The Effect of Caffeine on Movement-Related Cortical Potential Morphology and Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup
2.3. Recordings
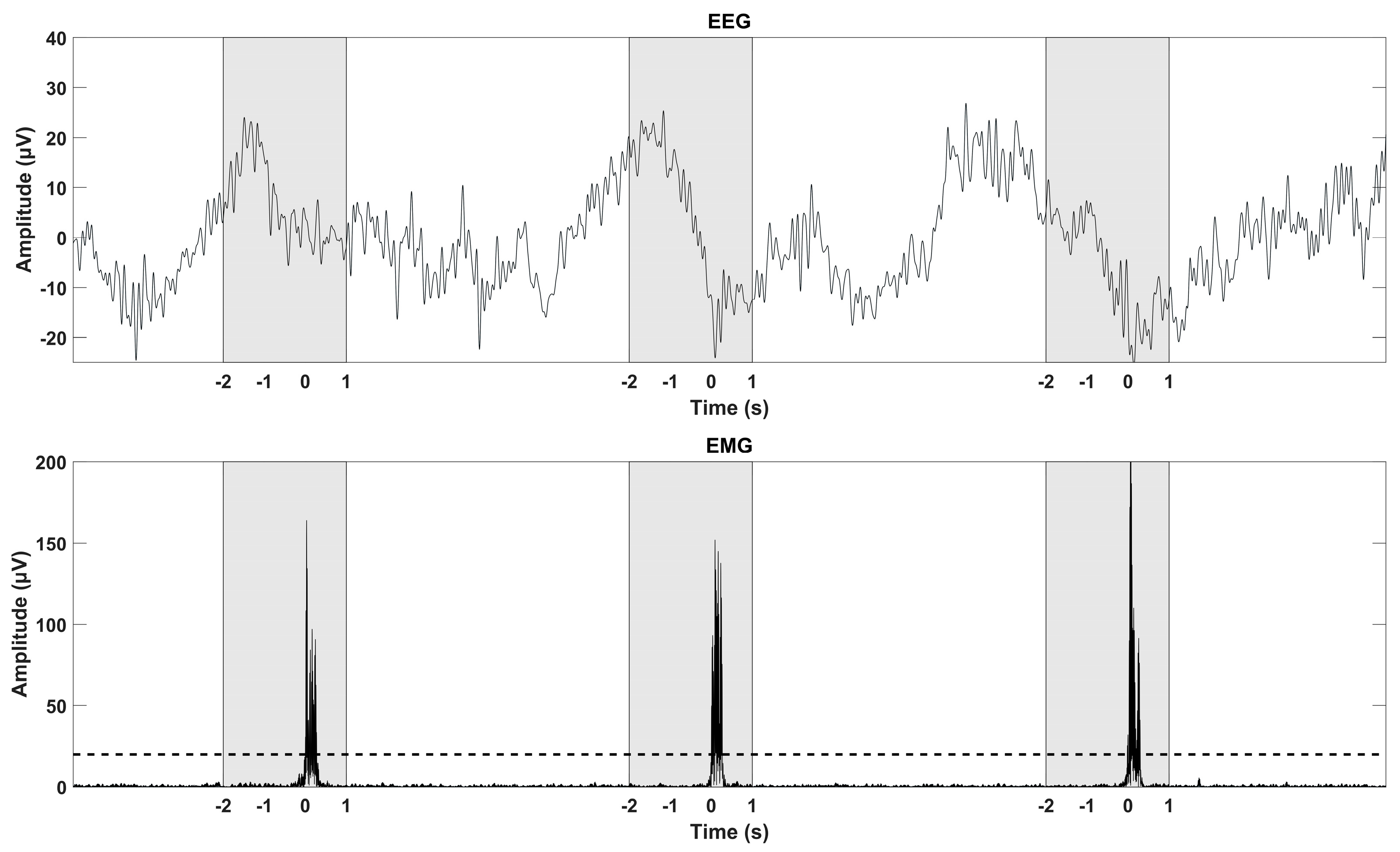
2.3.1. EEG
2.3.2. EMG
2.4. Data Analysis—EMG Detection
2.5. Data Analysis—MRCP Morphology
2.6. Data Analysis—MRCP Detection
2.6.1. Pre-Processing
2.6.2. Feature Extraction and Classification
2.7. Statistical Analysis
3. Results
3.1. MRCP Morphology
3.2. MRCP Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olsen, S.; Alder, G.; Williams, M.; Chambers, S.; Jochumsen, M.; Signal, N.; Rashid, U.; Niazi, I.K.; Taylor, D. Electroencephalographic recording of the movement-related cortical potential in ecologically valid movements: A scoping review. Front. Neurosci. 2021, 15, 721387. [Google Scholar] [CrossRef] [PubMed]
- Jochumsen, M.; Niazi, I.K.; Mrachacz-Kersting, N.; Jiang, N.; Farina, D.; Dremstrup, K. Comparison of spatial filters and features for the detection and classification of movement-related cortical potentials in healthy individuals and stroke patients. J. Neural Eng. 2015, 12, 056003. [Google Scholar] [CrossRef] [PubMed]
- Jochumsen, M.; Niazi, I.K.; Taylor, D.; Farina, D.; Dremstrup, K. Detecting and classifying movement-related cortical potentials associated with hand movements in healthy subjects and stroke patients from single-electrode, single-trial EEG. J. Neural Eng. 2015, 12, 056013. [Google Scholar] [CrossRef] [PubMed]
- Niazi, I.K.; Jiang, N.; Tiberghien, O.; Nielsen, J.F.; Dremstrup, K.; Farina, D. Detection of movement intention from single-trial movement-related cortical potentials. J. Neural Eng. 2011, 8, 066009. [Google Scholar] [CrossRef] [PubMed]
- Jochumsen, M.; Shafique, M.; Hassan, A.; Niazi, I.K. Movement intention detection in adolescents with cerebral palsy from single-trial EEG. J. Neural Eng. 2018, 15, 066030. [Google Scholar] [CrossRef] [PubMed]
- Ofner, P.; Schwarz, A.; Pereira, J.; Wyss, D.; Wildburger, R.; Müller-Putz, G.R. Attempted Arm and Hand Movements can be Decoded from Low-Frequency EEG from Persons with Spinal Cord Injury. Sci. Rep. 2019, 9, 7134. [Google Scholar] [CrossRef]
- Leerskov, K.; Rehman, M.; Niazi, I.; Cremoux, S.; Jochumsen, M. Investigating the feasibility of combining EEG and EMG for controlling a hybrid human computer interface in patients with spinal cord injury. In Proceedings of the 2020 IEEE 20th International Conference on Bioinformatics and Bioengineering (BIBE), Cincinnati, OH, USA, 26–28 October 2020; pp. 403–410. [Google Scholar]
- Xu, R.; Jiang, N.; Vuckovic, A.; Hasan, M.; Mrachacz-Kersting, N.; Allan, D.; Fraser, M.; Nasseroleslami, B.; Conway, B.; Dremstrup, K. Movement-related cortical potentials in paraplegic patients: Abnormal patterns and considerations for BCI-rehabilitation. Front. Neuroeng. 2014, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Savic, A.; Aliakbaryhosseinabadi, S.; Blicher, J.; Farina, D.; Mrachacz-Kersting, N.; Dosen, S. Online control of an assistive active glove by slow cortical signals in patients with amyotrophic lateral sclerosis. J. Neural Eng. 2021, 18, 046085. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Farina, D.; Murguialday, A.R.; Dremstrup, K.; Montoya, P.; Birbaumer, N. Offline identification of imagined speed of wrist movements in paralyzed ALS patients from single-trial EEG. Front. Neurosci. 2009, 3, 664. [Google Scholar]
