Abstract
Pulse oximeters are widely used in hospitals and homes for measurement of blood oxygen saturation level (SpO2) and heart rate (HR). Concern has been raised regarding a possible bias in obtaining pulse oximeter measurements from different fingertips and the potential effect of skin pigmentation (white, brown, and dark). In this study, we obtained 600 SpO2 measurements from 20 volunteers using three UK NHS-approved commercial pulse oximeters alongside our custom-developed sensor, and used the Munsell colour system (5YR and 7.5YR cards) to classify the participants’ skin pigmentation into three distinct categories (white, brown, and dark). The statistical analysis using ANOVA post hoc tests (Bonferroni correction), a Bland–Altman plot, and a correlation test were then carried out to determine if there was clinical significance in measuring the SpO2 from different fingertips and to highlight if skin pigmentation affects the accuracy of SpO2 measurement. The results indicate that although the three commercial pulse oximeters had different means and standard deviations, these differences had no clinical significance.
1. Introduction
Pulse oximetry is a non-invasive method of measuring blood oxygen saturation (SpO2), which has revolutionised the monitoring of oxygenated and de-oxygenated haemoglobin [1,2]. Due to it being a non-invasive method, it is widely used in all healthcare settings including hospitals and was adopted as the international standard for patient monitoring when administering anaesthesia in early 1990 [3]. Since the COVID-19 pandemic, SpO2 has become one of the most important vital parameters of health to monitor clinical status.
It is well known that the accuracy of pulse oximeters is influenced by various factors, such as the fingertip selection, motion artefacts, and light interference [4,5,6]. In particular, the effect of skin colour and finger placement on blood oxygen saturation measurement has attracted significant attention in recent years. Evidence-based research shows that pulse oximeters (POs) may perform less accurately in darker-pigment patients in comparison to patients with lighter pigment or other skin colours [7,8,9,10,11,12]. For example, it was shown that some colours of nail polish (blue, green, and black) absorb more light, hence decreasing the accuracy of POs [8,13,14]. Considering that SpO2 level is a vital parameter for diagnosing many conditions, the topic has attracted significant attention recently. Shi et al. [15] investigated the effect of skin pigmentation on pulse oximeter accuracy, and showed that SpO2 was overestimated in people with darker skin. Bickler et al. [16] studied the effect of skin pigmentation on pulse oximeter accuracy at low oxygen saturation and showed an overestimated SpO2 in darkly pigmented individuals during hypoxia [16]. Adler et al. [17] investigated the effect of skin pigmentation on pulse oximeter accuracy but the results from their work showed that skin pigmentation did not affect the performance of POs. The inaccuracy in oxygen saturation readings has a significant impact, with black patients having more than a 2-fold probability of a falsely high oxygen reading compared to white patients in the ICU [18]. Occult hypoxemia was in turn associated with higher mortality and organ failures [19].
Although there is substantial work on IoT-based pulse oximeters [20,21,22,23], the focus is often on the design and implementation aspects of a particular component, such as communication, calibration, or user application. In particular, Mahgoub et al. [23] concentrate on the design of a health monitoring system with remote alert capability. Chugh et al. [21] focus on a low-cost calibration-free pulse oximeter, while ref. [23] studies the wireless body area network aspect of pulse oximeters. However, the accuracy evaluation of such designs in realistic conditions and their comparison with NHS-approved instruments have not received the required attention.
In this research, we review the physical principles of pulse oximetry and evaluate the performance of our custom pulse oximeter based on an off-the-shelf photodetector and 3D-printed enclosure developed in earlier research [24]. We then compare its performance with that of the three different UK NHS-approved pulse oximeters using extensive measurements and statistical analysis, including the investigation of the impact of skin pigmentation on SpO2 measurement accuracy. During the measurements, blood oxygen saturation measurements were taken from 20 healthy volunteers on each finger of both left and right hands, using different sensors. As SpO2 is normally measured on the middle or index fingers [25], one of our objectives was to understand how the sensor placement on a particular finger affects the accuracy. A statistical analysis and data visualisation using a Bonferroni test and Bland–Altman plot were used to demonstrate the differences between the devices. Our results indicate that although the commercial pulse oximeters had different means and standard deviations, these differences had no statistical significance.
The rest of the paper is structured as follows. Section 2 describes related work focusing on the impact of skin pigmentation on blood saturation measurement. Section 3 presents the physical principles of pulse oximetry, including the Beer–Lambert law and the construction of pulse oximeters. The coverage of these principles and the subsequent evaluation methodology is geared towards researchers working on IoT systems. Section 4 contains our methodology, including study protocol and recruitment of volunteers, followed by results and conclusions in Section 5 and Section 6, respectively.
