An Innovative EEG-Based Pain Identification and Quantification: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures
2.2.1. Procedure for Group 1: Healthy Participants— “No Pain” and “with Pain”
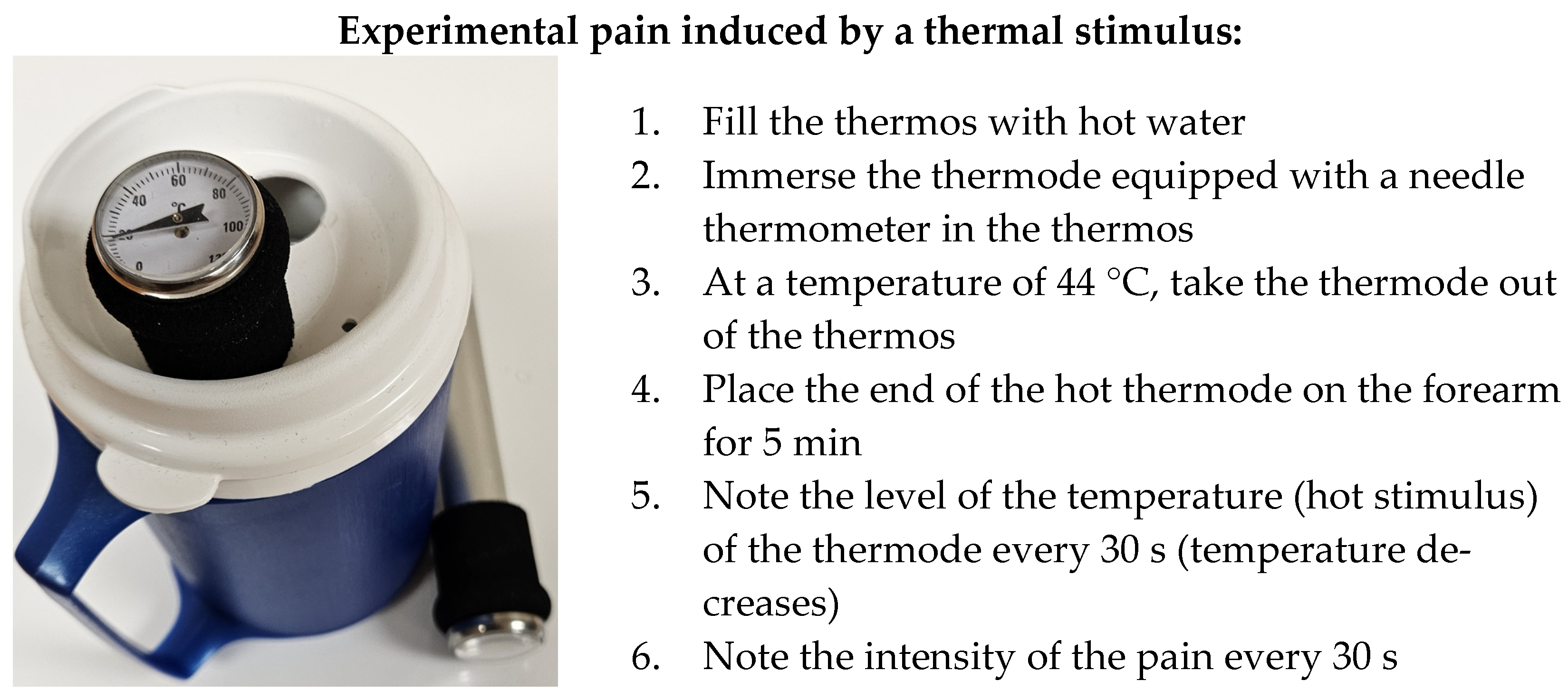
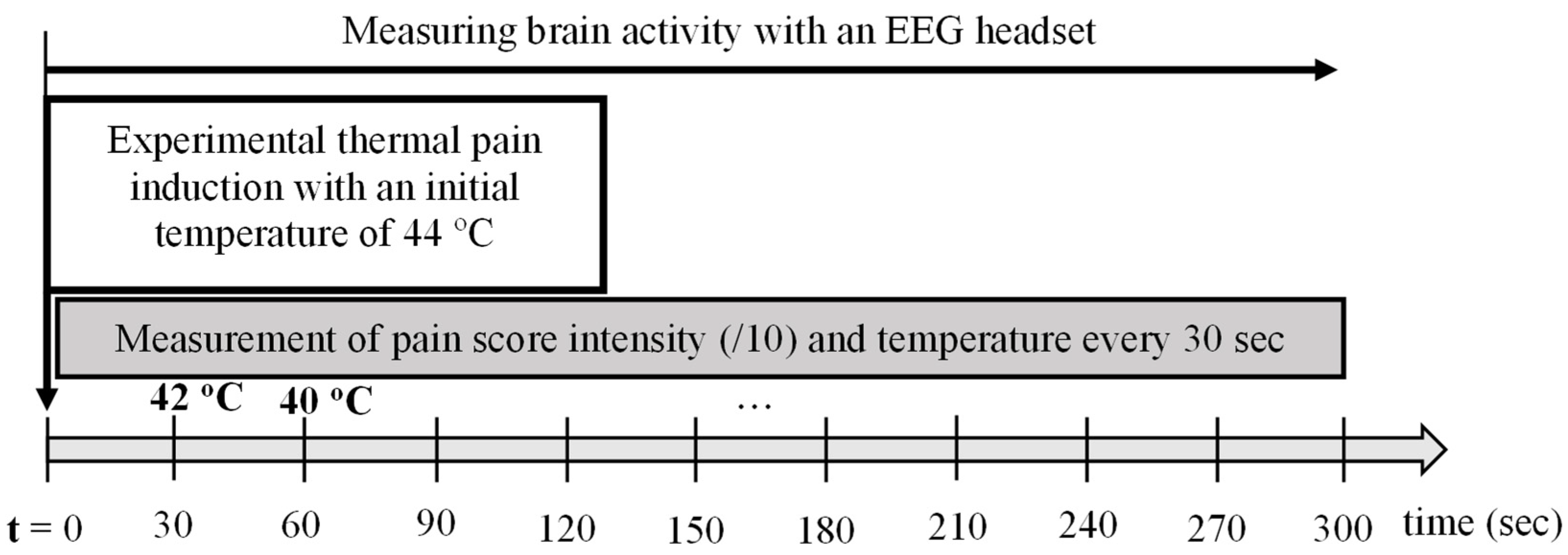
2.2.2. Procedure for Group 2: Healthy Participants Subjected to Thermal Experimental Pain
2.2.3. Procedure for Group 3: Participants Living with Chronic Pain
2.3. EEG Data Acquisition