- Aliakbaryhosseinabadi, S.; Dosen, S.; Savic, A.; Blicher, J.; Farina, D.; Mrachacz-Kersting, N. Participant-specific classifier tuning increases the performance of hand movement detection from EEG in patients with Amyotrophic Lateral Sclerosis. J. Neural Eng. 2021, 18, 056023. [Google Scholar] [CrossRef]
- Kornhuber, H.H.; Deecke, L. Hirnpotentialänderrungen bei Willkürbewegungen und passiven Bewegungen des Menschen: Bereitschaftspotential und reafferente Potentiale. Pflügers Arch. Ges. Physiol. 1965, 284, 1–17. [Google Scholar] [CrossRef]
- Walter, W.G.; Cooper, R.; Aldridge, V.J.; McCallum, W.C.; Winter, A.L. Contingent negative variation: An electric sign of sensorimotor association and expectancy in the human brain. Nature 1964, 203, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, H.; Hallett, M. What Is Bereitschaftspotential? Clin. Neurophysiol. 2006, 117, 2341–2356. [Google Scholar] [CrossRef] [PubMed]
- Jochumsen, M.; Niazi, I.K. Detection and classification of single-trial movement-related cortical potentials associated with functional lower limb movements. J. Neural Eng. 2020, 17, 035009. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, O.F.; Dremstrup Nielsen, K.; Voigt, M. Movement-related parameters modulate cortical activity during imaginary isometric plantar-flexions. Exp. Brain Res. 2006, 171, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Arai, N.; Tsai, C.; Ziemann, U. Movement related cortical potentials of cued versus self-initiated movements: Double dissociated modulation by dorsal premotor cortex versus supplementary motor area rTMS. Hum. Brain Mapp. 2011, 33, 824–839. [Google Scholar] [CrossRef] [PubMed]
- Kæseler, R.L.; Johansson, T.W.; Struijk, L.N.A.; Jochumsen, M. Feature and classification analysis for detection and classification of tongue movements from single-trial pre-movement EEG. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Slobounov, S.; Hallett, M.; Newell, K.M. Perceived effort in force production as reflected in motor-related cortical potentials. Clin. Neurophysiol. 2004, 115, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Ofner, P.; Schwarz, A.; Pereira, J.; Müller-Putz, G.R. Upper limb movements can be decoded from the time-domain of low-frequency EEG. PLoS ONE 2017, 12, e0182578. [Google Scholar] [CrossRef]
- Lopez-Larraz, E.; Montesano, L.; Gil-Agudo, A.; Minguez, J. Continuous decoding of movement intention of upper limb self-initiated analytic movements from pre-movement EEG correlates. J. Neuroeng. Rehabil. 2014, 11, 153. [Google Scholar] [CrossRef]
- Jochumsen, M.; Niazi, I.K.; Dremstrup, K.; Kamavuako, E.N. Detecting and classifying three different hand movement types through electroencephalography recordings for neurorehabilitation. Med. Biol. Eng. Comput. 2015, 54, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Jochumsen, M.; Niazi, I.K.; Mrachacz-Kersting, N.; Farina, D.; Dremstrup, K. Detection and classification of movement-related cortical potentials associated with task force and speed. J. Neural Eng. 2013, 10, 056015. [Google Scholar] [CrossRef] [PubMed]