2. Related Work
Monitoring of blood oxygen saturation (SpO2) is vital as a diagnostic of many cardiovascular and respiratory conditions, such as heart attack, chronic obstructive pulmonary disease COPD, asthma, lung cancer, and pneumonia. Maintaining adequate SpO2 levels is critical to sustaining life, and SpO2 is under constant homeostatic control by the brain, which controls it at a subconscious level. When a person is unwell, the body may not be able to respond adequately and may require medical assistance and intervention. In these circumstances, it is critically important that the SpO2 level is accurately measured and external assistance provided to ensure the level does not go below the safe threshold. The normal range for SpO2 in humans ranges from 95% up to 100% [26]. When the SpO2 level drops below 92%, this is known as ’hypoxemia’ and usually results in observable changes in a person’s physical status including a variety of symptoms, such as shortness of breath, fingernails and lips that can appear blue, rapid breathing, and an increased heart rate. Without prompt medical intervention, a person can become confused, lose the ability to communicate, or lose consciousness and die. Therefore, accurate measurement and timely intervention are critical for ensuring that patients survive.
Pulse oximeters estimate the arterial oxygen saturation (SaO2) from the oxygenated and total haemoglobin ratio. Shi et al. [15] investigated the effect of skin pigmentation on pulse oximeter accuracy using 6505 participants over 32 studies with all their datasets obtained from hospital settings. Fifteen of their studies measured skin pigmentation and compared SaO2 and pulse oximeter measurements in dark-skinned subjects. The research showed that SpO2 was overestimated in people with darker skin [15].
Adler et al. [17] studied the effect of skin pigmentation on pulse oximeter accuracy in an emergency department. The research was carried out in an emergency department section of a hospital, collecting SpO2 readings from 284 patients with three skin pigments. The authors determined skin pigmentation through colour swatches with controlled lighting. The arterial blood sample of the volunteers was taken simultaneously as the pulse oximeter measured the SpO2 values. The results from their work showed that skin pigmentation did not affect the performance of POs [17].
Bickler et al. [16] showed the effects of skin pigmentation on the pulse oximeter accuracy at low oxygen saturation by evaluating the SaO2 level of 11 healthy dark-skin-pigmented subjects. The subjects were placed in a semi-supine position and were allowed to inhale an air mixture of nitrogen, carbon dioxide, and oxygen through a mouthpiece. The method estimated breath-by-breath SaO2 using an end-tidal oxygen and carbon dioxide concentration, which was determined by a mass spectrometer. During the measurement, an operator controlled the inspiration of gas to achieve a stable plateau of desaturation. Additionally, three commercially available POs were used to measure the difference between SaO2 and SpO2. Their findings showed overestimated SaO2 in darkly pigmented individuals during hypoxia [16]. For a more comprehensive review of the topic, the reader is referred to the works by Shi et al. [15,27], Al-Halawani [28], and Cabanas [29].
3. Principle of Pulse Oximetry
Pulse oximeters use photoplethysmography (PPG) to detect variations in blood volume within human biological tissues resulting from the heartbeat [30]. PPG is a non-invasive technique that employs a light-emitting source, such as an LED, and a photodetector. The light-emitting source radiates red or infrared light to the skin, and then, measures the amount of light either reflected from the skin surface or transmitted through biological tissue depending on the measurement mode. In reflection mode, the light detected by a photodetector is a result of scattering and reflection from biological tissue. As shown in Figure 1, the LED is placed adjacent to the photodiode within a minimum spacing distance between the diode and skin. The distance should accurately represent the pulsatile PPG component and ranges from 0.5 to 18 mm. There is also separation between the LED and the photodiode to prevent the direct incidence of light from the LED to the photodiode. The precise mounting of this opaque material, fitting the sensor firmly on the skin, and ensuring the area of application is plain and spacious, are important for accurate measurement [31]. The reflective method is convenient for measurement from different parts of the body as both the light source and the photodetector are located close to each other rather than on the opposite sides of the biological tissue, which is the case in transmission mode.

Figure 1.
Reflection measurement mode [32].
3.1. Relationship between Haemoglobin and Absorption of Light
Pulse oximetry is based on the fact that in the red and infrared spectrum, light absorption is different for oxygenated haemoglobin (O2Hb) and de-oxygenated haemoglobin (HHb) [2]. Compared to blue, green, yellow, and far infrared wavelengths, which are absorbed by water and vascular tissues, both red and near infrared (NIR) light have deeper penetration depth in biological tissues. The amount of light absorption depends on both haemoglobin type and the wavelength: oxygenated haemoglobin O2Hb absorbs more infrared light and less red light, while de-oxygenated haemoglobin HHb absorbs more red light. Consequently, pulse oximeters utilise two wavelengths: NIR at 940 nm and red light at 660 nm [2,33,34]. It should be noted that the light absorption directly depends on the arterial blood volume, which oscillates with the cardiac cycle, rising in systole and descending in diastole, compared to the volume of blood in the capillaries, veins, skin area, fat, and bones, which remains constant.