2.3.1. EEG Data Preprocessing
EEG Signals Filtering
EEG Frequency Band Selection
EEG Signal Normalization
2.3.2. Extracting Pain-Related Feature from EEG Signals to Pain Identification and Quantification Indicator (Piq)
- (1)
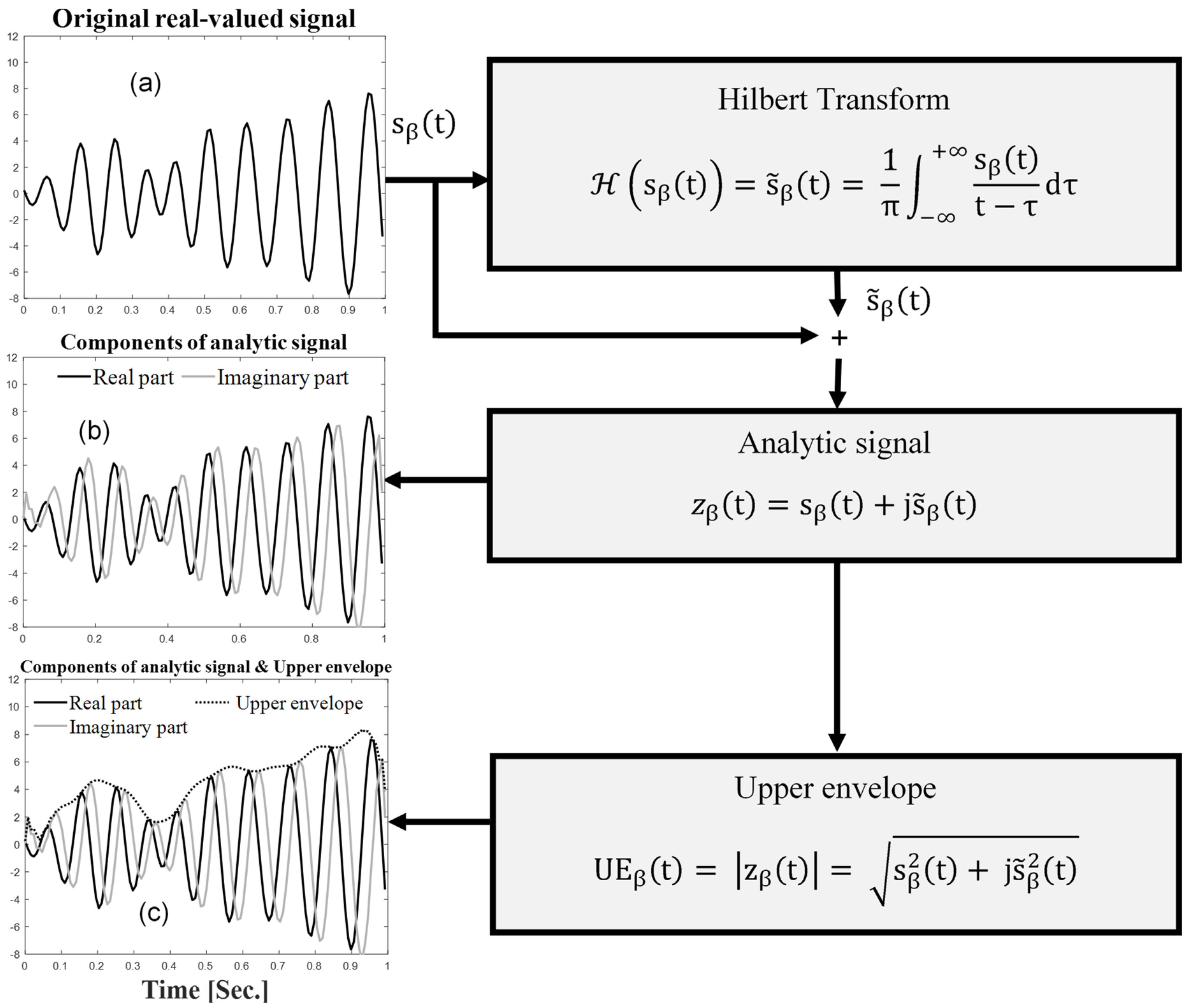
- The first step was the estimation of the analytic EEG signals for the beta frequency band () using Hilbert transform, as follows:where represents Hilbert transform, the steps of which are illustrated in Figure 4.
