Dual-Planar Monopole Antenna-Based Remote Sensing System for Microwave Medical Applications
Abstract
1. Introduction
1.1. Microwave Sensing and Imaging (MSI) Systems
1.2. Wearable and Portable MSI Prototypes
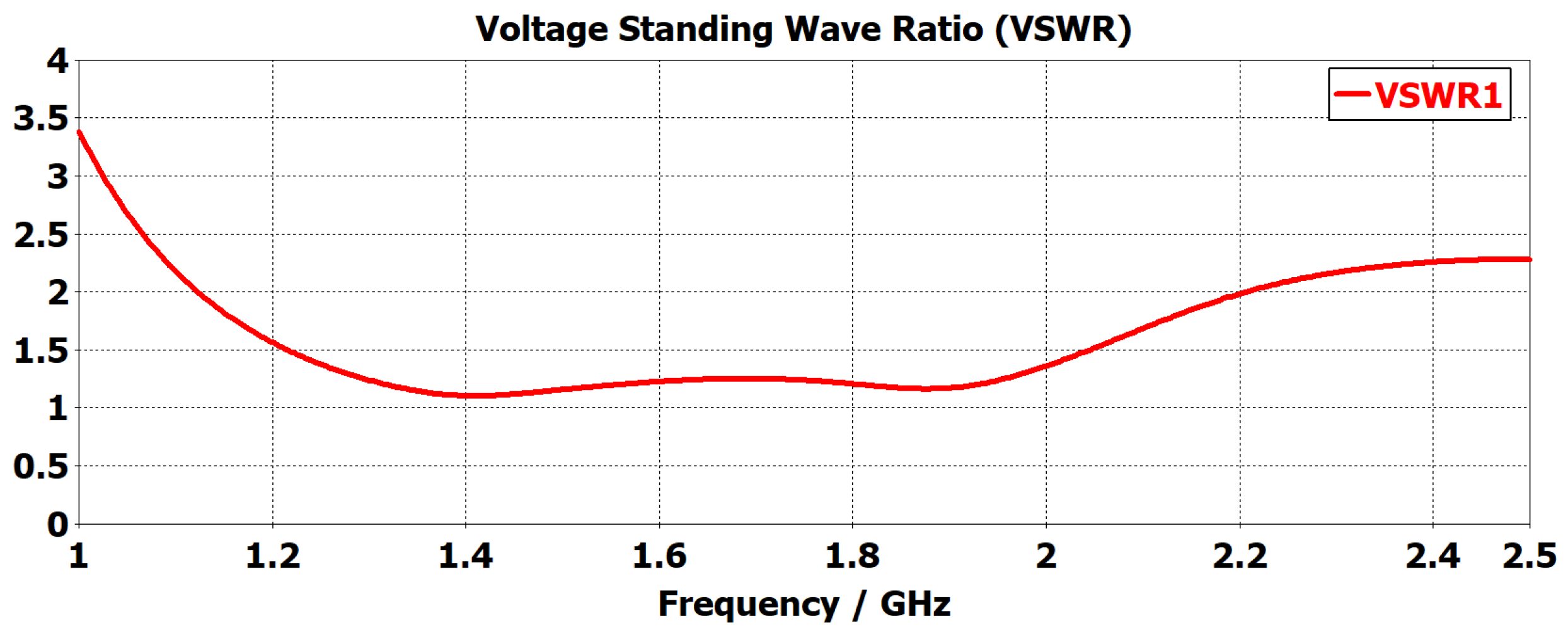
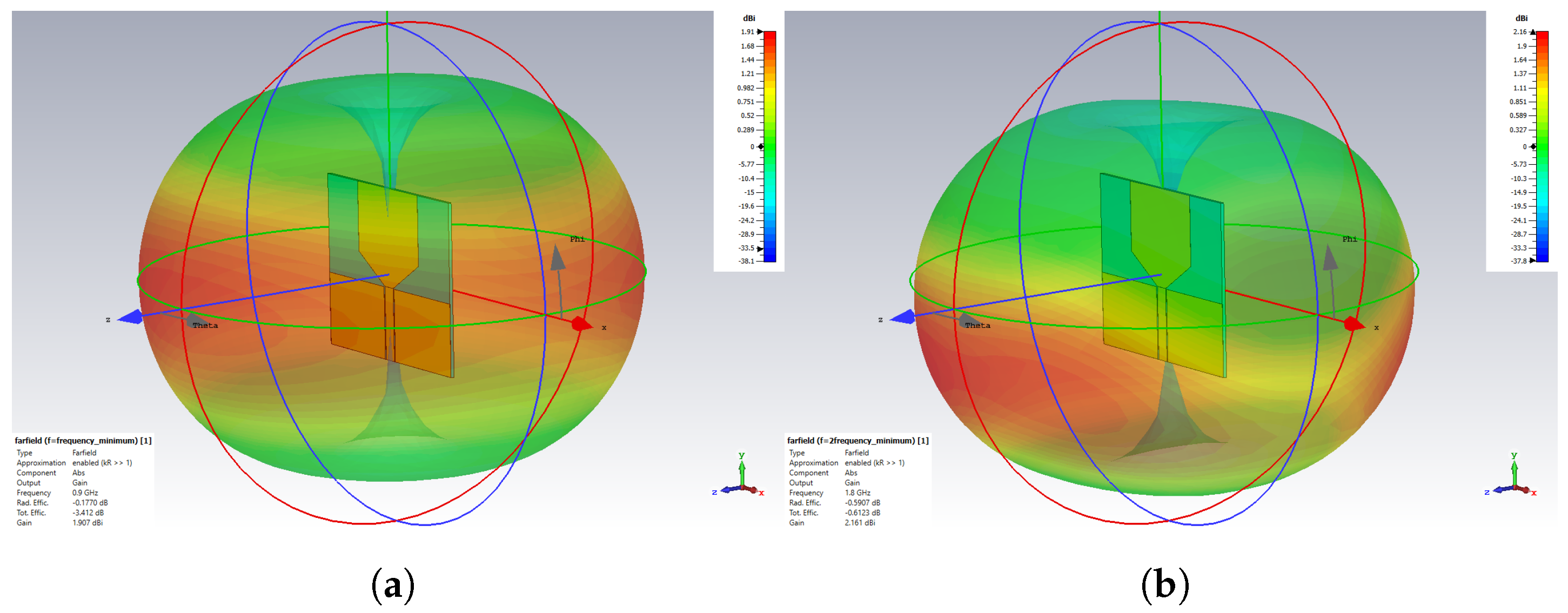
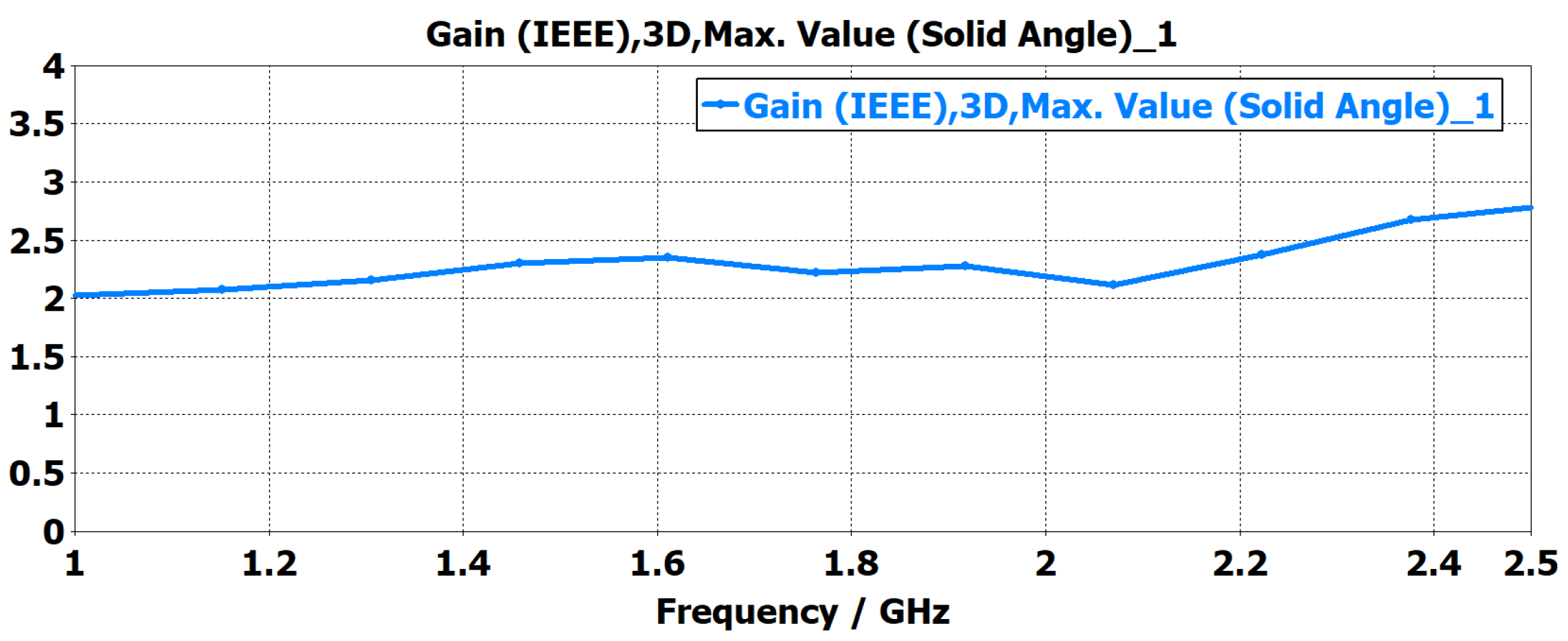
- A customized planar monopole antenna was designed for ND monitoring. The antenna features a low profile and wideband characteristics, operating within the frequency range of 1 to 2.5 GHz to ensure sufficient penetration depth for detecting LVE in the brain’s lateral regions. The wideband capability was achieved through a tapered structure at the radiating patch and the feeding line. The antenna’s near-field performance, when placed close to brain phantoms, was evaluated in terms of time domain characterization, E-field distribution, and S-parameter. The Specific Absorption Rate (SAR) was also assessed to ensure the antenna adheres to safety limits.
- A remote-controlling circuit was designed for remotely accessing and commanding the antenna pairs. The circuit includes a microcontroller, one-pole-four-throw (1P4T), Bluetooth module and mobile app. The S-parameter data, specifically the reflection coefficients, were collected when the antenna pairs were positioned in close proximity to brain phantoms. Two data sets were collected: one was collected manually, and one was collected using the controlling circuit. The two sets of data were then compared and processed with a radar-based algorithm to verify the effectiveness of the proposed system.