The light received by the photodetector consists of pulsatile (AC) and stable non-pulsatile components (DC) [2]. The pulsatile component depends not only on the systolic blood pressure (SBP) in the vascular tissue but also on the number of illuminated tissues in the artery [35]. The pulse oximeter uses the peak amplitude of absorbance to evaluate the modulation ratio of red and NIR [2]: , where R is the ratio of pulsatile to non-pulsatile components of Red–IR light absorption, and A represents the absorbance. The R-value can be used to evaluate the SpO2 level [7]:
The Oxygen in the blood is carried by the haemoglobin within red blood cells (RBCs); as the RBCs travel around the body, the oxygen is released by the haemoglobin. The ratio of oxygenated haemoglobin to the total quantity of haemoglobin in the blood is referred to as the oxygen saturation in arterial blood, SaO2. It is important to note that other forms of haemoglobin, such as methaemoglobin and carboxyhaemoglobin, affect the accuracy of the pulse oximeter. Methaemoglobin is formed when the iron in the heme group is in the ferric state () instead of the normal ferrous state (). The methaemoglobin cannot transport oxygen around the body and has a blue-brown pigmentation. Carboxyhaemoglobin develops on inhalation or exposure to carbon monoxide [36,37]. Carbon monoxide has a higher affinity with haemoglobin than oxygen, hence the CO is not released as it becomes attached to the haemoglobin, which reduces the amount of oxygen the blood carries [38] (Figure 2).

Figure 2.
The extinction coefficients for the four different types of haemoglobin and their relationships within red and IR light wavelengths [2].
The amount of absorbed light is proportional to the Hb concentration in the blood. The relationship between light absorption and Hb is described by the Beer–Lambert law [36], which is the merging of two laws: Beer’s law describes the direct relationship between concentration and absorbance, whereas Lambert’s law describes the direct relationship between absorbance and path length [39]. The Beer–Lambert law is given as [39]
where A is the absorbance of light by a sample, l is path length, c is concentration level, is the molar extinction coefficient, and C is the concentration of the absorbing tissue.
For incident light at a given wavelength and with intensity I0 propagating through a concentrated solution c, the intensity of transmitted light can be deduced as
Since we are considering a pulse oximeter, where the measurement area is the fingertip, the fingertip can be regarded as the solution with concentration c. In the transmission of incident light Ii at a given wavelength, the light intensity varies, with the change depending on the absorption properties of the fingertip and the path length of the finger, Figure 3 [40,41,42]. To accurately evaluate the total absorbance, a summation of venous and arterial absorbance would be required [2].

Figure 3.
Beer–Lambert model and component [33].
A PO evaluates absorbance with time, a first-order derivative of the above equation concerning time [2]:
The ratio R of light transmitted to incident light from the red and IR wavelengths of the light source is expressed as
From the R-value extinction coefficient of haemoglobin, SaO2 can be calculated. The mathematical relationship between the extinction coefficient and haemoglobin is denoted as [43]
where is the haemoglobin extinction coefficient, is the extinction coefficient of the oxygenated haemoglobin, and and are the two separate wavelengths of light [43]. As the value of R decreases the SpO2 increases. The peak–peak amplitude of the pulse wave at a high percentage of SpO 2 is higher in the IR. The low level of SpO2 percentage amplitude is higher with red than with infrared. At low SpO2, there is an increase in de-oxyhaemoglobin, which absorbs red light better than oxyhaemoglobin.
3.2. Empirical Calibration of SpO2
As the Beer–Lambert law does not model the scattering of light through the skin surface, a calibration is required. Traditionally, pulse oximeters implement an empirical calibration equation for the estimation of SpO2 from derived data from a vast group of healthy normothermic volunteers (non-smokers). This empirical calibration results in a pulse oximeter that depends on a fixed calibration curve to estimate the SpO2 level from a PPG-measured signal [44]. Every pulse oximeter manufacturer has a tailor-made calibration curve based on the experiment carried out on their volunteers [45,46]. Some patented algorithms have been obtained, where the R ratio is evaluated from the ratio of the derivatives of the IR and red lights’ intensities, which is then employed in an empirical equation for the computation of the average SpO2 percentage [47]. The calibration and validation correct the errors emerging from the Beer–Lambert R-curve model. The comparison curves of the Beer–Lambert law and empirical calibration are available in [48] (shown in fig. 86.4).