- (2)
- The second step was the extraction of the upper envelope (UE) of the EEG signals for the beta frequency band (), defined as the absolute value of the analytic signal, as follows:
- (3)
- The third step was the estimation of the coefficient of variation of the upper envelope in the beta EEG frequency band (CVUEβ). To this end, the mean and standard deviation (std) of were computed in each epoch to obtain CVUEβ as follows:
- (4)
- The fourth step consisted of smoothing using a 15th-order Savitzky–Golay filter.
- (5)
- The fifth step was the calculation of the pain identification and quantification (Piq) indicator in the beta frequency band (Piqβ).
2.4. Statistical Analysis
3. Results
3.1. New Approach to Identify and Quantify Pain
3.2. Descriptive Statistics of Groups to Meet Objective (2) i.e., Determine the Threshold Quantified for the Identification of the Presence of Pain
3.2.1. Group 1: Healthy Participants—“No Pain” and “with Pain”
3.2.2. Group 2: Healthy Participants Submitted to Thermal Pain
3.2.3. Group 3: Participants Living with Chronic Pain
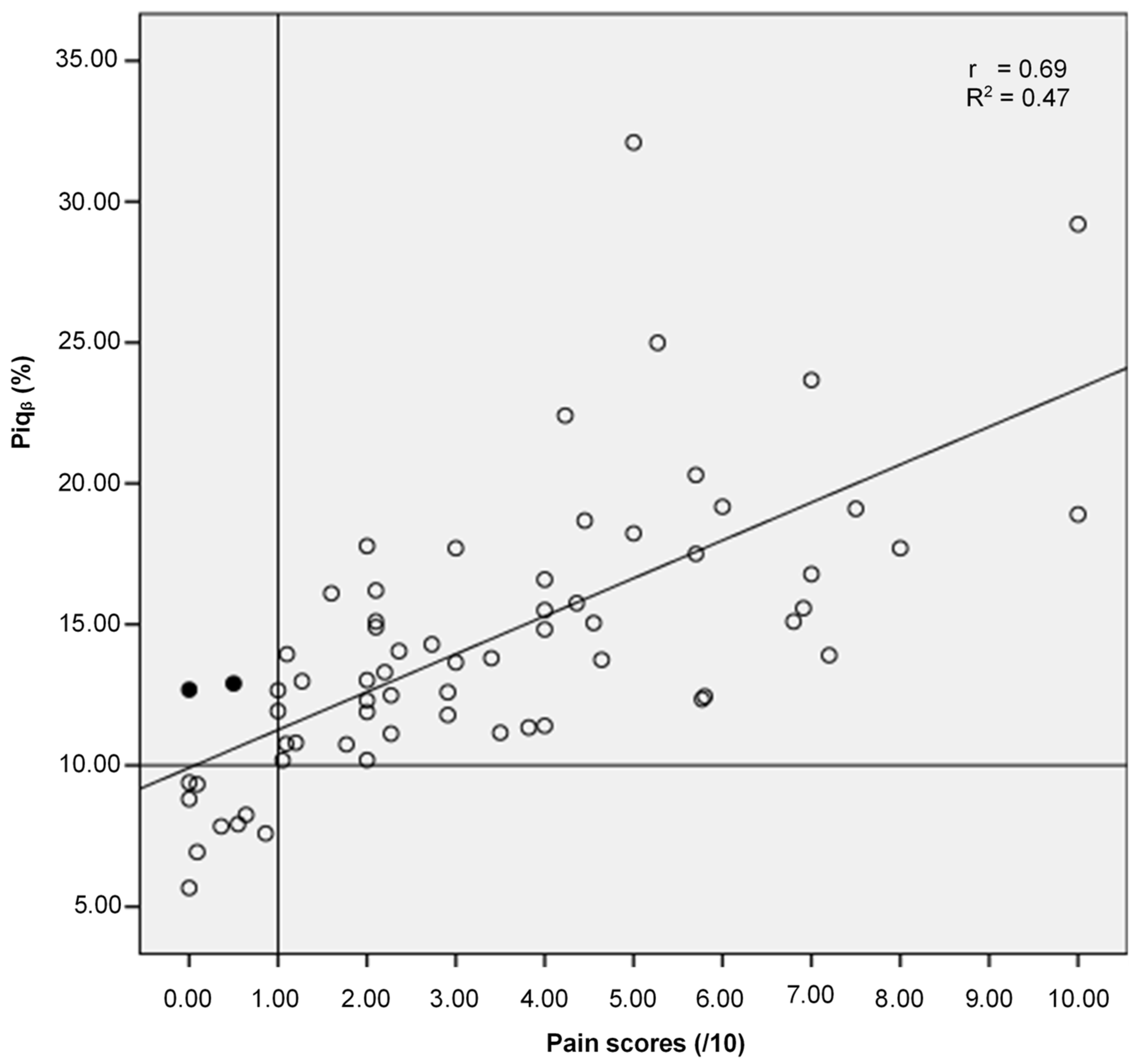
3.3. Results for Objective (3) i.e., the Relationship between the Proposed Approach for the Identification and Quantification of Pain (Piqβ) and Self-Reported Pain (Score/10)
3.4. Results for the Secondary Objective, i.e., the Effect Size of Medication Acting on Brain Activity on the New Approach
4. Discussion
4.1. New Methodological Approach for Pain Identification and Quantification
4.2. Identification of Pain
4.3. Quantification of Pain
4.4. Effect Size of Brain-Acting Medication on Pain Measurement (Piqβ (%))
4.5. Perspective
4.6. Limits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2022, 163, e328–e332. [Google Scholar] [CrossRef]
- Zimmer, Z.; Fraser, K.; Grol-Prokopczyk, H.; Zajacova, A. A global study of pain prevalence across 52 countries: Examining the role of country-level contextual factors. Pain 2022, 163, 1740–1750. [Google Scholar] [CrossRef]
- Sá, K.N.; Moreira, L.; Baptista, A.F.; Yeng, L.T.; Teixeira, M.J.; Galhardoni, R.; de Andrade, D.C. Prevalence of chronic pain in developing countries: Systematic review and meta-analysis. Pain Rep. 2019, 4, e779. [Google Scholar] [CrossRef]
- Shieh, J.-S.; Dai, C.-Y.; Wen, Y.-R.; Sun, W.-Z. A novel fuzzy pain demand index derived from patient-controlled analgesia for postoperative pain. IEEE Trans. Biomed. Eng. 2007, 54, 2123–2132. [Google Scholar] [CrossRef]
- Kamdar, M.M. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain; Mary Ann Liebert, Inc.: Larchmont, NY, USA, 2010. [Google Scholar]
- Walsh, T. Practical problems in pain measurements. Pain 1984, 19, 96–98. [Google Scholar] [CrossRef]
- Schnakers, C.; Zasler, N.D. Pain assessment and management in disorders of consciousness. Curr. Opin. Neurol. 2007, 20, 620–626. [Google Scholar] [CrossRef]
- Herr, K.; Coyne, P.J.; McCaffery, M.; Manworren, R.; Merkel, S. Pain assessment in the patient unable to self-report: Position statement with clinical practice recommendations. Pain Manag. Nurs. 2011, 12, 230–250. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Nanda, S.; Arya, S.; Kumar, U.; Sharma, R.; Kumaran, S.S.; Bhatia, R. Correlating cognition and cortical excitability with pain in fibromyalgia: A case control study. Adv. Rheumatol. 2021, 61, 10. [Google Scholar] [CrossRef]
- Jenkins, L.C.; Chang, W.-J.; Buscemi, V.; Liston, M.; Skippen, P.; Cashin, A.G.; McAuley, J.H.; Schabrun, S.M. Low somatosensory cortex excitability in the acute stage of low back pain causes chronic pain. J. Pain 2022, 23, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Desmeules, J.A.; Cedraschi, C.; Rapiti, E.; Baumgartner, E.; Finckh, A.; Cohen, P.; Dayer, P.; Vischer, T. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003, 48, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.A.; Gracely, R.H. Biology and therapy of fibromyalgia. Functional magnetic resonance imaging findings in fibromyalgia. Arthritis Res. Ther. 2007, 8, 224. [Google Scholar] [CrossRef][Green Version]