- Ofner, P.; Muller-Putz, G.R. Using a Noninvasive Decoding Method to Classify Rhythmic Movement Imaginations of the Arm in Two Planes. IEEE Trans. Biomed. Eng. 2015, 62, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Aliakbaryhosseinabadi, S.; Kostic, V.; Pavlovic, A.; Radovanovic, S.; Kamavuako, E.N.; Jiang, N.; Petrini, L.; Dremstrup, K.; Farina, D.; Mrachacz-Kersting, N. Influence of attention alternation on movement-related cortical potentials in healthy individuals and stroke patients. Clin. Neurophysiol. 2017, 128, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Hatta, A.; Nishihira, Y.; Higashiura, T.; Kim, S.R.; Kaneda, T. Long-term motor practice induces practice-dependent modulation of movement-related cortical potentials (MRCP) preceding a self-paced non-dominant handgrip movement in kendo players. Neurosci. Lett. 2009, 459, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, D.; Lange, F.; Seer, C.; Kopp, B.; Jahanshahi, M. Movement-related potentials in Parkinson’s disease. Clin. Neurophysiol. 2016, 127, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Yamashiro, K.; Onishi, H.; Yasuhiro, B.; Shimoyama, Y.; Maruyama, A. Whole-hand water flow stimulation increases motor cortical excitability: A study of transcranial magnetic stimulation and movement-related cortical potentials. J. Neurophysiol. 2015, 113, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Thacker, J.S.; Middleton, L.E.; McIlroy, W.E.; Staines, W.R. The influence of an acute bout of aerobic exercise on cortical contributions to motor preparation and execution. Physiol. Rep. 2014, 2, e12178. [Google Scholar] [CrossRef] [PubMed]
- de Tommaso, M.; Serpino, C.; Ricci, K.; Franco, G.; Devitofrancesco, V.; Livrea, P. Effects of low and high frequency repetitive transcranial magnetic stimulation of the primary motor cortex on contingent negative variations in normal subjects. Neurosci. Lett. 2012, 509, 39–43. [Google Scholar] [CrossRef]
- Lu, M.; Bliem, B.; Jung, P.; Arai, N.; Tsai, C.; Ziemann, U. Modulation of preparatory volitional motor cortical activity by paired associative transcranial magnetic stimulation. Hum. Brain. Mapp. 2009, 30, 3645–3656. [Google Scholar] [CrossRef]
- Wright, D.J.; Holmes, P.S.; Smith, D. Using the movement-related cortical potential to study motor skill learning. J. Mot. Behav. 2011, 43, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Jochumsen, M.; Christensen, C.R.; Christensen, H.R.; Cremoux, S.; Signal, N.; Allen, K.; Taylor, D.; Niazi, I.K. Quantification of movement-related EEG correlates associated with motor training: A study on movement-related cortical potentials and sensorimotor rhythms. Front. Hum. Neurosci. 2017, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Niazi, I.K.; Kersting, N.M.; Jiang, N.; Dremstrup, K.; Farina, D. Peripheral Electrical Stimulation Triggered by Self-Paced Detection of Motor Intention Enhances Motor Evoked Potentials. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Jiang, N.; Mrachacz-Kersting, N.; Lin, C.; Asin, G.; Moreno, J.; Pons, J.; Dremstrup, K.; Farina, D. A Closed-Loop Brain-Computer Interface Triggering an Active Ankle-Foot Orthosis for Inducing Cortical Neural Plasticity. IEEE Trans. Biomed. Eng. 2014, 20, 2092–2101. [Google Scholar]
