06 | −35.92 | −7.16 | −10.67 | −0.97 | −8.79 | 5.83 | −1.31 | |
| RPIQ | 1.23 | 1.4 | 1.67 | 1.43 | 0.9 | 1.21 | 1.56 | 1.22 | 1.77 | 1.93 | 2.26 | 1.99 | |
| Cu | R2 | 0.24 | 0.45 | 0.45 | 0.38 | 0.06 | 0.5 | 0.5 | 0.35 | 0.74 | 0.53 | 0.56 | 0.61 |
| RMSE | 0.35 | 1.03 | 0.1 | 0.49 | 0.36 | 1 | 0.1 | 0.49 | 0.19 | 0.95 | 0.09 | 0.41 | |
| bias | −0.02 | 0.04 | 0 | 0.01 | 0 | 0.08 | 0 | 0.03 | −0.01 | −0.01 | 0 | −0.01 | |
| RPIQ | 0.87 | 1.33 | 1.36 | 1.19 | 0.39 | 1.38 | 1.61 | 1.13 | 2.48 | 1.7 | 1.86 | 2.01 | |
| Zn | R2 | 0.51 | 0.47 | 0.56 | 0.51 | 0.42 | 0.41 | 0.64 | 0.49 | 0.72 | 0.6 | 0.71 | 0.68 |
| RMSE | 2.59 | 1.43 | 0.72 | 1.58 | 2.84 | 1.46 | 0.65 | 1.65 | 1.86 | 1.23 | 0.59 | 1.23 | |
| bias | −0.09 | 0.05 | 0 | −0.01 | −0.07 | 0 | 0 | −0.02 | −0.02 | 0.01 | −0.01 | −0.01 | |
| RPIQ | 1.02 | 1.34 | 1.54 | 1.30 | 0.91 | 1.29 | 1.98 | 1.39 | 1.23 | 1.73 | 2.03 | 1.66 | |
| pH | R2 | 0.32 | 0.69 | 0.67 | 0.56 | 0.6 | 0.74 | 0.77 | 0.70 | 0.81 | 0.77 | 0.79 | 0.79 |
| RMSE | 0.23 | 0.6 | 0.5 | 0.44 | 0.21 | 0.56 | 0.42 | 0.40 | 0.12 | 0.53 | 0.4 | 0.35 | |
| bias | −0.01 | 0.01 | 0 | 0.00 | 0.01 | −0.03 | 0 | −0.01 | 0 | 0 | 0 | 0.00 | |
| RPIQ | 1.18 | 2.11 | 2.41 | 1.90 | 1.69 | 2.46 | 2.91 | 2.35 | 3.37 | 2.45 | 3.01 | 2.94 | |
References
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C sequestration as a biological negative emission strategy. Front. Clim. 2019, 1, 482133. [Google Scholar] [CrossRef]
- Verdouw, C.; Tekinerdogan, B.; Beulens, A.; Wolfert, S. Digital twins in smart farming. Agric. Syst. 2021, 189, 103046. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Heller, D.; Chudnovsky, A. A novel method of classifying soil profiles in the field using optical means. Soil Sci. Soc. Am. J. 2008, 72, 1113–1123. [Google Scholar] [CrossRef]
- Rooney, D.; Lowery, B. A profile cone penetrometer for mapping soil horizons. Soil Sci. Soc. Am. J. 2000, 64, 2136–2139. [Google Scholar] [CrossRef]
- Rooney, D.J.; Norman, J.; Grunwald, S. Soil imaging penetrometer: A tool for obtaining real-time-in-situ soil images. In Proceedings of the ASAE Annual Meeting, Sacramento, CA, USA, 29 July–1 August 2001; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2001. [Google Scholar] [CrossRef]
- Poggio, M.; Brown, D.J.; Bricklemyer, R.S. Laboratory-based evaluation of optical performance for a new soil penetrometer visible and near-infrared (VisNIR) foreoptic. Comput. Electron. Agric. 2015, 115, 12–20. [Google Scholar] [CrossRef]
- Grunwald, S. Artificial intelligence and soil carbon modeling demystified: Power, potentials, and perils. Carbon Footpr. 2022, 1, 1–23. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Adamchuk, V.I.; Sudduth, K.A.; McKenzie, N.J.; Lobsey, C. Proximal soil sensing: An effective approach for soil measurements in space and time. Adv. Agron. 2011, 113, 243–291. [Google Scholar] [CrossRef]
- Brown, D.J.; Shepherd, K.D.; Walsh, M.G.; Mays, M.D.; Reinsch, T.G. Global soil characterization with VNIR diffuse reflectance spectroscopy. Geoderma 2006, 132, 273–290. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Behrens, T.; Ben-Dor, E.; Brown, D.J.; Demattê, J.A.M.; Shepherd, K.D.; Shi, Z.; Stenberg, B.; Stevens, A.; Adamchuk, V.; et al. A global spectral library to characterize the world’s soil. Earth-Sci. Rev. 2016, 155, 198–230. [Google Scholar] [CrossRef]