- Misra, G.; Ofori, E.; Chung, J.W.; Coombes, S.A. Pain-related suppression of beta oscillations facilitates voluntary movement. Cereb. Cortex 2017, 27, 2592–2606. [Google Scholar] [CrossRef]
- Nickel, M.M.; May, E.S.; Tiemann, L.; Schmidt, P.; Postorino, M.; Dinh, S.T.; Gross, J.; Ploner, M. Brain oscillations differentially encode noxious stimulus intensity and pain intensity. Neuroimage 2017, 148, 141–147. [Google Scholar] [CrossRef]
- Diers, M.; de Vos, C.C.; Gandhi, W.; Hoeppli, M.E.; Becker, S.; Bock, E.; Baillet, S.; Schweinhardt, P. Induced oscillatory signaling in the beta frequency of top-down pain modulation. Pain Rep. 2020, 5, e806. [Google Scholar] [CrossRef]
- Teixeira, M.; Mancini, C.; Wicht, C.A.; Maestretti, G.; Kuntzer, T.; Cazzoli, D.; Mouthon, M.; Annoni, J.-M.; Chabwine, J.N. Beta electroencephalographic oscillation is a potential GABAergic biomarker of chronic peripheral neuropathic pain. Front. Neurosci. 2021, 15, 594536. [Google Scholar] [CrossRef]
- Segning, C.M.; Harvey, J.; Ezzaidi, H.; Fernandes, K.B.P.; da Silva, R.A.; Ngomo, S. Towards the objective identification of the presence of pain based on electroencephalography signals’ analysis: A proof-of-concept. Sensors 2022, 22, 6272. [Google Scholar] [CrossRef]
- Díaz, J.; Razeto-Barry, P.; Letelier, J.-C.; Caprio, J.; Bacigalupo, J. Amplitude modulation patterns of local field potentials reveal asynchronous neuronal populations. J. Neurosci. 2007, 27, 9238–9245. [Google Scholar] [CrossRef]
- Díaz, J.A.; Arancibia, J.M.; Bassi, A.; Vivaldi, E.A. Envelope analysis of the airflow signal to improve polysomnographic assessment of sleep disordered breathing. Sleep 2014, 37, 199–208. [Google Scholar] [CrossRef]
- Hidalgo, V.M.; Díaz, J.; Mpodozis, J.; Letelier, J.-C. Envelope analysis of the human alpha rhythm reveals EEG gaussianity. IEEE Trans. Biomed. Eng. 2022, 70, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Salinsky, M.C.; Oken, B.S.; Storzbach, D.; Dodrill, C.B. Assessment of CNS effects of antiepileptic drugs by using quantitative EEG measures. Epilepsia 2003, 44, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef]
- Bouffard, J.; Bouyer, L.J.; Roy, J.-S.; Mercier, C. Pain induced during both the acquisition and retention phases of locomotor adaptation does not interfere with improvements in motor performance. Neural Plast. 2016, 2016, 8539096. [Google Scholar] [CrossRef]
- Lomond, K.V.; Côté, J.N. Movement timing and reach to reach variability during a repetitive reaching task in persons with chronic neck/shoulder pain and healthy subjects. Exp. Brain Res. 2010, 206, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Strulov, L.; Zimmer, E.Z.; Granot, M.; Tamir, A.; Jakobi, P.; Lowenstein, L. Pain catastrophizing, response to experimental heat stimuli, and post–cesarean section pain. J. Pain 2007, 8, 273–279. [Google Scholar] [CrossRef]
- Granot, M.; Lowenstein, L.; Yarnitsky, D.; Tamir, A.; Zimmer, E.Z. Postcesarean section pain prediction by preoperative experimental pain assessment. J. Am. Soc. Anesthesiol. 2003, 98, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.H.; Coghill, R.; Houle, T.T.; Seid, M.H.; Lindel, W.M.; Parker, R.L.; Washburn, S.A.; Harris, L.; Eisenach, J.C. Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. J. Am. Soc. Anesthesiol. 2006, 104, 417–425. [Google Scholar] [CrossRef]
- Willis Jr, W. The pain system: The neural basis of nociceptive transmission in the mammalian nervous system. Pain Headache 1985, 8, 1–346. [Google Scholar] [PubMed]