2. Antenna Design, Simulation and Fabrication
3. Near-Field Analysis
3.1. Time Domain Characterization
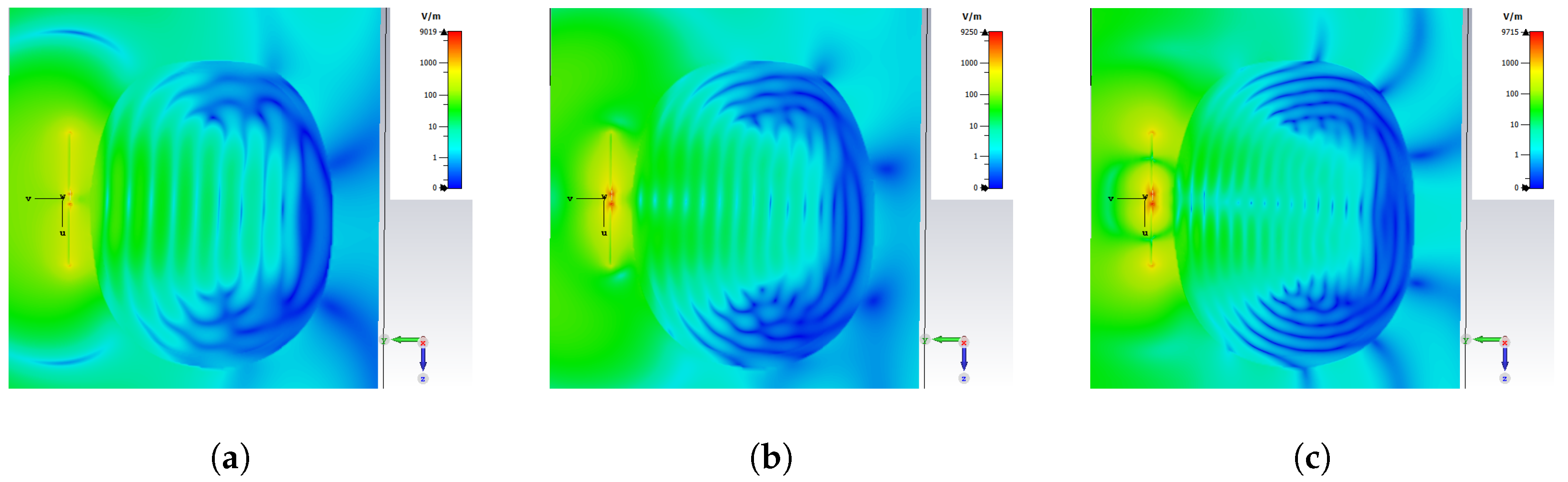
3.2. E-Field Distribution and Scattering Behaviour
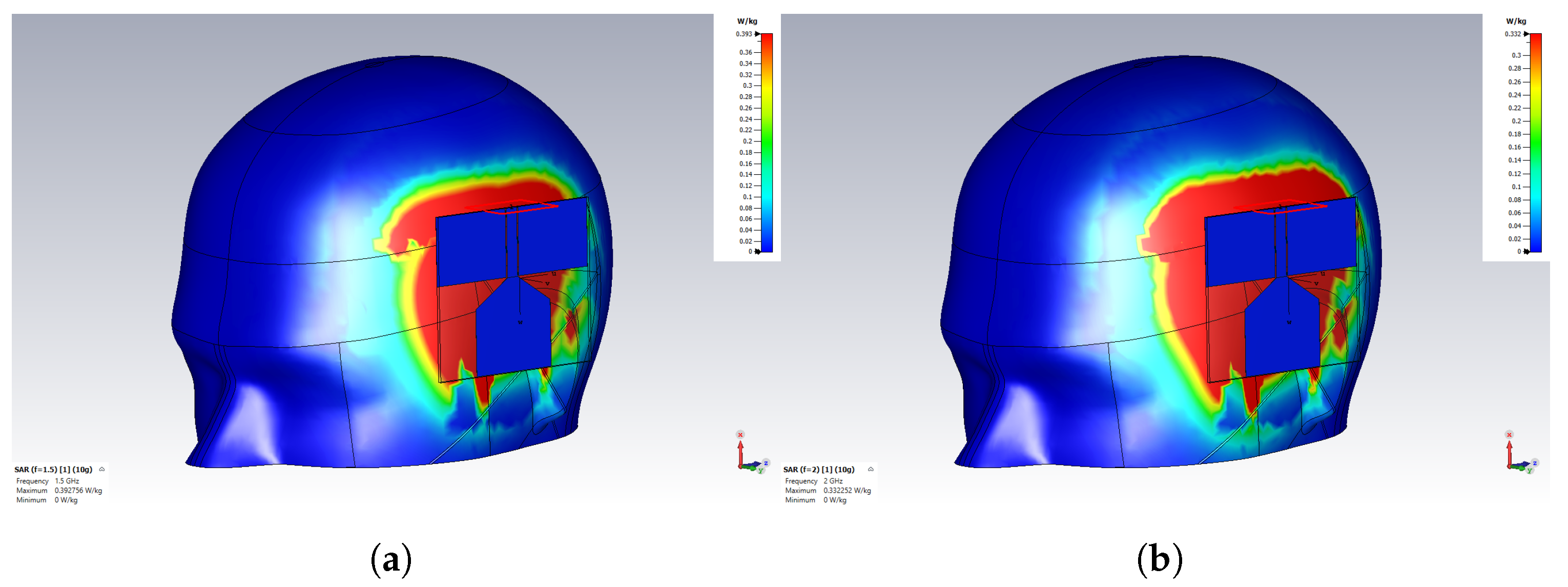
3.3. Health and Safety Evaluation
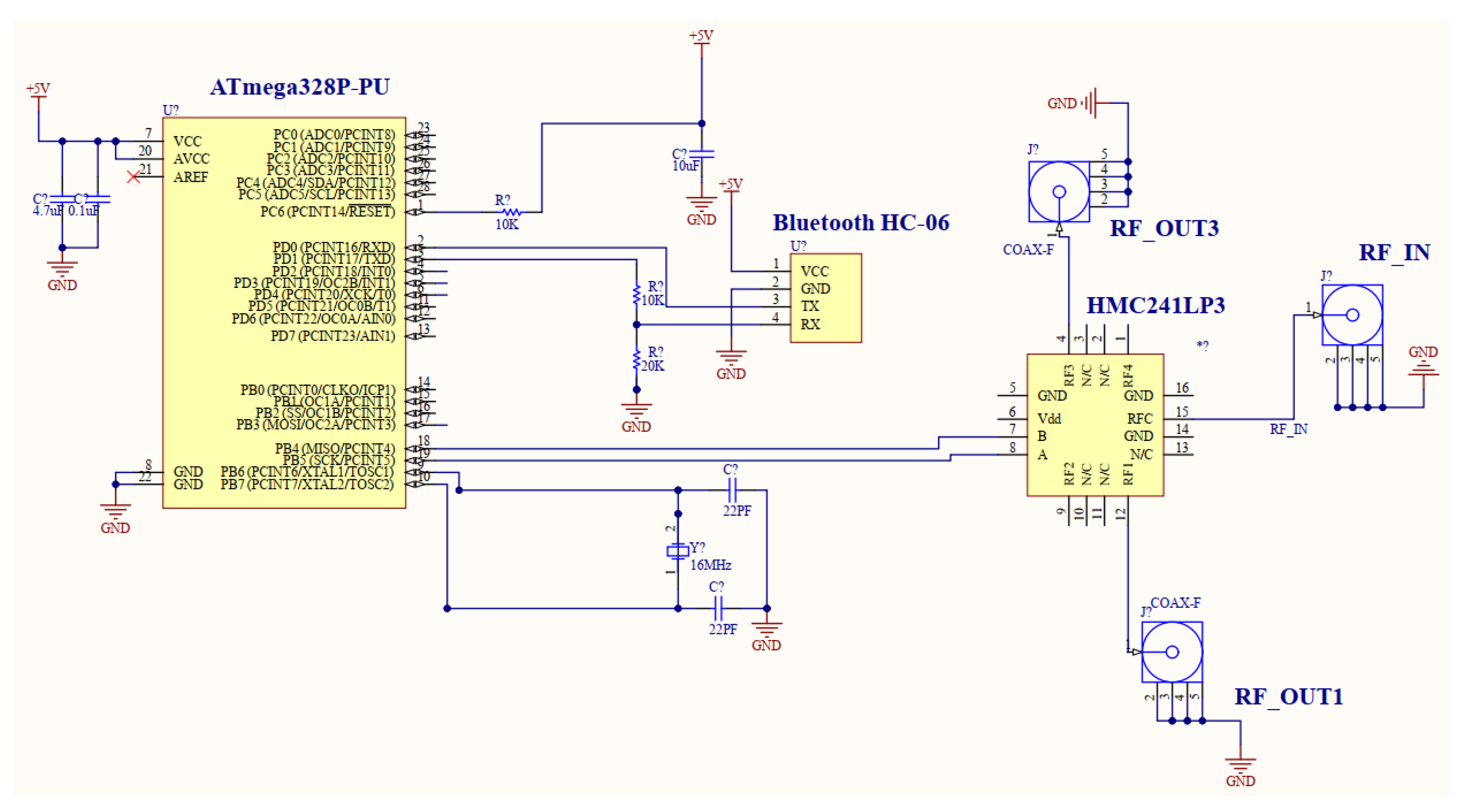
4. Controlling Circuit Design
5. Experimental Validation
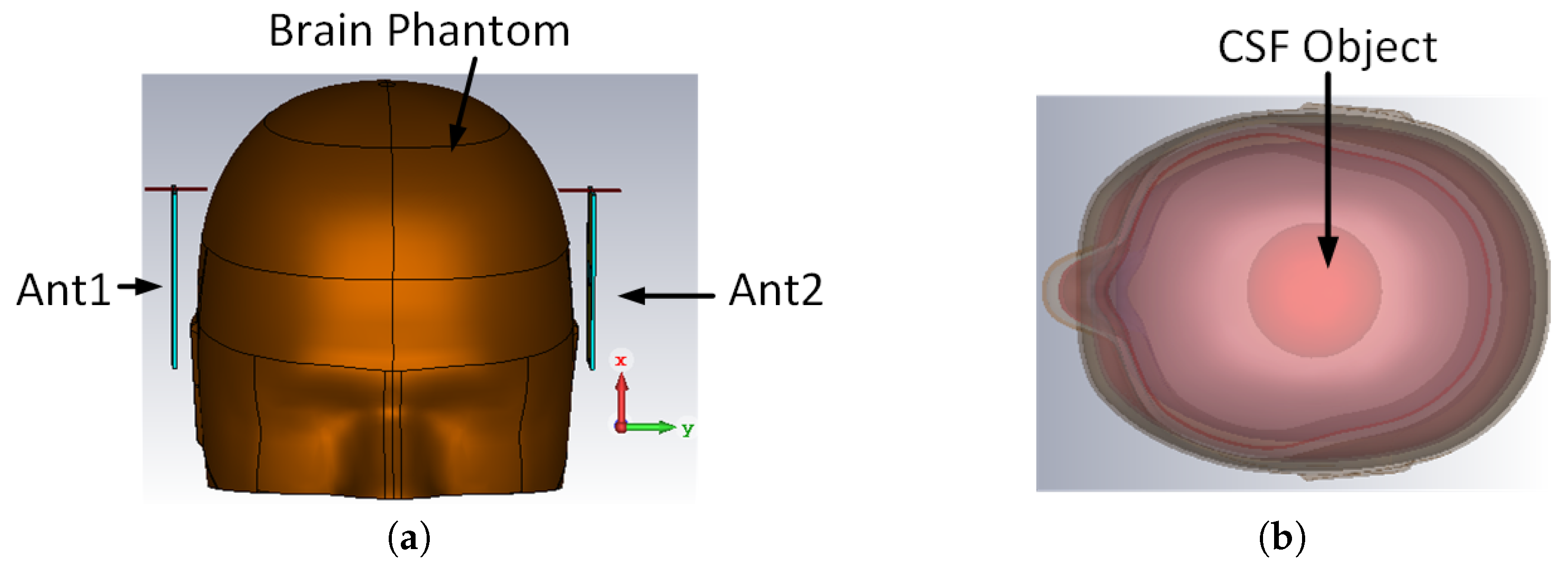
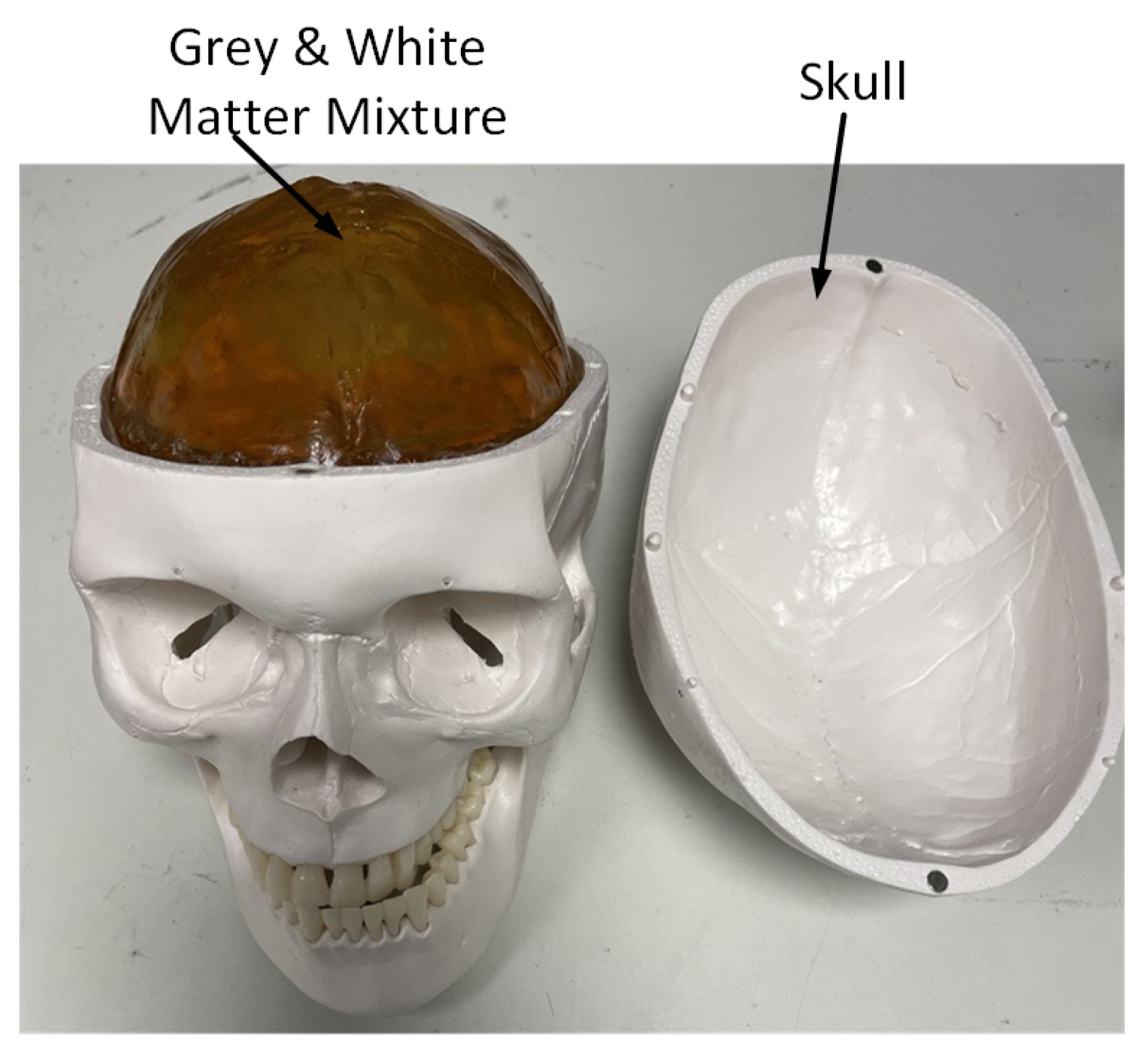
5.1. Brain Phantom
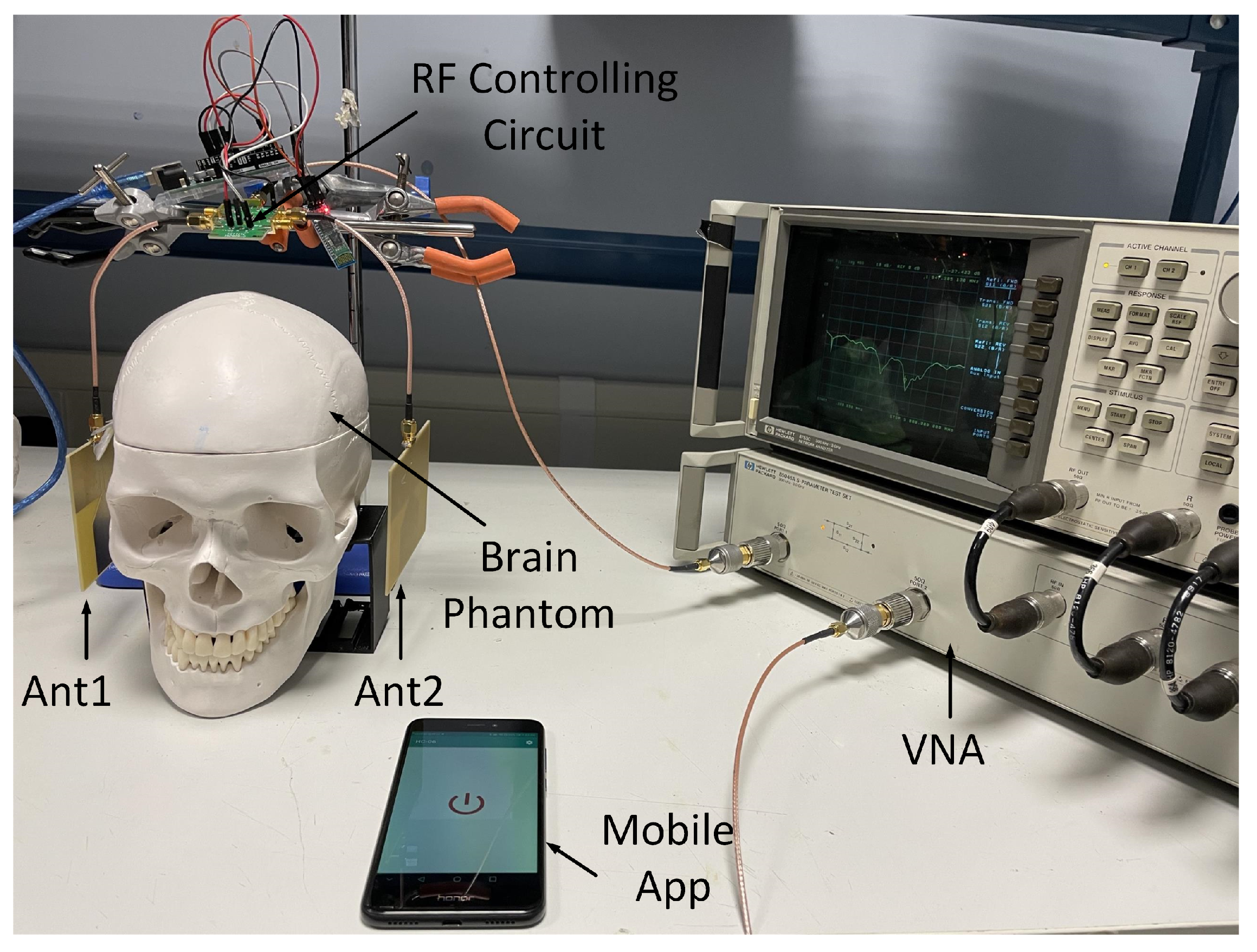
5.2. Measurement Set-Up
6. Results and Discussion
6.1. Switching Circuit Evaluation
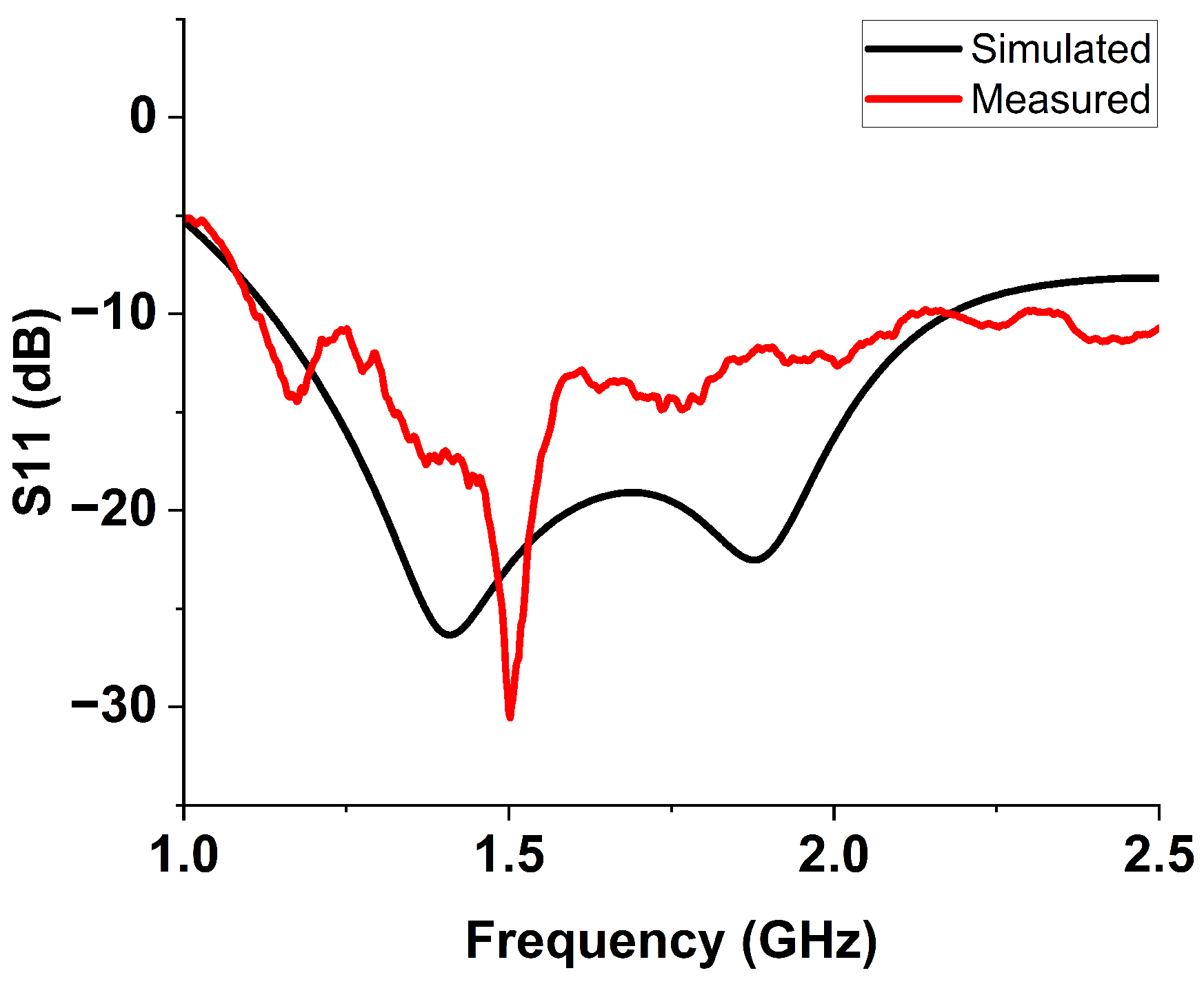
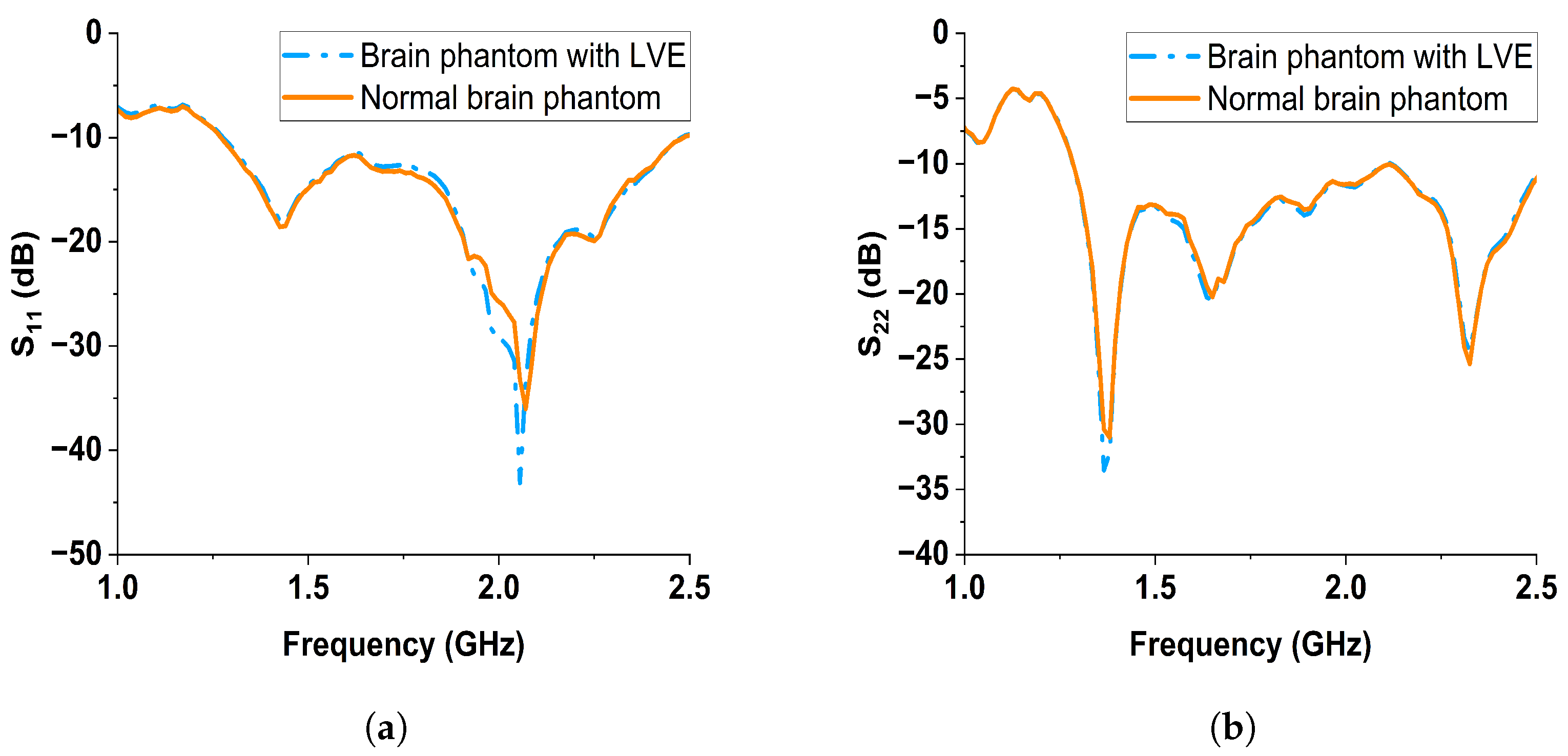
6.2. S-Parameter Results Discussion
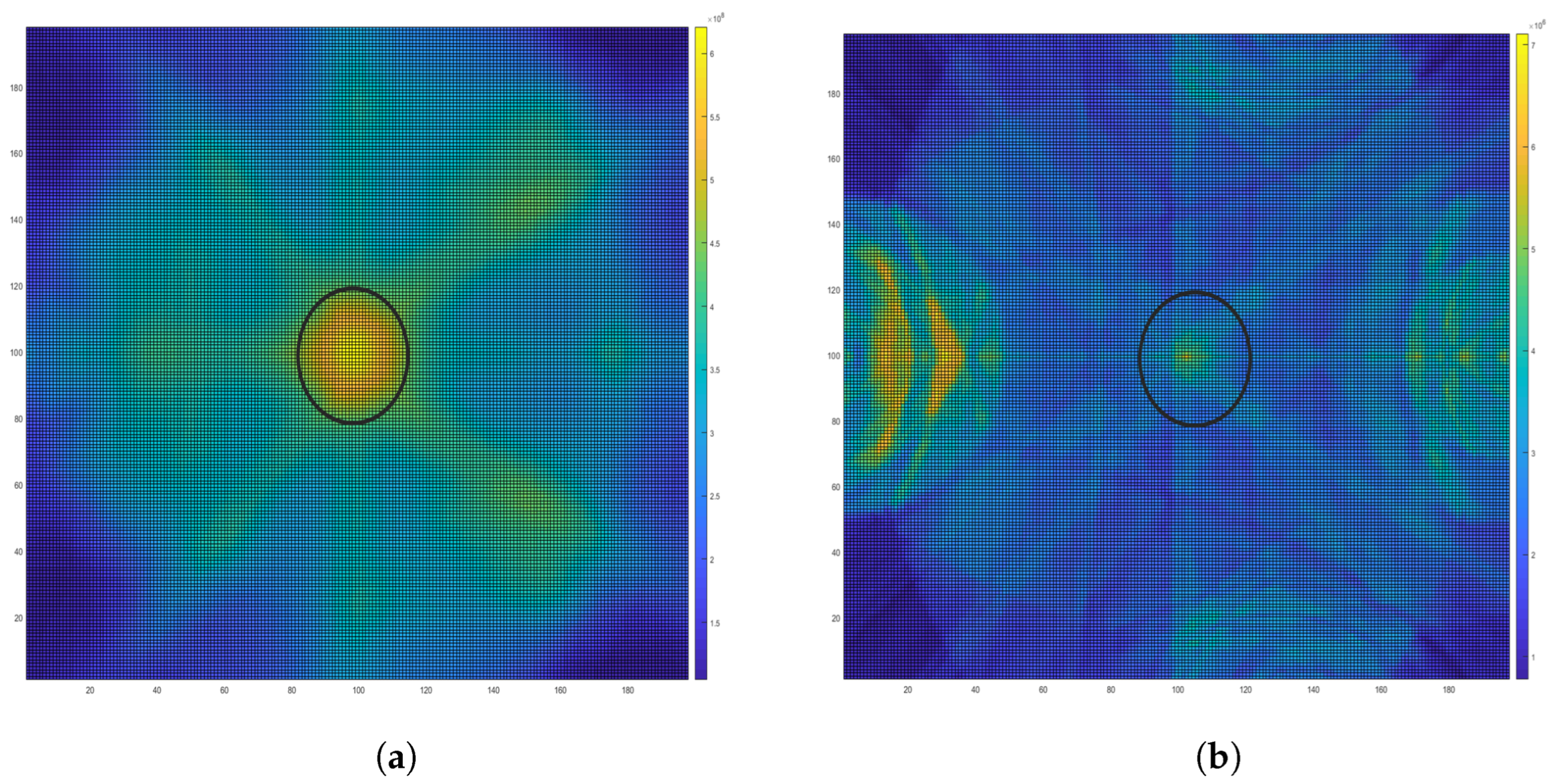
6.3. Image Reconstruction
7. Conclusions & Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ND | Neurodegenerative |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| MSI | Microwave Sensing and Imaging |
| MIST | Microwave Imaging via Space–Time |
| SMA | SubMiniature version A |
| LVE | Lateral Ventricular Enlargement |
| CSF | Cerebrospinal Fluid |
| CPW | Coplanar Waveguide |
| SAR | Specific Absorption Rate |
| VNA | Vector Network Analyzer |
| 1P1T | One-Pole One-Throw |