- Knox, N.M.; Grunwald, S.; McDowell, M.L.; Bruland, G.L.; Myers, D.B.; Harris, W.G. Modelling soil carbon fractions with visible near-infrared (VNIR) and mid-infrared (MIR) spectroscopy. Geoderma 2015, 239–240, 229–239. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, D.; Wang, J.; Triantafilis, J. Soil organic carbon (SOC) prediction in Australian sugarcane fields using Vis–NIR spectroscopy with different model setting approaches. Geoderma Reg. 2022, 30, e00566. [Google Scholar] [CrossRef]
- Clingensmith, C.M.; Grunwald, S. Predicting soil properties and interpreting Vis-NIR models from across continental United States. Sensors 2022, 22, 3187. [Google Scholar] [CrossRef]
- Ng, W.; Minasny, B.; Montazerolghaem, M.; Padarian, J.; Ferguson, R.; Bailey, S.; McBratney, A.B. Convolutional neural network for simultaneous prediction of several soil properties using visible/near-infrared, mid-infrared, and their combined spectra. Geoderma 2019, 352, 251–267. [Google Scholar] [CrossRef]
- Demattê, J.A.M.; Paiva, A.F.S.; Poppiel, R.R.; Rosin, N.A.; Ruiz, L.F.C.; Mello, F.A.O.; Minasny, B.; Grunwald, S.; Ge, Y.; Ben Dor, E.; et al. The Brazilian Soil Spectral Service (BraSpecS): A user-friendly system for global soil spectra communication. Remote Sens. 2022, 14, 740. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Q.L.; Peng, J.; Ji, W.; Liu, H.; Li, X.; Viscarra Rossel, R.A. Development of a national VNIR soil-spectral library for soil classification and prediction of organic matter concentrations. Sci. China Earth Sci. 2014, 57, 1671–1680. [Google Scholar] [CrossRef]
- Baumann, P.; Helfenstein, A.; Gubler, A.; Keller, A.; Meuli, R.G.; Wächter, D.; Lee, J.; Viscarra Rossel, R.; Six, J. Developing the Swiss mid-infrared soil spectral library for local estimation and monitoring. SOIL 2021, 7, 525–546. [Google Scholar] [CrossRef]
- Wijewardane, N.K.; Ge, Y.; Morgan, C.L.S. Prediction of soil organic and inorganic carbon at different moisture contents with dry ground VNIR: A comparative study of different approaches. Eur. J. Soil Sci. 2016, 67, 605–615. [Google Scholar] [CrossRef]
- Karray, E.; Elmannai, H.; Toumi, E.; Gharbia, M.H.; Meshoul, S.; Ben Rabah, Z. Evaluating the potentials of PLSR and SVR models for soil properties prediction using field imaging, laboratory VNIR spectroscopy and their combination. Comput. Model. Eng. Sci. 2023, 136, 1399–1425. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B.; Tranter, G.; Murphy, B.W. Using soil knowledge for the evaluation of mid-infrared diffuse reflectance spectroscopy for predicting soil physical and mechanical properties. Eur. J. Soil Sci. 2008, 59, 960–971. [Google Scholar] [CrossRef]
- Tsimpouris, E.; Tsakiridis, N.L.; Theocharis, J.B. Using autoencoders to compress soil VNIR–SWIR spectra for more robust prediction of soil properties. Geoderma 2021, 393, 114967. [Google Scholar] [CrossRef]
- Zhou, N.; Hong, J.; Song, B.; Wu, S.; Wei, Y.; Wang, T. Feature variable selection based on VIS-NIR spectra and soil moisture content prediction model construction. J. Spectrosc. 2024, 2024, e8180765. [Google Scholar] [CrossRef]
- Dangal, S.R.S.; Sanderman, J.; Wills, S.; Ramirez-Lopez, L. Accurate and precise prediction of soil properties from a large mid-infrared spectral library. Soil Syst. 2019, 3, 11. [Google Scholar] [CrossRef]