- LaMotte, R.H.; Thalhammer, J.G.; Robinson, C.J. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: A comparison of neural events in monkey with sensory judgments in human. J. Neurophysiol. 1983, 50, 1–26. [Google Scholar] [CrossRef]
- Athif, M.; Ren, H. WaveCSP: A robust motor imagery classifier for consumer EEG devices. Australas. Phys. Eng. Sci. Med. 2019, 42, 159–168. [Google Scholar] [CrossRef]
- Ngomo, S.; Mercier, C.; Bouyer, L.J.; Savoie, A.; Roy, J.-S. Alterations in central motor representation increase over time in individuals with rotator cuff tendinopathy. Clin. Neurophysiol. 2015, 126, 365–371. [Google Scholar] [CrossRef]
- Farzan, F.; Bortoletto, M. Identification and verification of a ‘true’ TMS evoked potential in TMS-EEG. J. Neurosci. Methods 2022, 378, 109651. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Nguyen, J.-P. A practical algorithm for using rTMS to treat patients with chronic pain. Neurophysiol. Clin. 2019, 49, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Segning, C.M.; Ezzaidi, H.; da Silva, R.A.; Ngomo, S. A Neurophysiological Pattern as a Precursor of Work-Related Musculoskeletal Disorders Using EEG Combined with EMG. Int. J. Environ. Res. Public Health 2021, 18, 2001. [Google Scholar] [CrossRef] [PubMed]
- Power, J.D.; Lynch, C.J.; Dubin, M.J.; Silver, B.M.; Martin, A.; Jones, R.M. Characteristics of respiratory measures in young adults scanned at rest, including systematic changes and “missed” deep breaths. Neuroimage 2020, 204, 116234. [Google Scholar] [CrossRef] [PubMed]
- Liberati, G.; Klöcker, A.; Algoet, M.; Mulders, D.; Maia Safronova, M.; Ferrao Santos, S.; Ribeiro Vaz, J.-G.; Raftopoulos, C.; Mouraux, A. Gamma-band oscillations preferential for nociception can be recorded in the human insula. Cereb. Cortex 2018, 28, 3650–3664. [Google Scholar] [CrossRef] [PubMed]
- Nir, R.-R.; Sinai, A.; Moont, R.; Harari, E.; Yarnitsky, D. Tonic pain and continuous EEG: Prediction of subjective pain perception by alpha-1 power during stimulation and at rest. Clin. Neurophysiol. 2012, 123, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Nir, R.-R.; Sinai, A.; Raz, E.; Sprecher, E.; Yarnitsky, D. Pain assessment by continuous EEG: Association between subjective perception of tonic pain and peak frequency of alpha oscillations during stimulation and at rest. Brain Res. 2010, 1344, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ploner, M.; May, E.S. Electroencephalography and magnetoencephalography in pain research—Current state and future perspectives. Pain 2018, 159, 206–211. [Google Scholar] [CrossRef]
- Shao, S.; Shen, K.; Yu, K.; Wilder-Smith, E.P.; Li, X. Frequency-domain EEG source analysis for acute tonic cold pain perception. Clin. Neurophysiol. 2012, 123, 2042–2049. [Google Scholar] [CrossRef]
- Aboalayon, K.A.; Almuhammadi, W.S.; Faezipour, M. A comparison of different machine learning algorithms using single channel EEG signal for classifying human sleep stages. In Proceedings of the 2015 Long Island Systems, Applications and Technology, Farmingdale, NY, USA, 1 May 2015; pp. 1–6. [Google Scholar]
- Zhang, X.; Yao, L.; Zhang, D.; Wang, X.; Sheng, Q.Z.; Gu, T. Multi-person brain activity recognition via comprehensive EEG signal analysis. In Proceedings of the 14th EAI International Conference on Mobile and Ubiquitous Systems: Computing, Networking and Services, Melbourne, Australia, 7–10 November 2017; pp. 28–37. [Google Scholar]
- Zhao, Y.; Cen, Y. Data Mining Applications with R; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Cohen, M.X. MATLAB for Brain and Cognitive Scientists; MIT Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Zamm, A.; Debener, S.; Bauer, A.K.R.; Bleichner, M.G.; Demos, A.P.; Palmer, C. Amplitude envelope correlations measure synchronous cortical oscillations in performing musicians. Ann. N. Y. Acad. Sci. 2018, 1423, 251–263. [Google Scholar] [CrossRef]