- Jochumsen, M.; Cremoux, S.; Robinault, L.; Lauber, J.; Arceo, J.; Navid, M.; Nedergaard, R.; Rashid, U.; Haavik, H.; Niazi, I. Investigation of Optimal Afferent Feedback Modality for Inducing Neural Plasticity with A Self-Paced Brain-Computer Interface. Sensors 2018, 18, 3761. [Google Scholar] [CrossRef] [PubMed]
- Mrachacz-Kersting, N.; Kristensen, S.R.; Niazi, I.K.; Farina, D. Precise temporal association between cortical potentials evoked by motor imagination and afference induces cortical plasticity. J. Physiol. 2012, 590, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Wentrup, M.; Mattia, D.; Oweiss, K. Using brain-computer interfaces to induce neural plasticity and restore function. J. Neural Eng. 2011, 8, 025004. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, J.; Serrano, J.I.; Del Castillo, M.D.; Monge-Pereira, E.; Molina-Rueda, F.; Alguacil-Diego, I.; Pons, J.L. Detection of the onset of upper-limb movements based on the combined analysis of changes in the sensorimotor rhythms and slow cortical potentials. J. Neural Eng. 2014, 11, 056009. [Google Scholar] [CrossRef]
- Lew, E.; Chavarriaga, R.; Silvoni, S.; Millán, J.R. Detection of self-paced reaching movement intention from EEG signals. Front. Neuroeng. 2012, 5, 13. [Google Scholar] [CrossRef]
- Lorist, M.M.; Tops, M. Caffeine, fatigue, and cognition. Brain Cogn. 2003, 53, 82–94. [Google Scholar] [CrossRef]
- Ashton, H.; Millman, J.E.; Telford, R.; Thompson, J.W. The effect of caffeine, nitrazepam and cigarette smoking on the contingent negative variation in man. Electroencephalogr. Clin. Neurophysiol. 1974, 37, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Barthel, T.; Mechau, D.; Wehr, T.; Schnittker, R.; Liesen, H.; Weiss, M. Readiness potential in different states of physical activation and after ingestion of taurine and/or caffeine containing drinks. Amino Acids 2001, 20, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.; Mattie, H.; Plooij-van Gorsel, P.C.; Werre, P.F. The effects of a depressant and a stimulant drug on the contingent negative variation. Biol. Psychol. 1978, 6, 209–218. [Google Scholar] [CrossRef] [PubMed]
- de Morree, H.M.; Klein, C.; Marcora, S.M. Cortical substrates of the effects of caffeine and time-on-task on perception of effort. J. Appl. Physiol. 2014, 117, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, A.; Benedetti, F.; Carlino, E. Placebo-induced decrease in fatigue: Evidence for a central action on the preparatory phase of movement. Eur. J. Neurosci. 2015, 41, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.; Yang, D.; Li, C.; An, G.; Wang, J.; Shao, Y.; Fan, R.; Ma, Q. Effects of caffeine on event-related potentials and neuropsychological indices after sleep deprivation. Front. Behav. Neurosci. 