Instead of a linear decrease in SpO2, the empirical calibration shows a non-linear decrease of 70% in oxygen saturation. At a ratio value of 1.25 R, the data becomes linearised. The Beer–Lambert model only displays oxygen saturation in the range of 30–95%. Empirical calibration makes it possible to represent 100% oxygen saturation. Mapping of SaO2 values is performed using the following expression [49]:
where is a multiple of the ratio of red to infrared light deflected by the body tissues. The empirically derived coefficient K is related to the different extinctions of Hb and HbO2. In practice, the relation between SpO2 and R is the same. Equation (3) can be normalised to
where and are derived through empirical calibration; in the referenced figure, K1 = 110 and K2 = −25 [49].
3.3. Using RMS Value for Estimation of R-Value
The combination of the RMS AC signal with the mean DC signal can be used to evaluate the R-value for the calibration of SpO2 in a pulse oximeter [50]:
In order to evaluate the AC component, the signal is filtered through a bandpass filter with a bandpass of 0.67–10 Hz. An array of RMS synchronous to the detected peak beat–beat of the rolling RMS signal. The advantage of this method is the elimination of the requirement to find a pure signal from noise. Consequently, the peak is used as a non-pulsatile signal and might display an AC component because of the filter.
4. Methods
This research study is conducted to investigate the effect of skin pigmentation and finger placement on pulse oximeter SpO2 accuracy.
4.1. System Design and Prototype
The blood pressure measurement was obtained non-invasively from participants’ fingertips through a 3D-shaped fingertip casing furnished with an IR LED and photodiode. The PPG signal was obtained using a commercial biosensor ProtoCentral Electronics (Bengaluru, India) ProtoCentral Pulse Express optical PPsensor with MAX30102 and MAX32664D biosensor modules. The optoelectronic unit was set up with red and IR LEDs and a single photodiode, with the connection implemented in reflectance mode. The photodiode has a peak wavelength sensitivity of 950 nm and an angle of intensity of 100. The current IF flowing through the LED or PD is directly proportional to the potential difference V (voltage) and inversely proportional to the resistance R1 (ohms) from the LED or PD [51]: . The resistor was adjusted to vary the LED brightness level. The amount of light passing through the fingertips varies with the expansion and contraction of the heart, which causes variation in blood volume in the fingertips through the increase and decrease of blood flow. As blood flows through the arteries and capillaries of the fingertips, the PD absorbs less light as some of the light is scattered, which leads to higher resistance of the photodetector. The output produced by the PD has a combination of DC and AC components, where the DC component is not beneficial, while the pulsatile AC component carries important information which is synchronous to the heart rate. The overall architecture of the system was designed to be modular for future scalability in accuracy and efficiency.
The sensor was housed in a 3D-printed finger case for better accuracy and for blocking out ambient light. The early version of the 3D case had to be fastened to the waist with a waist strap, while the other part was to be attached to the index finger of the right hand, as shown in Figure 4a. Due to the bulky size of the initial version, a miniaturised version had to be developed, Figure 4b. Further testing revealed that the looseness of the Velcro strap affected the accuracy of the HR measurements in the miniaturised version. This problem was resolved in the final prototype, which had compact dimensions, a 0.91-inch OLED display, and remote monitoring capability, as shown in Figure 4c. The final prototype performed optimally in comparison to the earlier versions. A more detailed description of the sensor design including the communication capability is available in Nwibor et al. [24].
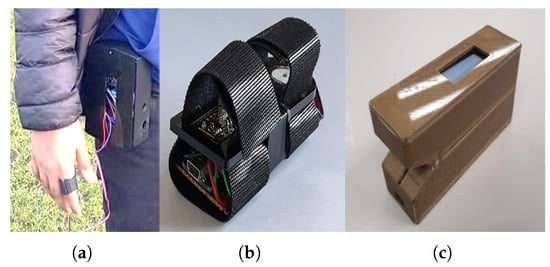
Figure 4.
The sensor prototype development timeline [24]. (a) Alpha version of the sensor using Velcro tape and bulky 3D case; (b) beta version of the sensor; (c) the final version of the sensor with 0.91-inch OLED display and remote monitoring capacity.
4.2. Ethics Approval
The study was conducted by a multi-disciplinary team comprising an electronic engineering team and medical professionals from a large University Teaching Hospital in the UK. The lead researcher obtained ethics approval and Institutional Review Board (IRB) authorisation for conducting this study on neuro-typical healthy volunteers. Reference of the ethics code: REC Project ID: 2882. The study was performed in the laboratory of Microwave Photonics and Sensors at the Department of Electronic Engineering, School of Engineering, Physical and Mathematical Sciences, Royal Holloway, University of London.