- Schreier, P.J.; Scharf, L.L. Statistical Signal Processing of Complex-Valued Data: The Theory of Improper and Noncircular Signals; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Díaz, J.; Bassi, A.; Coolen, A.; Vivaldi, E.A.; Letelier, J.-C. Envelope analysis links oscillatory and arrhythmic EEG activities to two types of neuronal synchronization. Neuroimage 2018, 172, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, J.; Bauer, W.; Zagórowska, M.; Piątek, P. On digital realizations of non-integer order filters. Circuits Syst. Signal Process. 2016, 35, 2083–2107. [Google Scholar] [CrossRef]
- Liu, J. Smoothing filter-based intensity modulation: A spectral preserve image fusion technique for improving spatial details. Int. J. Remote Sens. 2000, 21, 3461–3472. [Google Scholar] [CrossRef]
- Jahani, S.; Setarehdan, S.K.; Boas, D.A.; Yücel, M.A. Motion artifact detection and correction in functional near-infrared spectroscopy: A new hybrid method based on spline interpolation method and Savitzky–Golay filtering. Neurophotonics 2018, 5, 015003. [Google Scholar] [CrossRef]
- Pander, T. EEG signal improvement with cascaded filter based on OWA operator. Signal Image Video Process. 2019, 13, 1165–1171. [Google Scholar] [CrossRef]
- Puzdrowska, P. Signal filtering method of the fast-varying diesel exhaust gas temperature. Combust. Engines 2018, 57, 48–52. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Marston, L.; Spencer, S.; De Souza, L.; Wand, B. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst. Rev. 2018, 3, CD008208. [Google Scholar]
- Wiffen, P.J.; Derry, S.; Bell, R.F.; Rice, A.S.; Tölle, T.R.; Phillips, T.; Moore, R.A. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 6, CD007938. [Google Scholar] [CrossRef]
- Taran, S.; Bajaj, V. Emotion recognition from single-channel EEG signals using a two-stage correlation and instantaneous frequency-based filtering method. Comput. Methods Programs Biomed. 2019, 173, 157–165. [Google Scholar] [CrossRef]
- Alazrai, R.; Al-Rawi, S.; Alwanni, H.; Daoud, M.I. Tonic cold pain detection using Choi–Williams time-frequency distribution analysis of EEG signals: A feasibility study. Appl. Sci. 2019, 9, 3433. [Google Scholar] [CrossRef]
- Elsayed, M.; Swee, S.K.; Chiang, T.S. The Pain Signals: A Systematic Review on the Electroencephalogram of the Nociceptive Pain. Eng. Lett. 2023, 31, 1747–1758. [Google Scholar]
- Mussigmann, T.; Bardel, B.; Lefaucheur, J.-P. Resting-state electroencephalography (EEG) biomarkers of chronic neuropathic pain. A systematic review. NeuroImage 2022, 258, 119351. [Google Scholar] [CrossRef] [PubMed]
- Athavipach, C.; Pan-Ngum, S.; Israsena, P. A wearable in-ear EEG device for emotion monitoring. Sensors 2019, 19, 4014. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, L.D.; Sjoquist, D.L.; Stephan, P.E. Understanding Regression Analysis: An Introductory Guide; Sage Publications: New York, NY, USA, 2016; Volume 57. [Google Scholar]
- Maempel, J.; Clement, N.; Brenkel, I.; Walmsley, P. Validation of a prediction model that allows direct comparison of the Oxford Knee Score and American Knee Society clinical rating system. Bone Jt. J. 2015, 97, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.; Ghert, M.; Simpson, A. Interpreting regression models in clinical outcome studies. Bone Jt. Res. 2015, 4, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Bazzari, A.H.; Bazzari, F.H. Advances in targeting central sensitization and brain plasticity in chronic pain. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 38. [Google Scholar] [CrossRef]