| 1P4T | One-Pole Four-Throw |
| RL | Return Loss |
| IL | Insertion Loss |
References
- Scheltens, P.; Blennow, K.; Breteler, M.M.; De Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.F.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Xu, W.; Li, J.Q.; Wang, J.; Lai, T.J.; et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Raji, C.A.; Lopez, O.; Kuller, L.; Carmichael, O.; Becker, J. Age, Alzheimer disease, and brain structure. Neurology 2009, 73, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Pini, L.; Pievani, M.; Bocchetta, M.; Altomare, D.; Bosco, P.; Cavedo, E.; Galluzzi, S.; Marizzoni, M.; Frisoni, G.B. Brain atrophy in Alzheimer’s disease and aging. Ageing Res. Rev. 2016, 30, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Nestor, S.M.; Rupsingh, R.; Borrie, M.; Smith, M.; Accomazzi, V.; Wells, J.L.; Fogarty, J.; Bartha, R.; Initiative, A.D.N. Ventricular enlargement as a possible measure of Alzheimer’s disease progression validated using the Alzheimer’s disease neuroimaging initiative database. Brain 2008, 131, 2443–2454. [Google Scholar] [CrossRef]
- Apostolova, L.G.; Green, A.E.; Babakchanian, S.; Hwang, K.S.; Chou, Y.Y.; Toga, A.W.; Thompson, P.M. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment and Alzheimer’s disease. Alzheimer Dis. Assoc. Disord. 2012, 26, 17. [Google Scholar] [CrossRef]