- Davari, M.; Karimi, S.A.; Bahrami, H.A.; Hammond, S.M.; Fahmideh, S. Simultaneous prediction of several soil properties related to engineering uses based on laboratory Vis-NIR reflectance spectroscopy. Catena 2021, 197, 104987. [Google Scholar] [CrossRef]
- Grunwald, S.; Rooney, D.J.; McSweeney, K.; Lowery, B. Development of pedotransfer functions for a profile cone penetrometer. Geoderma 2001, 100, 25–47. [Google Scholar] [CrossRef]
- Thomas, P.; Mondal, S.; Roy, D.; Meena, M.; Aggarwal, B.; Sharma, A.; Behera, U.; Das, T.; Jatav, R.; Chakraborty, D. Exploring the relationships between penetration resistance, bulk density, and water content in cultivated soils. J. Agric. Phys. 2020, 20, 22. [Google Scholar]
- Viscarra Rossel, R.A.; Lobsey, C.R.; Sharman, C.; Flick, P.; McLachlan, G. Novel proximal sensing for monitoring soil organic C stocks and condition. Environ. Sci. Technol. 2017, 51, 5630–5641. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; McBratney, A.B.; Minasny, B. Proximal Soil Sensing (Progress in Soil Science); Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Borůvka, L.; Saberioon, M.; Vašát, R. Visible, near-infrared, and mid-infrared spectroscopy applications for soil assessment with emphasis on soil organic matter content and quality: State-of-the-art and key issues. Appl. Spectrosc. 2013, 67, 1349–1362. [Google Scholar] [CrossRef]
- Gubler, A. Quantitative Estimations of Soil Properties by VNIR Spectroscopy: Applications for Laboratory and Field Measurements; Südwestdeutscher Verlag für Hochschulschriften: London, UK, 2012. [Google Scholar] [CrossRef]
- Branco de Freitas Maia, C.M.; Novotny, E.H.; Rittl, T.F.; Bermingham Hayes, M.H. Soil organic matter: Chemical and physical characteristics and analytical methods. A review. Curr. Org. Chem. 2013, 17, 2985–2990. [Google Scholar] [CrossRef]
- Stenberg, B.; Viscarra Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Chapter five—Visible and near infrared spectroscopy in soil science. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 107, pp. 163–215. [Google Scholar] [CrossRef]
- Bowers, S.A.; Hanks, R.J. Reflection of radiant energy from soils. Soil Sci. 1965, 100, 130–138. [Google Scholar] [CrossRef]
- Hunt, G.R.; Vincent, R.K. The behavior of spectral features in the infrared emission from particulate surfaces of various grain sizes. J. Geophys. Res. 1968, 73, 6039–6046. [Google Scholar] [CrossRef]
- Bänninger, D.; Lehmann, P.; Flühler, H. Modelling the effect of particle size, shape and orientation of light transfer through porous media. Eur. J. Soil Sci. 2006, 57, 906–915. [Google Scholar] [CrossRef]
- Sadeghi, M.; Babaeian, E.; Tuller, M.; Jones, S.B. Particle size effects on soil reflectance explained by an analytical radiative transfer model. Remote Sens. Environ. 2018, 210, 375–386. [Google Scholar] [CrossRef]
- Norouzi, S.; Sadeghi, M.; Liaghat, A.; Tuller, M.; Jones, S.B.; Ebrahimian, H. Information depth of NIR/SWIR soil reflectance spectroscopy. Remote Sens. Environ. 2021, 256, 112315. [Google Scholar] [CrossRef]
- Cierniewski, J.; Gdala, T.; Karnieli, A. A hemispherical–directional reflectance model as a tool for understanding image distinctions between cultivated and uncultivated bare surfaces. Remote Sens. Environ. 2004, 90, 505–523. [Google Scholar] [CrossRef]
- Wu, C.Y.; Jacobson, A.R.; Laba, M.; Baveye, P.C. Alleviating moisture content effects on the visible near-infrared diffuse-reflectance sensing of soils. Soil Sci. 2009, 174, 456. [Google Scholar] [CrossRef]