- Jutzeler, C.; Curt, A.; Kramer, J. Relationship between chronic pain and brain reorganization after deafferentation: A systematic review of functional MRI findings. NeuroImage Clin. 2015, 9, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Volz, M.S.; Medeiros, L.F.; da Graça Tarragô, M.; Vidor, L.P.; DallAgnol, L.; Deitos, A.; Brietzke, A.; Rozisky, J.R.; Rispolli, B.; Torres, I.L. The relationship between cortical excitability and pain catastrophizing in myofascial pain. J. Pain 2013, 14, 1140–1147. [Google Scholar] [CrossRef]
- Vidor, L.P.; Torres, I.L.; Medeiros, L.F.; Dussán-Sarria, J.A.; Dall’Agnol, L.; Deitos, A.; Brietzke, A.; Laste, G.; Rozisky, J.R.; Fregni, F. Association of anxiety with intracortical inhibition and descending pain modulation in chronic myofascial pain syndrome. BMC Neurosci. 2014, 15, 42. [Google Scholar] [CrossRef]
- Caumo, W.; Deitos, A.; Carvalho, S.; Leite, J.; Carvalho, F.; Dussán-Sarria, J.A.; Lopes Tarrago, M.d.G.; Souza, A.; Torres, I.L.d.S.; Fregni, F. Motor cortex excitability and BDNF levels in chronic musculoskeletal pain according to structural pathology. Front. Hum. Neurosci. 2016, 10, 357. [Google Scholar] [CrossRef]
- Botelho, L.M.; Morales-Quezada, L.; Rozisky, J.R.; Brietzke, A.P.; Torres, I.L.; Deitos, A.; Fregni, F.; Caumo, W. A framework for understanding the relationship between descending pain modulation, motor corticospinal, and neuroplasticity regulation systems in chronic myofascial pain. Front. Hum. Neurosci. 2016, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Dickenson, A.; Ghandehari, J. Anti-convulsants and anti-depressants. In Analgesia; Springer: Berlin/Heidelberg, Germany, 2007; pp. 145–177. [Google Scholar]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Giusti, G.D.; Reitano, B.; Gili, A. Pain assessment in the Emergency Department. Correlation between pain rated by the patient and by the nurse. An observational study. Acta Biomed. 2018, 89, 64. [Google Scholar]
- Stoopler, M.; Choinière, M.; Nam, A.; Guigui, A.; Walfish, L.; Mohamed, N.; Vigouroux, M.; González-Cárdenas, V.-H.; Ingelmo, P. Chronic pain-related consultations to the emergency department of children with complex pain conditions: A retrospective analysis of healthcare utilization and costs. Can. J. Pain 2022, 6, 86–94. [Google Scholar] [CrossRef] [PubMed]


| Group 1 (n = 7) | Group 2 (n = 15) | Group 3 (n = 66) | |||
|---|---|---|---|---|---|
| Condition—Capsaicin | Condition—Thermal Stimulus | Condition—Chronic Pain | |||
| No Pain | With Pain | Thermal Pain | Centrally Acting Medication (n = 36) | Other Treatment (n = 30) | |
| Average age (years) | 32.40 | 31.70 | 41.17 | 52.10 | |
| Average of pain duration (months) | – | – | 55.34 | 105.03 | |
| Average pain scores (Numerical scale/10) | 0.00 | 4.00 | Decrease in pain from 6.1 to 0.7 | 2.63 | 4.10 |
| ID | Sex: 1 = Male 2 = Female | Age (Years) | Pain Duration (Month) | Pain Scores (/10) | Piqβ (%) | ||
|---|---|---|---|---|---|---|---|
| No Pain Condition | With Pain Condition | No Pain Condition | With Pain Condition | ||||
| 1 | 1 | 35 | – | 0 | 5 | 7.13 | 12.35 |
| 2 | 2 | 45 | – | 0 | 5 | 8.28 | 11.23 |
| 3 | 1 | 33 | – | 0 | 4 | 7.16 | 13.00 |
| 4 | 1 | 30 | – | 0 | 2 | 8.15 | 12.54 |
| 5 | 1 | 28 | – | 0 | 5 | 8.24 | 13.56 |
| 6 | 1 | 24 | – | 0 | 5 | 7.13 | 14.12 |
| 7 | 1 | 26 | – | 0 | 2 | 9.00 | 10.15 |
| Mean (SD) | 31.6 (7.0) | 0 (0) | 4 (1.4) | 7.83 (0.74) | 12.42 (1.4) | ||
| ID | Sex: 1 = Male 2 = Female | Age (Years) | Pain Duration (Month) | Pain Score (/10) | Piqβ (%) | Medication with Central Effect: 1 = Yes 0 = Other Medications |
|---|---|---|---|---|---|---|
| 1 | 1 | 21 | 30 | 0.00 | 8.81 | 0 |
| 2 | 2 | 40 | 37 | 4.36 | 15.75 | 1 |
| 3 | 1 | 32 | 18 | 2.36 | 14.05 | 1 |