- Johnson, K.A.; Fox, N.C.; Sperling, R.A.; Klunk, W.E. Brain imaging in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006213. [Google Scholar] [CrossRef]
- McGinnis, S.M. Neuroimaging in neurodegenerative dementias. In Proceedings of the Seminars in Neurology; Thieme Medical Publishers: New York, NY, USA, 2012; Volume 32, pp. 347–360. [Google Scholar]
- Bao, W.; Xie, F.; Zuo, C.; Guan, Y.; Huang, Y.H. PET neuroimaging of Alzheimer’s disease: Radiotracers and their utility in clinical research. Front. Aging Neurosci. 2021, 13, 624330. [Google Scholar] [CrossRef]
- Chandra, R.; Zhou, H.; Balasingham, I.; Narayanan, R.M. On the opportunities and challenges in microwave medical sensing and imaging. IEEE Trans. Biomed. Eng. 2015, 62, 1667–1682. [Google Scholar] [CrossRef]
- Semenov, S. Microwave tomography: Review of the progress towards clinical applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 3021–3042. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Alqadami, A.S.; Abbosh, A. Stroke Diagnosis Using Microwave Techniques: Review of Systems and Algorithms. IEEE J. Electromagn. Microwaves Med. Biol. 2022, 7, 122–135. [Google Scholar] [CrossRef]
- Susskind, C. Possible use of microwaves in the management of lung disease. Proc. IEEE 1973, 61, 673–674. [Google Scholar] [CrossRef]
- Shao, W.; McCollough, T. Advances in microwave near-field imaging: Prototypes, systems, and applications. IEEE Microw. Mag. 2020, 21, 94–119. [Google Scholar] [CrossRef]