- Piekarczyk, J.; Kaźmierowski, C.; Królewicz, S.; Cierniewski, J. Effects of soil surface roughness on soil reflectance measured in laboratory and outdoor conditions. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2016, 9, 827–834. [Google Scholar] [CrossRef]
- Angelopoulou, T.; Balafoutis, A.; Zalidis, G.; Bochtis, D. From laboratory to proximal sensing spectroscopy for soil organic carbon estimation—A review. Sustainability 2020, 12, 443. [Google Scholar] [CrossRef]
- Hedley, C.; Roudier, P.; Maddi, L. VNIR soil spectroscopy for field soil analysis. Commun. Soil Sci. Plant Anal. 2015, 46 (Suppl. S1), 104–121. [Google Scholar] [CrossRef]
- Chang, C.W.; Laird, D.A.; Hurburgh, C.R.J. Influence of soil moisture on near-infrared reflectance spectroscopic measurement of soil properties. Soil Sci. 2005, 170, 244. [Google Scholar] [CrossRef]
- Rienzi, E.A.; Mijatovic, B.; Mueller, T.G.; Matocha, C.J.; Sikora, F.J.; Castrignanò, A.M. Prediction of soil organic carbon under varying moisture levels using reflectance spectroscopy. Soil Sci. Soc. Am. J. 2014, 78, 958–967. [Google Scholar] [CrossRef]
- Seidel, M.; Vohland, M.; Greenberg, I.; Ludwig, B.; Ortner, M.; Thiele-Bruhn, S.; Hutengs, C. Soil moisture effects on predictive VNIR and MIR modeling of soil organic carbon and clay content. Geoderma 2022, 427, 116103. [Google Scholar] [CrossRef]
- Knadel, M.; Castaldi, F.; Barbetti, R.; Ben-Dor, E.; Gholizadeh, A.; Lorenzetti, R. Mathematical techniques to remove moisture effects from visible–near-infrared–shortwave-infrared soil spectra—Review. Appl. Spectrosc. Rev. 2023, 58, 629–662. [Google Scholar] [CrossRef]
- Lobell, D.B.; Asner, G.P. Moisture effects on soil reflectance. Soil Sci. Soc. Am. J. 2002, 66, 722–727. [Google Scholar] [CrossRef]
- Tan, Y.; Jiang, Q.; Yu, L.; Liu, H.; Zhang, B. Reducing the moisture effect and improving the prediction of soil organic matter with VIS-NIR spectroscopy in black soil area. IEEE Access 2021, 9, 5895–5905. [Google Scholar] [CrossRef]
- Cambou, A.; Allory, V.; Cardinael, R.; Vieira, L.C.; Barthes, B.G. Comparison of soil organic carbon stocks predicted using visible and near infrared reflectance (VNIR) spectra acquired in situ vs. on sieved dried samples: Synthesis of different studies. Soil Secur. 2021, 5, 100024. [Google Scholar] [CrossRef]
- Dhawale, N.M.; Adamchuk, V.I.; Prasher, S.O.; Viscarra Rossel, R.A. Evaluating the precision and accuracy of proximal soil vis–NIR sensors for estimating soil organic matter and texture. Soil Syst. 2021, 5, 48. [Google Scholar] [CrossRef]
- Hutengs, C.; Ludwig, B.; Jung, A.; Eisele, A.; Vohland, M. Comparison of portable and bench-top spectrometers for mid-infrared diffuse reflectance measurements of soils. Sensors 2018, 18, 993. [Google Scholar] [CrossRef]
- Hutengs, C.; Seidel, M.; Oertel, F.; Ludwig, B.; Vohland, M. In situ and laboratory soil spectroscopy with portable visible-to-near-infrared and mid-infrared instruments for the assessment of organic carbon in soils. Geoderma 2019, 355, 113900. [Google Scholar] [CrossRef]
- Hutengs, C.; Eisenhauer, N.; Schaedler, M.; Lochner, A.; Seidel, M.; Vohland, M. VNIR and MIR spectroscopy of PLFA-derived soil microbial properties and associated soil physicochemical characteristics in an experimental plant diversity gradient. Soil Biol. Biochem. 2021, 160, 108319. [Google Scholar] [CrossRef]