| 4 | 2 | 59 | 29 | 1.05 | 10.18 | 0 |
| 5 | 2 | 64 | 120 | 1.09 | 10.76 | 1 |
| 6 | 2 | 63 | 396 | 6.00 | 19.17 | 1 |
| 7 | 2 | 58 | 5 | 2.73 | 14.29 | 1 |
| 8 | 2 | 63 | 36 | 0.64 | 8.25 | 1 |
| 9 | 1 | 30 | 4 | 5.27 | 24.99 | 0 |
| 10 | 1 | 68 | 120 | 4.55 | 15.05 | 1 |
| 11 | 1 | 69 | 24 | 0.09 | 9.33 | 0 |
| 12 | 1 | 78 | 6 | 1.00 | 12.66 | 0 |
| 13 | 1 | 30 | 5 | 0.36 | 7.84 | 0 |
| 14 | 2 | 17 | 6 | 4.64 | 13.74 | 0 |
| 15 | 2 | 28 | 84 | 3.00 | 13.65 | 0 |
| 16 | 2 | 21 | 12 | 1.00 | 11.92 | 0 |
| 17 | 2 | 22 | 6 | 0.55 | 7.92 | 0 |
| 18 | 2 | 26 | 84 | 4.45 | 18.68 | 0 |
| 19 | 2 | 54 | 4 | 5.77 | 12.34 | 0 |
| 20 | 1 | 42 | 22 | 3.82 | 11.34 | 0 |
| 21 | 1 | 66 | 72 | 2.00 | 10.19 | 0 |
| 22 | 2 | 59 | 84 | 0.09 | 6.93 | 1 |
| 23 | 1 | 21 | 4 | 2.27 | 11.12 | 0 |
| 24 | 2 | 19 | 7 | 2.00 | 12.30 | 0 |
| 25 | 1 | 23 | 1 | 1.27 | 12.98 | 0 |
| 26 | 1 | 22 | 204 | 2.91 | 12.59 | 0 |
| 27 | 2 | 60 | 2 | 1.77 | 10.74 | 0 |
| 28 | 2 | 57 | 1 | 2.91 | 11.78 | 1 |
| 29 | 2 | 31 | 8 | 4.23 | 22.41 | 1 |
| 30 | 2 | 52 | 0.75 | 2.27 | 12.48 | 0 |
| 31 | 2 | 33 | 7 | 6.91 | 15.57 | 0 |
| 32 | 2 | 37 | 12 | 0.86 | 7.59 | 1 |
| 33 | 2 | 22 | 120 | 2.00 | 11.89 | 0 |
| 34 | 2 | 36 | 2 | 0.00 | 9.39 | 0 |
| 35 | 2 | 59 | 84 | 2.00 | 13.02 | 1 |
| 36 | 2 | 63 | 6 | 4.00 | 14.82 | 0 |
| 37 | 2 | 64 | 3 | 0.00 | 5.66 | 1 |
| 38 | 2 | 15 | 6 | 4.00 | 16.59 | 0 |
| 39 | 1 | 32 | 16 | 10.00 | 18.9 | 1 |
| 40 | 1 | 45 | 96 | 8.00 | 17.7 | 1 |
| 41 | 2 | 44 | 24 | 2.10 | 14.9 | 1 |
| 42 | 2 | 45 | 240 | 7.50 | 19.1 | 1 |
| 43 | 2 | 46 | 240 | 4.00 | 15.5 | 0 |
| 44 | 2 | 47 | 48 | 4.00 | 11.4 | 0 |
| 45 | 2 | 32 | 216 | 0.50 | 12.9 | 0 |
| 46 | 2 | 45 | 24 | 5.70 | 17.5 | 1 |
| 47 | 2 | 71 | 120 | 10.0 | 29.2 | 1 |
| 48 | 1 | 56 | 4 | 3.50 | 11.15 | 0 |
| 49 | 2 | 27 | 60 | 1.60 | 16.1 | 1 |
| 50 | 1 | 68 | 120 | 6.80 | 15.1 | 1 |
| 51 | 2 | 73 | 24 | 3.40 | 13.8 | 0 |
| 52 | 2 | 55 | 240 | 2.10 | 15.1 | 1 |
| 53 | 1 | 45 | 48 | 5.70 | 20.3 | 0 |
| 54 | 2 | 62 | 60 | 2.10 | 16.2 | 1 |
| 55 | 1 | 66 | 144 | 3.00 | 17.7 | 0 |
| 56 | 1 | 65 | 3 | 2.20 | 13.3 | 1 |
| 57 | 2 | 49 | 192 | 1.20 | 10.8 | 0 |
| 58 | 2 | 61 | 360 | 7.20 | 13.9 | 1 |
| 59 | 1 | 60 | 84 | 5.80 | 12.44 | 1 |
| 60 | 1 | 53 | 5 | 0.00 | 12.68 | 0 |
| 61 | 2 | 44 | 48 | 7.00 | 16.78 | 1 |
| 62 | 2 | 59 | 120 | 1.10 | 13.94 | 1 |
| 63 | 2 | 59 | 300 | 2.00 | 17.78 | 0 |
| 64 | 2 | 24 | 96 | 7.00 | 23.67 | 1 |
| 65 | 2 | 61 | 516 | 5.00 | 32.10 | 1 |
| 66 | 2 | 27 | 24 | 5.00 | 18.23 | 0 |
| Mean (SD) | 46.1 (17.3) | 77.9 (105.9) | 3.30 (2.50) | 14.3 (4.8) |
| Variables | Sub-Groups | p Value | Effect Size | Clinical Difference Δ (%) | |
|---|---|---|---|---|---|
| Medication with Centrally Acting (n = 36) | Medication without Centrally Acting (n = 30) | ||||
| Pain scores (/10) | 2.63 (1.87) IC: 1.9–3.2 | 4.10 (2.91) IC: 3.0–5.1 | 0.016 * | 0.61 | 55.8% |
| Piqβ (%) | 13.23 (3.66) IC: 11.9–14.6 | 15.68 (5.80) IC: 13.5–17.8 | 0.041 * | 0.51 | 18.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segning, C.M.; da Silva, R.A.; Ngomo, S. An Innovative EEG-Based Pain Identification and Quantification: A Pilot Study. Sensors 2024, 24, 3873. https://doi.org/10.3390/s24123873
Segning CM, da Silva RA, Ngomo S. An Innovative EEG-Based Pain Identification and Quantification: A Pilot Study. Sensors. 2024; 24(12):3873. https://doi.org/10.3390/s24123873
Chicago/Turabian StyleSegning, Colince Meli, Rubens A. da Silva, and Suzy Ngomo. 2024. "An Innovative EEG-Based Pain Identification and Quantification: A Pilot Study" Sensors 24, no. 12: 3873. https://doi.org/10.3390/s24123873
APA StyleSegning, C. M., da Silva, R. A., & Ngomo, S. (2024). An Innovative EEG-Based Pain Identification and Quantification: A Pilot Study. Sensors, 24(12), 3873. https://doi.org/10.3390/s24123873