- O’Loughlin, D.; O’Halloran, M.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave breast imaging: Clinical advances and remaining challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Rezaeieh, S.; Abbosh, A. Lung cancer detection using frequency-domain microwave imaging. Electron. Lett. 2015, 51, 740–741. [Google Scholar] [CrossRef]
- Pride, G.L.; Fraser, J.F.; Gupta, R.; Alberts, M.J.; Rutledge, J.N.; Fowler, R.; Ansari, S.A.; Abruzzo, T.; Albani, B.; Arthur, A.; et al. Prehospital care delivery and triage of stroke with emergent large vessel occlusion (ELVO): Report of the Standards and Guidelines Committee of the Society of Neurointerventional Surgery. J. Neurointerventional Surg. 2017, 9, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Brogan, R.J.; Kontojannis, V.; Garara, B.; Marcus, H.J.; Wilson, M.H. Near-infrared spectroscopy (NIRS) to detect traumatic intracranial haematoma: A systematic review and meta-analysis. Brain Inj. 2017, 31, 581–588. [Google Scholar] [CrossRef]
- Alqadami, A.S.; Zamani, A.; Trakic, A.; Abbosh, A. Flexible electromagnetic cap for three-dimensional electromagnetic head imaging. IEEE Trans. Biomed. Eng. 2021, 68, 2880–2891. [Google Scholar] [CrossRef]
- Saied, I.M.; Arslan, T. Noninvasive wearable RF device towards monitoring brain atrophy and lateral ventricle enlargement. IEEE J. Electromagn. RF Microwaves Med. Biol. 2019, 4, 61–68. [Google Scholar] [CrossRef]