- Semella, S.; Hutengs, C.; Seidel, M.; Ulrich, M.; Schneider, B.; Ortner, M.; Thiele-Bruhn, S.; Ludwig, B.; Vohland, M. Accuracy and reproducibility of laboratory diffuse reflectance measurements with portable VNIR and MIR spectrometers for predictive soil organic carbon modeling. Sensors 2022, 22, 2749. [Google Scholar] [CrossRef] [PubMed]
- Sharififar, A.; Sarmadian, F.; Malone, B.P.; Minasny, B. Addressing the issue of digital mapping of soil classes with imbalanced class observations. Geoderma 2019, 350, 84–92. [Google Scholar] [CrossRef]
- Goodwin, D.J.; Kane, D.A.; Dhakal, K.; Covey, K.R.; Bettigole, C.; Hanle, J.; Ortega-S., J.A.; Perotto-Baldivieso, H.L.; Fox, W.E.; Tolleson, D.R. Can low-cost, handheld spectroscopy tools coupled with remote sensing accurately estimate soil organic carbon in semi-arid grazing lands? Soil Syst. 2022, 6, 38. [Google Scholar] [CrossRef]
- Mitu, S.M.; Smith, C.; Sanderman, J.; Ferguson, R.R.; Shepherd, K.; Ge, Y. Evaluating consistency across multiple NeoSpectra (compact Fourier transform near-infrared) spectrometers for estimating common soil properties. Soil Sci. Soc. Am. J. 2024, 88, 1324–1339. [Google Scholar] [CrossRef]
- Murad, M.O.F.; Ackerson, J.; Tolles, C.; Meissner, K.; Morgan, C.L.S.; Ge, Y. Estimating soil organic carbon content at variable moisture contents using a low-cost spectrometer. Geoderma 2023, 440, 116723. [Google Scholar] [CrossRef]
- Murad, M.O.F.; Jones, E.J.; Minasny, B.; McBratney, A.B.; Wijewardane, N.; Ge, Y. Assessing a VisNIR penetrometer system for in-situ estimation of soil organic carbon under variable soil moisture conditions. Biosyst. Eng. 2022, 224, 197–212. [Google Scholar] [CrossRef]
- Grunwald, S.; Vasques, G.M.; Rivero, R.G. Fusion of soil and remote sensing data to model soil properties. Adv. Agron. 2015, 131, 1–109. [Google Scholar] [CrossRef]
- Farzamian, M.; Paz, M.C.; Paz, A.M.; Castanheira, N.L.; Gonçalves, M.C.; Monteiro Santos, F.A.; Triantafilis, J. Mapping soil salinity using electromagnetic conductivity imaging—A comparison of regional and location-specific calibrations. Land Degrad. Dev. 2019, 30, 1393–1406. [Google Scholar] [CrossRef]
- Tavares, T.R.; Nunes, L.C.; Alves, E.E.N.; Almeida, E.; Maldaner, L.F.; Krug, F.J.; Carvalho, H.W.P.; Molin, J.P. Simplifying sample preparation for soil fertility analysis by X-ray fluorescence spectrometry. Sensors 2019, 19, 5066. [Google Scholar] [CrossRef]
- Schmidinger, J.; Barkov, V.; Tavakoli, H.; Correa, J.E.; Ostermann, M.; Atzmueller, M.; Gebbers, R.; Vogel, S. Which and How Many Soil Sensors Are Ideal to Predict Key Soil Properties: A Case Study with Seven Sensors. Available online: https://ssrn.com/abstract=4844780 (accessed on 19 October 2024).
- Chen, Y.; Gao, S.; Jones, E.J.; Singh, B. Prediction of soil clay content and cation exchange capacity using visible near-infrared spectroscopy, portable X-ray fluorescence, and X-ray diffraction techniques. Environ. Sci. Technol. 2021, 55, 4629–4637. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.R.; Molin, J.P.; Nunes, L.C.; Wei, M.C.F.; Krug, F.J.; de Carvalho, H.W.P.; Mouazen, A.M. Multi-sensor approach for tropical soil fertility analysis: Comparison of individual and combined performance of VNIR, XRF, and LIBS spectroscopies. Agronomy 2021, 11, 1028. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, R.; Li, S.; Chen, S.; Jiang, Q.; Zhou, L.; Shi, Z. Multi-sensor fusion for the determination of several soil properties in the Yangtze River Delta, China. Eur. J. Soil Sci. 2019, 70, 162–173. [Google Scholar] [CrossRef]