2020, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Díaz, C.F.; Guerrero-Méndez, C.D.; Bastos-Filho, T.; Jaramillo-Isaza, S.; Ruiz-Olaya, A.F. Effects of the concentration level, eye fatigue and coffee consumption on the performance of a BCI system based on visual ERP-P300. J. Neurosci. Methods 2022, 382, 109722. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.R.; Struve, F.A.; Patrick, G. The effects of caffeine withdrawal on cognitive P300 auditory and visual evoked potentials. Clin. Electroencephalogr. 1999, 30, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Mundahl, J.H.; Streitz, T.D.; Maile, K.; Gulachek, N.S.; He, J.; He, B. Effects of soft drinks on resting state EEG and brain–computer interface performance. IEEE Access 2017, 5, 18756–18764. [Google Scholar] [CrossRef]
- Myrden, A.; Chau, T. Effects of user mental state on EEG-BCI performance. Front. Hum. Neurosci. 2015, 9, 308. [Google Scholar] [CrossRef]
- Li, S.; Duan, J.; Sun, Y.; Sheng, X.; Zhu, X.; Meng, J. Exploring fatigue effects on performance variation of intensive brain–computer interface practice. Front. Neurosci. 2021, 15, 773790. [Google Scholar] [CrossRef] [PubMed]


| Channel | Component | Pre 1 (µV) | Pre 2 (µV) | Post (µV) |
|---|---|---|---|---|
| Fz | RP | −1.4 ± 0.4 | −2.0 ± 0.4 | −1.5 ± 0.4 |
| NS | −6.5 ± 0.7 | −7.2 ± 0.6 | −6.2 ± 0.6 | |
| MP | −7.9 ± 0.8 | −8.6 ± 0.8 | −8.2 ± 0.8 | |
| FCz | RP | −0.5 ± 0.5 | −1.3 ± 0.4 | −0.8 ± 0.4 |
| NS | −8.2 ± 0.7 | −9.5 ± 0.7 | −8.6 ± 0.7 | |
| MP | −10.3 ± 1.0 | −11.6 ± 1.0 | −11.2 ± 0.9 | |
| Cz | RP | −0.5 ± 0.6 | −1.4 ± 0.4 | −0.7 ± 0.4 |
| NS | −9.0 ± 0.9 | −10.0 ± 0.8 | −9.1 ± 0.9 | |
| MP | −10.8 ± 1.3 | −11.8 ± 1.3 | −11.5 ± 1.3 |
| Classifier | Pre 1 (%) | Pre 2 (%) | Post (%) | |
|---|---|---|---|---|
| Random forest | Mean ± standard error | 79.3 ± 1.5 | 80.1 ± 1.9 | 82.3 ± 1.8 |
| Range [min–max] | [65.0–94.5] | [61.5–96.0] | [55.0–98.0] | |
| Linear discriminant analysis | Mean ± standard error | 78.8 ± 1.9 | 81.0 ± 2.0 | 82.4 ± 1.7 |
| Range [min max] | [62.5–97.0] | [58.0–98.0] | [63.5–99.0] | |
| K-nearest neighbors | Mean ± standard error | 71.3 ± 2.1 | 73.6 ± 2.4 | 72.8 ± 2.5 |
| Range [min max] | [51.6–94.0] | [55.5–96.0] | [50.5–96.0] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jochumsen, M.; Lavesen, E.R.; Griem, A.B.; Falkenberg-Andersen, C.; Jensen, S.K.G. The Effect of Caffeine on Movement-Related Cortical Potential Morphology and Detection. Sensors 2024, 24, 4030. https://doi.org/10.3390/s24124030
Jochumsen M, Lavesen ER, Griem AB, Falkenberg-Andersen C, Jensen SKG. The Effect of Caffeine on Movement-Related Cortical Potential Morphology and Detection. Sensors. 2024; 24(12):4030. https://doi.org/10.3390/s24124030
Chicago/Turabian StyleJochumsen, Mads, Emma Rahbek Lavesen, Anne Bruun Griem, Caroline Falkenberg-Andersen, and Sofie Kirstine Gedsø Jensen. 2024. "The Effect of Caffeine on Movement-Related Cortical Potential Morphology and Detection" Sensors 24, no. 12: 4030. https://doi.org/10.3390/s24124030
APA StyleJochumsen, M., Lavesen, E. R., Griem, A. B., Falkenberg-Andersen, C., & Jensen, S. K. G. (2024). The Effect of Caffeine on Movement-Related Cortical Potential Morphology and Detection. Sensors, 24(12), 4030. https://doi.org/10.3390/s24124030