4.3. Recruitment of Volunteers
We recruited 20 healthy volunteers from (as declared) African, Asian, and Caucasian ethnicity. All our volunteers were between the ages of 25 and 60 years old and were required to be able to communicate (read and write) in the English language, comply with the study procedure, and to be willing to sign our debrief and consent form. All our volunteers were healthy as none of them showed signs of hypertension, hypotension, hypoxemia, bradycardia, or tachycardia. A qualified medical team (physician and nurses) ensured that the correct measurement standards were followed. The demographics of the volunteers are shown in Table 1.

Table 1.
Demographics of volunteers.
4.4. Study Protocol
At the time of taking the SpO2 reading from the volunteer, we recorded the skin pigmentation of the volunteer, medical history, lifestyle (smoker or non-smoker), and demographic information alongside the SpO2 output from the three commercial PO sensors, as well as our custom-developed sensor.
4.5. Measurement Procedure
We made our classified the skin pigment into three distinct categories through the comparison of the skin at the forehead of each volunteer using the Munsell colour system [52]. Our adopted method for assessing skin pigmentation is similar to the method adopted by Adler et al. [17]. This system describes the colour based on 3 axes or scales: hue, chroma, and value. The Munsell colour system is based on a numerical scale with a visually uniform sequence for each colour scale, with each colour having a visual relationship with other colours [53]. The hue axis is used to denote primary colours along the colour spectrum. Chroma denotes the saturated or intensity (weak or strength) of the colour within a colour group, while the value measures how dark or light a colour is. For example, the values of 0 and 10 describe the black and white colours, respectively. The value scale is constant across hue and chroma [17,54].
The Munsell colour system was printed on neutral grey cards with matte tiles mounted on them. We used the Munsell hue 5YR and 7.5YR cards as they closely resemble the human skin colour and provide a range of tiles for various chroma and value ranges. The cards represents a range of colours within the yellow–red hue family. Within this range, there are variations in lightness (value) and chroma, resulting in multiple distinct colours that all fall within the selected hue categories. The volunteers rested for 5 min from their time of arrival as they performed an indirect exercise by walking to the experiment area, and were seated in an upright position with the hand positioned on a table at the same level as the heart. The room was illuminated with a single fluorescent light with an open window for natural light. We compared the skin colour of each volunteer using the 5YR chart shown in Figure 5 and chose the tile closest to the volunteers’ skin pigment. The skin pigments of our volunteers were classified into three categories: dark skin pigment range, with a value of ≤5; brown skin value, with the value within the range 6–7; and white skin pigment, with a value of 8. The chroma colours were classified into 2 categories: category 1 represents skin with darker pigment and falls between chroma levels 1 and 2; while category 2 denotes lighter pigment, with a chroma level ≥ 3.
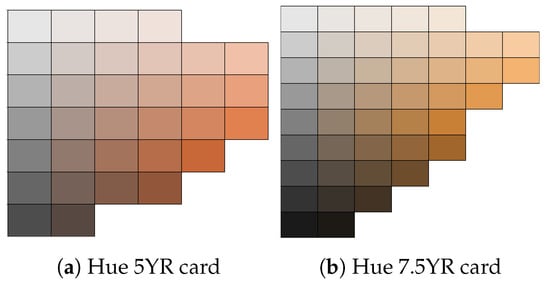
Figure 5.
Hue 5YR and 7.5YR hues were used to identify skin colour [54,55].
4.6. Finger Placement
We took a total of 600 SpO2 measurements from 20 volunteers, including measurements from each of the 10 different fingertips from both left and right hands (Figure 6). None of the volunteers used nail polish. The measurements were obtained using the UK NHS-approved pulse oximeters Acurio [56], Braun [57], and Ailie [58], which are all fingertip sensors.
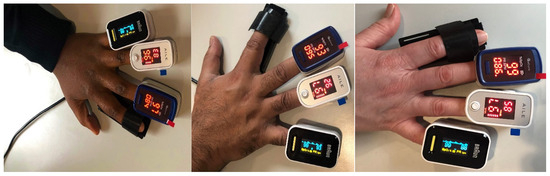
Figure 6.
Dark, brown, and white skin pigments with 3 different PO sensors placed on the fingertips of volunteers.
Additionally, we took measurements using our custom-developed pulse oximeter and recorded its readings 1 min after obtaining readings from the commercial sensor. After placing the finger in the PO, we waited for 45 s for the reading from the fingertip to stabilise. We then took the highest stable reading from the PO monitor measuring the SpO2. The volunteers were asked to rub their hands together to generate a bit of heat in their fingertips to prevent cold hands, which can lead to inaccurate readings.
4.7. Statistical Method
For comparing the best finger placement, we analysed the measurements from 10 different fingertips using ANOVA post hoc tests with a Bonferroni correction and an alpha value of 0.05. We also used the Shapiro–Wilk test to assess the normality of the measurements and the Bland–Altman plot to graphically represent the results.