- Vasques, G.M.; Rodrigues, H.M.; Coelho, M.R.; Baca, J.F.M.; Dart, R.O.; Oliveira, R.P.; Teixeira, W.G.; Ceddia, M.B. Field proximal soil sensor fusion for improving high-resolution soil property maps. Soil Syst. 2020, 4, 52. [Google Scholar] [CrossRef]
- Yurui, S.; Schulze Lammers, P.; Daokun, M.; Jianhui, L.; Qingmeng, Z. Determining soil physical properties by multi-sensor technique. Sens. Actuators A Phys. 2008, 147, 352–357. [Google Scholar] [CrossRef]
- Milella, A.; Reina, G.; Nielsen, M. A multi-sensor robotic platform for ground mapping and estimation beyond the visible spectrum. Precis. Agric. 2019, 20, 423–444. [Google Scholar] [CrossRef]
- Balan, T.; Dumitru, C.; Dudnik, G.; Alessi, E.; Lesecq, S.; Correvon, M.; Passaniti, F.; Licciardello, A. Smart multi-sensor platform for analytics and social decision support in agriculture. Sensors 2020, 20, 4127. [Google Scholar] [CrossRef]
- Van Wyck, N.; Anderson, G.; Farrington, S.; Rooney, D.; Wallace, W. In-Situ Near Infrared Sensor Unit and Method of Making the Same. U.S. Patent # 11 2023,686,676, June 2023. [Google Scholar]
- Topp, G.C.; Davis, J.L.; Annan, A.P. Electromagnetic determination of soil water content: Measurements in coaxial transmission lines. Water Resour. Res. 1980, 16, 574–582. [Google Scholar] [CrossRef]
- Ledieu, J.; Ridder, P.D.; Clerck, P.D.; Dautrebande, S. A method of measuring soil moisture by time-domain reflectometry. J. Hydrol. 1986, 88, 319–328. [Google Scholar] [CrossRef]
- Ferré, P.A.; Rudolph, D.L.; Kachanoski, R.G. Spatial averaging of water content by time domain reflectometry: Implications for twin rod probes with and without dielectric coatings. Water Resour. Res. 1996, 32, 271–279. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Villet, W.C.B.; Tringale, P.T.; Chan, C.K. Acoustic penetrometer for subsoil investigation. J. Acoust. Soc. Am. 1983, 74, 1095. [Google Scholar] [CrossRef]
- Goktepe, A.B.; Altun, S.; Sezer, A. Soil clustering by fuzzy c-means algorithm. Adv. Eng. Softw. 2005, 36, 691–698. [Google Scholar] [CrossRef]
- Houlsby, G.T.; Ruck, B.M. Interpretation of signals from an acoustic cone penetrometer. In Geotechnical Site Characterization; Robertson, Mayne, Eds.; Balkema: Rotterdam, The Netherlands, 1998; pp. 1–10. [Google Scholar]
- Domsch, H.; Ehlert, D.; Giebel, A.; Witzke, K.; Boess, J. Evaluation of the soil penetration resistance along a transect to determine the loosening depth. Precis. Agric. 2006, 7, 309–326. [Google Scholar] [CrossRef]
- Bai, X.; Jia, X.; Jia, Y.; Hu, W. Modeling long-term soil water dynamics in response to land-use change in a semi-arid area. J. Hydrol. 2020, 585, 124824. [Google Scholar] [CrossRef]
- Li, B.-B.; Li, P.-P.; Zhang, W.-T.; Ji, J.-Y.; Liu, G.-B.; Xu, M.-X. Deep soil moisture limits the sustainable vegetation restoration in arid and semi-arid Loess Plateau. Geoderma 2021, 399, 115122. [Google Scholar] [CrossRef]
- Wu, G.-L.; Cui, Z.; Huang, Z. Contribution of root decay process on soil infiltration capacity and soil water replenishment of planted forestland in semi-arid regions. Geoderma 2021, 404, 115289. [Google Scholar] [CrossRef]
- Paris, J.; Unverferth, M.; Farrington, S.; Hull, M.; Horton, R.; Rooney, D. Systems and Methods for Multispectral Landscape Mapping. U.S. Patent # 11,800,246, October 2023. [Google Scholar]