- Saied, I.M.; Chandran, S.; Arslan, T. Integrated flexible hybrid silicone-textile dual-resonant sensors and switching circuit for wearable neurodegeneration monitoring systems. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Anwar, U.; Arslan, T.; Hussain, A.; Russ, T.C.; Lomax, P. Design and Evaluation of Wearable Multimodal RF Sensing System for Vascular Dementia Detection. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.J.; Abbosh, A.M.; Mustafa, S.; Ireland, D. Microwave system for head imaging. IEEE Trans. Instrum. Meas. 2013, 63, 117–123. [Google Scholar] [CrossRef]
- Mobashsher, A.T.; Abbosh, A.M.; Wang, Y. Microwave system to detect traumatic brain injuries using compact unidirectional antenna and wideband transceiver with verification on realistic head phantom. IEEE Trans. Microw. Theory Tech. 2014, 62, 1826–1836. [Google Scholar] [CrossRef]
- Balanis, C.A. Antenna Theory: Analysis and Design; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Balanis, C.A. Modern Antenna Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave radiation and the brain: Mechanisms, current status, and future prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef]
- IEEE Std C95.1-2019 (Revision of IEEE Std C95.1-2005/Incorporates IEEE Std C95.1-2019/Cor 1-2019); IEEE Standard for Safety Levels with Respect to Human Exposure to Electric, Magnetic, and Electromagnetic Fields, 0 Hz to 300 GHz. 2019; pp. 1–312. Available online: https://ieeexplore.ieee.org/document/8859679 (accessed on 29 December 2023). [CrossRef]
- Midi, N.S.; Sasaki, K.; Ohyama, R.i.; Shinyashiki, N. Broadband complex dielectric constants of water and sodium chloride aqueous solutions with different DC conductivities. IEEJ Trans. Electr. Electron. Eng. 2014, 9, S8–S12. [Google Scholar] [CrossRef]
- Bond, E.J.; Li, X.; Hagness, S.C.; Van Veen, B.D. Microwave imaging via space-time beamforming for early detection of breast cancer. IEEE Trans. Antennas Propag. 2003, 51, 1690–1705. [Google Scholar] [CrossRef]








| Dimension Parameters | Symbol | Value |
|---|---|---|
| Substrate Length | 75.70 mm | |
| Substrate Width | 88.00 mm | |
| Substrate Thickness | 1.55 mm | |
| Patch Length | 30.89 mm | |
| Patch Width | 43.49 mm | |
| Ground Length | 32.00 mm | |
| Ground Width | 40.35 mm | |
| Feedline Length | 32.21 mm | |
| Feedline Width | 6.00 mm | |
| Copper Thickness | 0.035 mm | |
| Tapered Structure Angle | 146° |
| SAR (W/Kg) | 1.5 GHz | 2 GHz |
|---|---|---|
| 100 mW | 0.39 | 0.33 |
| 500 mW | 1.96 | 1.66 |
| Material | Quantity (mL) |
|---|---|
| Water | 700 |
| Sugar | 600 |
| Gelatin | 100 |
| RF Path | Return Loss (dB) | Insertion Loss (dB) |
|---|---|---|
| RF1 | −23.25 | −0.39 |
| RF3 | −23.77 | −0.76 |