Bland–Altman Plot
For comparative analysis and benchmarking of our custom-developed sensor with a commercial BP sensor, we used Bland–Altman plot analysis. This statistical tool assesses bias between measurements conducted by different instruments. In our data analysis, we set the limit of agreement to 95%. On each Bland–Altman plot, the X-axis represents mean values, with each point representing a mean of the two measurements [3,59,60,61]:
The Y-axis shows the difference between the measurements, with each point representing a difference between the two measurements:
There are three important lines in the plot [59]:
- The average difference between predicted and commercial BP values;
- The upper limit of agreement, representing the 95% confidence interval for the average difference;
- The lower limit of agreement, representing the 95% confidence interval for the average difference.
The upper and lower limits are computed based on the mean difference between two datasets and the standard deviation (SD) of that difference [60,61]. The SD of the differences between the custom and commercial sensors provides a suitable index for our comparison. The mean ±1.96 standard deviations (SDs) accounts for the 95% limits of agreement [60,61].
where LLoA and ULoA are the lower and upper limits of agreement, respectively.
5. Results and Discussion
5.1. Determining the Best Finger to Measure SpO2
We obtained SpO2 values from 10 different fingertips of each volunteer using different pulse oximeters, and then, calculated the mean and standard deviation. The calculated means of the SpO2 values from the three pulse oximeters were different for each device. The mapping of finger names to abbreviations is presented in Table 2.

Table 2.
Fingertip abbreviations.
A post hoc Bonferroni test was performed after the ANOVA test was performed to reveal the difference in measurements. We classified the fingers into 10 groups, as shown in Table 2. In each group, we measured 20 SpO2 values from each commercial sensor, and then, performed a one-way ANOVA test to investigate if there was any significant difference in SpO2 measurements with other fingers. We obtained three different p-values from the commercial sensors: Ailie PO, p-value = 0.44, F: 0.71; Acurio PO, p-value = 0.71, F: 1.00; Braun PO; p-value = 0.05, F: 2.76. From these we developed two hypotheses as follows. If p < 0.05, there is a significant difference between the means, and p ≥ 0.05 shows that there is no significant difference.
The Acurio PO’s lowest standard deviation value was found in R1 (95.80% ±1.85), the Ailie PO’s lowest standard deviation value was found in L4 (98.10% ±0.45), and the Braun PO’s lowest standard deviation value was found in L3 (98.85% ±0.49). We utilised the lowest standard deviation as it shows that the measured values are more spread out, hence why we chose the lowest standard deviation. We performed a post hoc Bonferroni correction test to see which finger groups were different from each other. We also performed an individual t-test and controlled the multiple comparisons using Bonferroni correction, i.e., we performed an individual t-test for all possible finger combinations.
After performing the repeated ANOVA test (Table 3, Table 4 and Table 5), we noticed that there was no significant difference in measuring SpO2 from different fingers. Post hoc analyses on the 45 comparisons, showed no significant difference between the commercial sensors (Ailie, Acurio, and Braun), as shown in the tables. We further constructed a bar chart with error bars to show the performance of the 10 fingertips, as shown in Figure 7.

Table 3.
The Ailie PO’s multiple finger comparison measurements with SpO2 mean and SD, using repeated two-way ANOVA test and post hoc test (Bonferroni-corrected at alpha p = 0.01).

Table 4.
The Acurio PO’s multiple finger comparison measurements with SpO2 mean and SD, using repeated two-way ANOVA test and post hoc test (Bonferroni-corrected at alpha p = 0.01).

Table 5.
The Braun PO’s multiple finger comparison measurements with SpO2 mean and SD, using repeated two-way ANOVA test and post hoc test (Bonferroni-corrected at alpha p = 0.01).
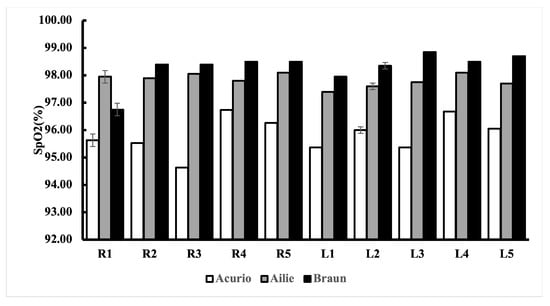
Figure 7.
SpO2 readings from the 10 different fingertips using different commercial POs, with error bars.
Additionally, Bland–Altman plots were created to show the degree of agreement between the commercial PO sensors and to show if there is clinical significance in the different SpO2 measurements between the sensors.
The Bland–Altman plot for the Ailie and Acurrio devices in Figure 8 shows a good degree of agreement, with only one outlier value seen outside the limits of agreement. The corresponding data point rallies around the bias point, hence a good degree of agreement is established. Hence, there is no clinically significant difference in the SpO2 readings from the two sensors. For the Braun and Ailie POs, the degree of agreement is also strong, as most data points are within the upper and lower limits of agreement, as shown in Figure 9. Hence, there is no clinically significant difference in the SpO2 readings between the two sensors.