- Rooney, D.; Dlott, J.; Farrington, S.; Wallace, W. Precision Site Characterization Using Digital Twin. U.S. Patent # 12,092,625, September 2024. [Google Scholar]
- Zhang, X.; Pourreza, A.; Cheung, K.H.; Zuniga-Ramirez, G.; Lampinen, B.D.; Shackel, K.A. Estimation of fractional photosynthetically active radiation from a canopy 3D model: Case study—Almond yield prediction. Front. Plant Sci. 2021, 12, 715361. [Google Scholar] [CrossRef] [PubMed]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- de Melo, R.H.C.; Conci, A. Succolarity: Defining a method to calculate this fractal measure. In Proceedings of the 2008 15th International Conference on Systems, Signals and Image Processing, Bratislava, Slovakia, 25–28 June 2008; pp. 291–294. [Google Scholar] [CrossRef]
- de Melo, R.H.C.; Conci, A. How Succolarity could be used as another fractal measure in image analysis. Telecommun. Syst. 2013, 52, 1643–1655. [Google Scholar] [CrossRef]
- Leavitt, B.; Pearce, A.; Van Wyck, N.; Kwayu, K.; Courville, Z.R.; Melendy, T.D.; Farrington, S. Use of a stable surrogate material and microscopy in the inference of bulk microstructural and strength properties of packed snow. Cold Regions Research and Engineering Laboratory, Hanover, NH, USA. 2022; submitted. [Google Scholar]
- Barrena-González, J.; Gabourel-Landaverde, V.A.; Mora, J.; Contador, J.F.L.; Fernández, M.P. Exploring soil property spatial patterns in a small grazed catchment using machine learning. Earth Sci. Inform. 2023, 16, 3811–3838. [Google Scholar] [CrossRef]
- Guindo, M.L.; Kabir, M.H.; Chen, R.; Liu, F. Potential of Vis-NIR to measure heavy metals in different varieties of organic-fertilizers using Boruta and deep belief network. Ecotoxicol. Environ. Saf. 2021, 228, 112996. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, T.; Xie, S.; Liu, Z.; Lin, C.; Hu, Y.; Wang, J.; Mao, X. Estimation of soil cations based on visible and near-infrared spectroscopy and machine learning. Agriculture 2023, 13, 1237. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature selection with the Boruta package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J.H.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: New York, NY, USA, 2009; Volume 2, pp. 1–758. [Google Scholar] [CrossRef]
- Keskin, H.; Grunwald, S. Regression kriging as a workhorse in the digital soil mapper’s toolbox. Geoderma 2018, 326, 22–41. [Google Scholar] [CrossRef]
- Keskin, H.; Grunwald, S.; Harris, W.G. Digital mapping of soil carbon fractions with machine learning. Geoderma 2019, 339, 40–58. [Google Scholar] [CrossRef]
- Beniston, J.W.; Lal, R.; Mercer, K.L. Assessing and managing soil quality for urban agriculture in a degraded vacant lot soil. Land Degrad. Dev. 2016, 27, 996–1006. [Google Scholar] [CrossRef]
- Xue, P.-P.; Carrillo, Y.; Pino, V.; Minasny, B.; McBratney, A.B. Soil properties drive microbial community structure in a large-scale transect in South Eastern Australia. Sci. Rep. 2018, 8, 11725. [Google Scholar] [CrossRef]

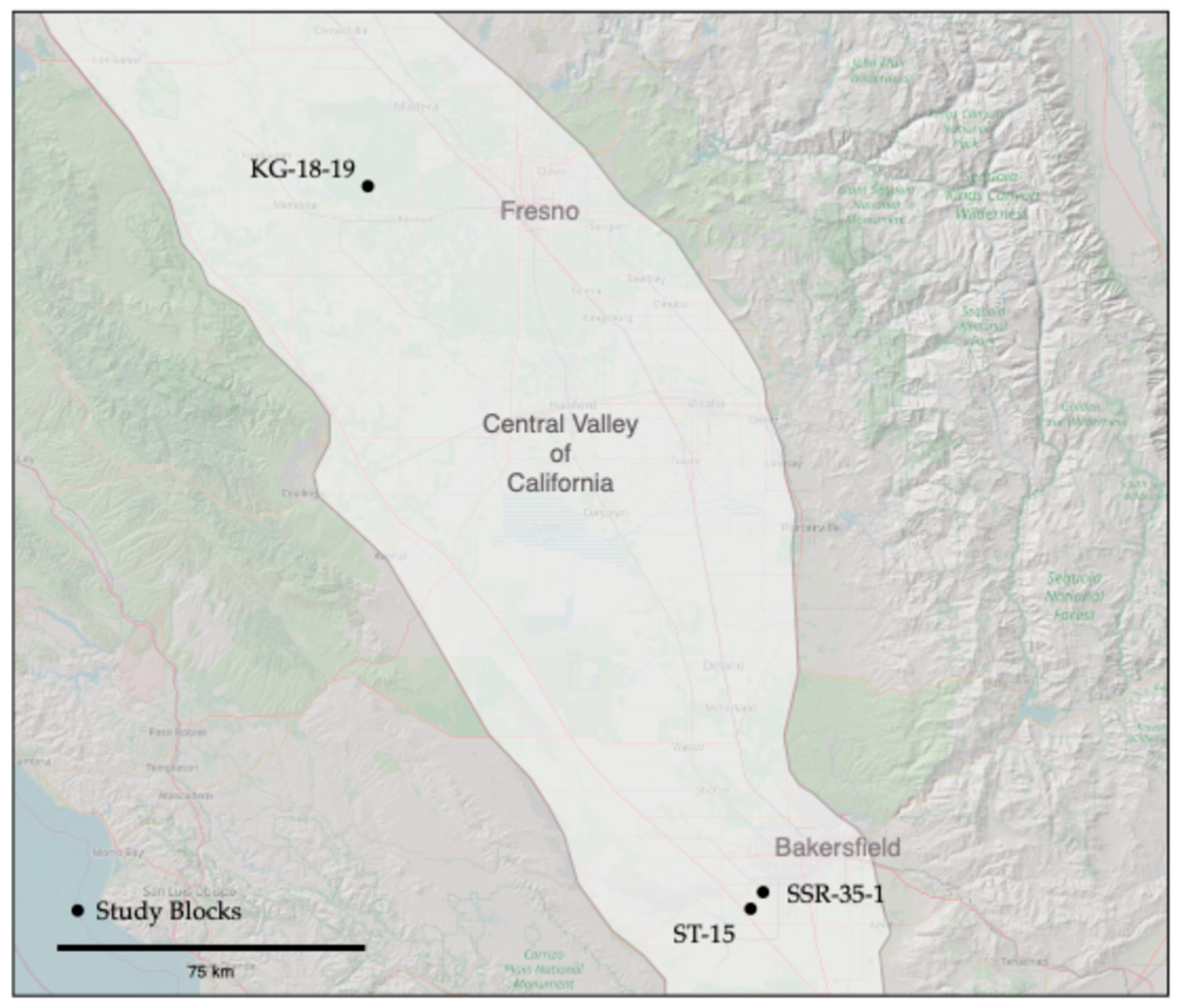

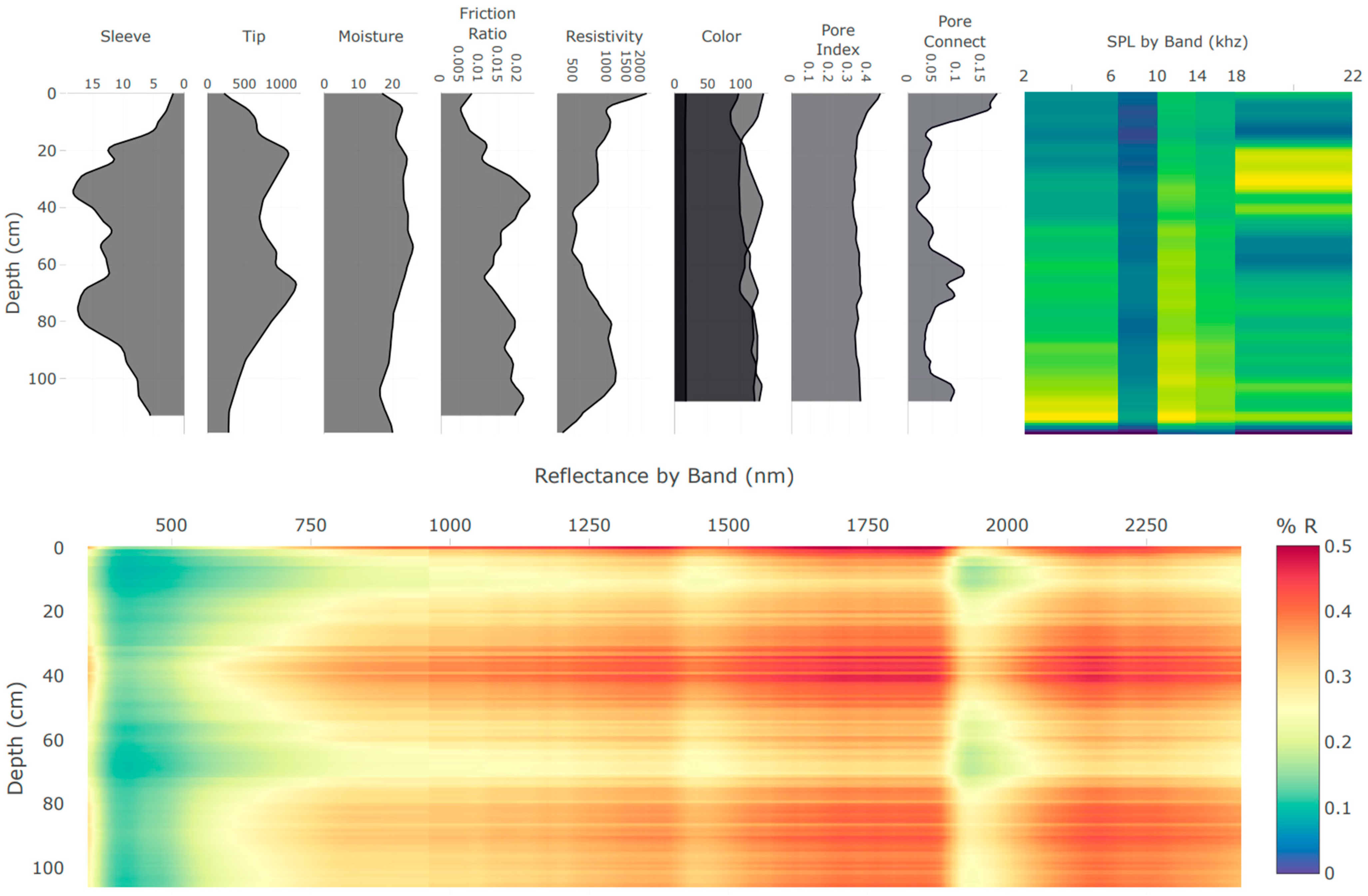



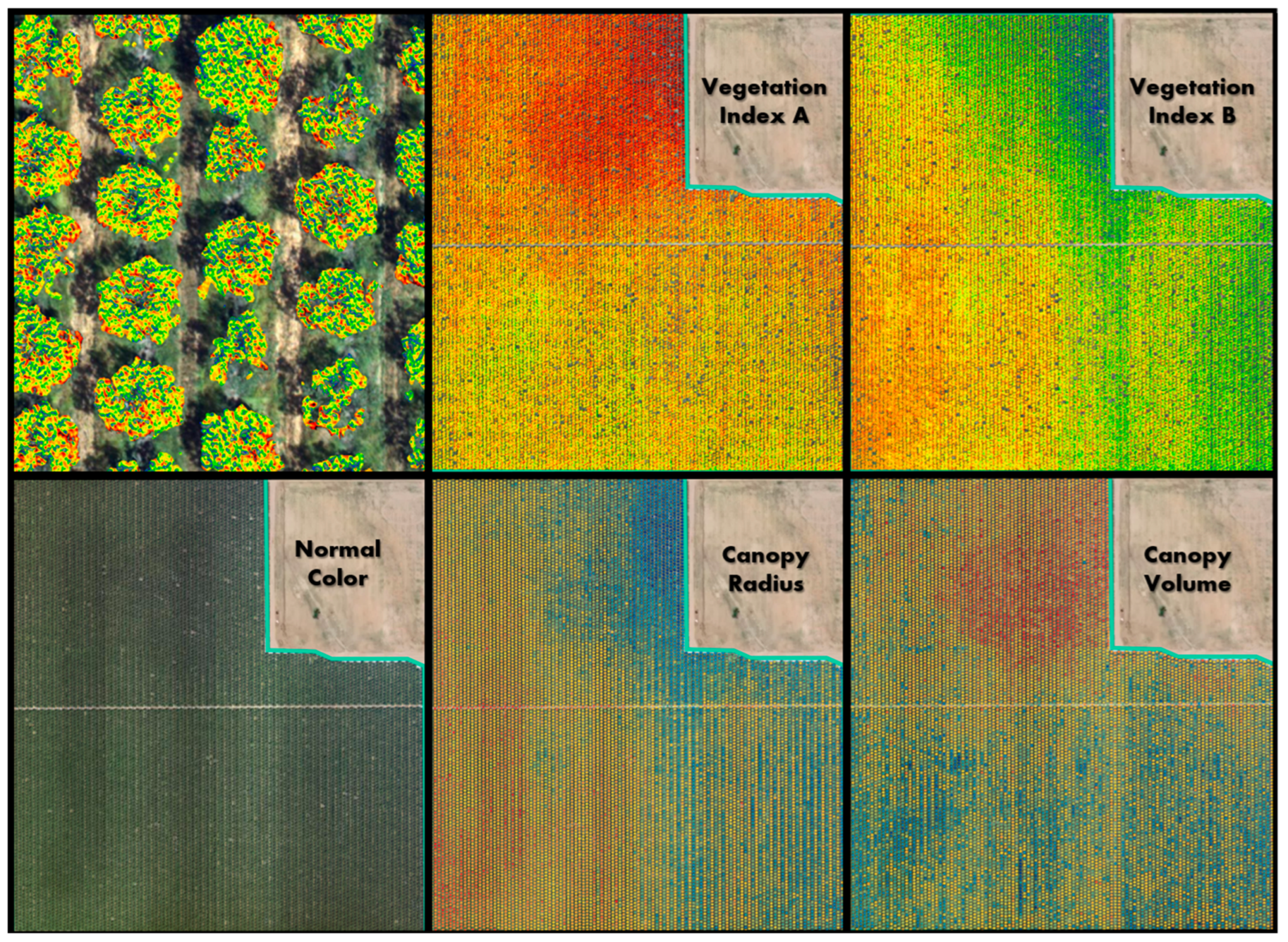



| Block | Location | Description | Samples |
|---|---|---|---|