| System | Platform | Antenna Type | Antenna Number | Frequency Band (GHz) | Targetting Disease |
|---|---|---|---|---|---|
| 20 | Wearable cap | Planar multi-slot | 24 | 0.9–2.5 | Stroke |
| 22 | Wearable hat | Planar monopole | 6 | 0.6–0.8 & 1.8–2.09 | ND |
| 23 | Wearable glass | Planar monopole | 4 | 1.2–2.5 | ND & Stroke |
| 24 | Circular platform | Vivaldi | 16 | 1–4 | Stroke |
| 25 | Rotatble platform | Slotted dipole | 1 | 1.1–3.4 | Brain injury |
| This Work | Portable platform | Planar monopole | 2 | 1.2–2.2 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Riaz, A.; Saied, I.M.; Shami, Z.; Arslan, T. Dual-Planar Monopole Antenna-Based Remote Sensing System for Microwave Medical Applications. Sensors 2024, 24, 328. https://doi.org/10.3390/s24020328
Zhao M, Riaz A, Saied IM, Shami Z, Arslan T. Dual-Planar Monopole Antenna-Based Remote Sensing System for Microwave Medical Applications. Sensors. 2024; 24(2):328. https://doi.org/10.3390/s24020328
Chicago/Turabian StyleZhao, Minghui, Asad Riaz, Imran M. Saied, Zain Shami, and Tughrul Arslan. 2024. "Dual-Planar Monopole Antenna-Based Remote Sensing System for Microwave Medical Applications" Sensors 24, no. 2: 328. https://doi.org/10.3390/s24020328
APA StyleZhao, M., Riaz, A., Saied, I. M., Shami, Z., & Arslan, T. (2024). Dual-Planar Monopole Antenna-Based Remote Sensing System for Microwave Medical Applications. Sensors, 24(2), 328. https://doi.org/10.3390/s24020328