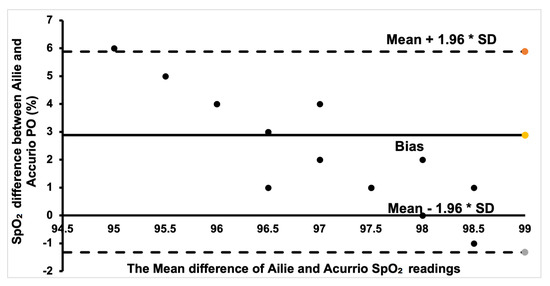
Figure 8.
Bland–Altman plot of Ailie and Acurio measurements.
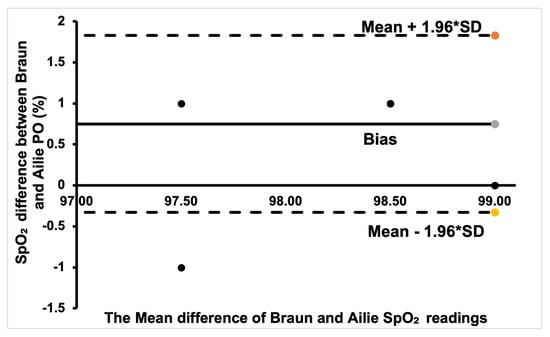
Figure 9.
Bland–Altman plot of Braun and Ailie measurements.
Finally, for the Braun and Acurio pair, shown in Figure 10, a good portion of the readings was within the positive bias. A few data points that are far from the bias points denote a weak agreement, with only one point being outside the lower limit of agreement. Therefore, there is no clinical difference in measuring SpO2 between the two commercial sensors.
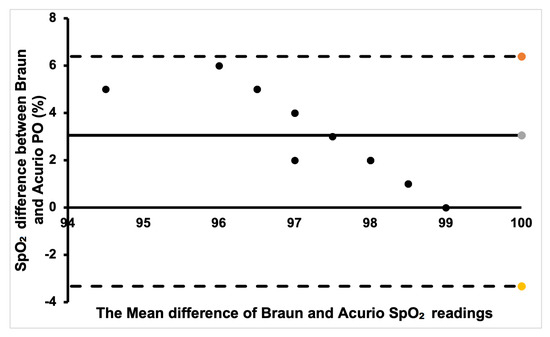
Figure 10.
Bland–Altman plot of Braun and Acurio measurements.
5.2. Comparison of Three Different Skin Colours
We classified the skin colours into three categories using the Munsell hue 5YR and 7.5YR cards, as they closely resemble the human skin colour and provide a range of tiles for various chroma and value ranges. We compared SpO2 readings from three skin colours, light, brown, and dark, as previously discussed. We carried out a repeated ANOVA test with post hoc analysis on three comparisons using the three different pulse oximeters, white vs. brown, brown vs. dark and dark vs. white, using the best measurement position. We further carried out a post hoc test (Bonferroni test) to further investigate if there was a significant difference between different skin colours. In our hypothesis, a value of p ≤ 0.05 indicated a significant difference between the SpO2 of the different skin colours. From our analysis, we obtained a p-value = 0.79, indicating no significant difference in the measurements and probably no clinical significance. Table 6 summarises the results from the post hoc analysis.

Table 6.
Measurement comparison using repeated two-way ANOVA test and post hoc test (Bonferroni-corrected at alpha p = 0.01).
5.3. Discussion
In this study, we investigated the relationship between the placement of pulse oximeters on different fingertips and their measurement accuracy. Our study showed that different fingers gave different SpO2 values, as shown in Table 3, Table 4 and Table 5, using the ANOVA test (p < 0.05) and Bonferroni-corrected test (p < 0.001). For the commercial sensors employed, the sensors showed higher SpO2 values in some fingers (Ailie = L4, Acurio = R1, Braun = L3). Although there was a difference in readings from the fingers, there was no significant difference in the SpO2 values between the fingertips. Additionally, in our study, we showed how three skin pigments (black, white, and brown) affected the SpO2 level of our users using different pulse oximeters.
All pulse oximeters performed differently with the fingertips, regardless of the finger measured. The mean and standard deviation in the readings between the fingers differed among the commercial pulse oximeter sensors. The commercial pulse oximeters (Ailie, Acurio, Braun) and our developed sensor did not show a significant difference in measuring from different fingers based on our hypothesis. Post hoc analyses on the 45 comparisons carried out on the fingers showed no significant difference between the commercial sensors and our developed sensor, as shown in the Table 6. Our study was limited to the specific models of pulse oximeters, hence the results might not be applicable to oximeters from different manufacturers.