| KG-18-19 | About 20 km southwest of Madera and less than 1 mi north of the San Joaquin River in Madera County, California | A 35.2 ha almond orchard, planted in 2017. Double-line drip irrigation. Soil map units are El Peco-Dinuba fine sandy loams and Grangeville sandy loam, with 0–1 percent slopes (leveled during planting). | 78 samples December 2023 |
| SSR-35-1 | About 8 km southwest of Bakersfield in Kern County, California | A 25.5 ha almond orchard, planted in 2012. Micro-sprinkler irrigation. Soil map units are primarily Kimberlina fine sandy loam with a small section of Granoso loamy sand adjacent to canal, with 0–2 percent slopes (leveled during planting). | 36 samples October 2023 |
| ST-15 | About 18 km southwest of Bakersfield in Kern County, California, and about 3 mi south of SSR-35-1 | A 31.2 ha almond orchard, planted in 2016. Double-line drip irrigation. Soil map units include Garces loam, Kimberlina fine sandy loam, Millox clay loam, and Tennco fine sandy loam. The field is split into two sections by a field road. The western section is adjacent to a canal. | 34 samples October 2023 |
| Property | Abbrev. | NAPT Method | Units | Method Comment |
|---|---|---|---|---|
| Organic Matter | OM | S9.20 | % | Loss on ignition |
| Sand | Sand | S14.10 | % | Hydrometer |
| Silt | Silt | S14.10 | % | Hydrometer |
| Clay | Clay | S14.10 | % | Hydrometer |
| Boron | B | S1.50 | mg/L | Saturated paste |
| Calcium | Ca | S5.10 | mg/kg | AA extraction |
| Copper | Cu | S6.10 | mg/kg | DTPA extraction |
| Zinc | Zn | S6.10 | mg/kg | DTPA extraction |
| pH | pH | S1.10 | pH units | Saturated paste |
| Method C | ||||||||||||
| CPI | Canopy Area (m2) | Canopy Volume (m3) | ||||||||||
| Fields | R2 | RMSE | Bias | RPIQ | R2 | RMSE | Bias | RPIQ | R2 | RMSE | Bias | RPIQ |
| St-15 | 0.67 | 6.34 | −0.07 | 0.63 | 0.67 | 4.79 | −0.15 | 0.58 | 0.68 | 14.56 | −0.06 | 0.7 |
| SSR-35-1 | 0.66 | 20.97 | −0.47 | 0.75 | 0.58 | 3.73 | 0.02 | 0.65 | 0.63 | 25.35 | −0.16 | 0.81 |
| KG-18-19 | 0.54 | 10.32 | −0.01 | 0.85 | 0.44 | 2.13 | 0 | 0.58 | 0.48 | 15.74 | −0.06 | 0.68 |
| Method D | ||||||||||||
| CPI | Canopy Area (m2) | Canopy Volume (m3) | ||||||||||
| Fields | R2 | RMSE | Bias | Fields | R2 | RMSE | Bias | Fields | R2 | RMSE | Bias | Fields |
| St-15 | 0.75 | 5.09 | −0.09 | 1.13 | 0.76 | 3.65 | −0.18 | 1.03 | 0.76 | 11.51 | −0.19 | 1.16 |
| SSR-35-1 | 0.74 | 17.93 | −0.41 | 1.27 | 0.72 | 2.94 | 0.01 | 1.06 | 0.73 | 21.2 | −0.15 | 1.21 |
| KG-18-19 | 0.72 | 8.15 | −0.08 | 1.64 | 0.65 | 1.72 | −0.01 | 1.33 | 0.70 | 12.23 | −0.11 | 1.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grunwald, S.; Murad, M.O.F.; Farrington, S.; Wallace, W.; Rooney, D. Multi-Sensor Soil Probe and Machine Learning Modeling for Predicting Soil Properties. Sensors 2024, 24, 6855. https://doi.org/10.3390/s24216855
Grunwald S, Murad MOF, Farrington S, Wallace W, Rooney D. Multi-Sensor Soil Probe and Machine Learning Modeling for Predicting Soil Properties. Sensors. 2024; 24(21):6855. https://doi.org/10.3390/s24216855
Chicago/Turabian StyleGrunwald, Sabine, Mohammad Omar Faruk Murad, Stephen Farrington, Woody Wallace, and Daniel Rooney. 2024. "Multi-Sensor Soil Probe and Machine Learning Modeling for Predicting Soil Properties" Sensors 24, no. 21: 6855. https://doi.org/10.3390/s24216855
APA StyleGrunwald, S., Murad, M. O. F., Farrington, S., Wallace, W., & Rooney, D. (2024). Multi-Sensor Soil Probe and Machine Learning Modeling for Predicting Soil Properties. Sensors, 24(21), 6855. https://doi.org/10.3390/s24216855