A survey of healthcare workers showed that 80% of healthcare workers measured SpO2 values from the index finger [62]. The authors of the study highlighted the fact that the index finger is fed through the deep palmar arcus, which is formed by the radial artery, whereas the middle finger receives blood from the radial and ulnar arteries. Hence, they concluded that the best-performing fingers for using a PO are the middle and index fingers [62]. The results are largely consistent with previous studies, such as [8,16]. Basaranoglu et al. [63], compared the SpO2 values for different fingertips and their results revealed that although there was a difference in SpO2 values from the finger, the difference had no clinical importance. These findings corroborate our result which showed a difference in mean measurement from the different fingers and that the difference had no clinical impact on SpO2 values. Bickler et al. [16] studied the effect of skin pigmentation on pulse oximeter accuracy at low saturation levels using three pulse oximeters. They observed no significant change in SpO2 values at normal SpO2 levels but observed a significant change in SpO2 levels in darker skin at low SpO2 levels (<80%). Their findings corroborate our observation, all our recruited volunteers were healthy with no sign of hypoxemia, hence there was no clinical significance of the effect of skin pigment in our study, as shown in Table 6.
6. Conclusions
Since the emergency of COVID-19, SpO2 values have become one of the most important vitals to monitor in various clinical areas such as home use, in-patient and out-patient use, hospitals, sports, and other medical uses. In this study, we first provided an overview of the physical principles of SpO2 measurement, including the Beer–Lambert law describing the intensity of light propagating through a biological tissue. We then measured SpO2 values from 20 volunteers with different skin colours on different fingertips of both hands using three commercially available NHS-approved sensors. We analysed the results statistically using a single-factor ANOVA test and Bonferroni-corrected test to statistically investigate if there was a clinical significance between our custom sensor and the commercial PO sensors. In our pilot study on the effect of skin pigmentation (light, brown, and dark), we concluded that there was no significance in SpO2 measurements in healthy individuals with different skin pigments. Through our experiments with our volunteer group, we also concluded that our sensor and three commercial sensors provided different SpO2 measurements, although the difference was of no significant importance. Similarly, although the measurements were different for each fingertip, the difference was not statistically significant.
Author Contributions
The research idea was proposed by S.H., who developed a benchmarking sensor measurement technology to compare it with UK NHS-approved commercial sensors. The measurement testing was then organised on volunteers, with the involvement of NHS experts. Additionally, S.H. contributed to the methodology and writing of the manuscript and supervised and dealt with the administration of the overall research project delivery. C.N. carried out the research experiment, validation, formal analysis, and partially wrote the manuscript. M.A. contributed on SpO2 hardware implementation and software implementation. M.S. contributed on NHS ethical approval, sensor testing, and measurement on volunteers, including writing the manuscript specifications on health application and impact of the SpO2 measurement discrepancies in NHS patients. K.S. helped on sensor measurement, testing and benchmarking on volunteers, ethical approval, recruitment of volunteers and study protocol, including writing the manuscript on the health-related areas. V.D. contributed on the input expertise on sensor technology, software implementation, and improving the focus of the research on the technology lead theme, including the manuscript’s overall presentation. S.N. supported the testing and measurement on volunteers with her expertise as an NHS nurse. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The lead researcher obtained ethics approval and Institutional Review Board (IRB) authorisation for conducting this study on neuro-typical healthy volunteers.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ortega, R.; Hansen, C.J.; Elterman, K.; Woo, A. Pulse Oximetry. N. Engl. J. Med. 2011, 364, e33. [Google Scholar] [CrossRef]
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse oximetry: Understanding its basic principles facilitates appreciation of its limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef]
- Allen, J.; Overbeck, K.; Nath, A.F.; Murray, A.; Stansby, G. A prospective comparison of bilateral photoplethysmography versus the ankle-brachial pressure index for detecting and quantifying lower limb peripheral arterial disease. J. Vasc. Surg. 2008, 47, 794–802. [Google Scholar] [CrossRef]
- Yelderman, M.; New, W. Evaluation of pulse oximetry. Anesthesiology 1983, 59, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Severinghaus, J.W.; Kelleher, J.F. Recent developments in pulse oximetry. Anesthesiology 1992, 76, 1018–1038. [Google Scholar]
- Hess, D.; Kacmarek, R. Techniques and devices for monitoring oxygenation: Oxygenation in the criticall ill patient. Respir. Care 1993, 38, 646–671. [Google Scholar]
- Elahi, S. COVID: Pulse Oxygen Monitors Work Less Well on Darker Skin, Experts Say. BBC News. 2022. Available online: https://www.bbc.com/news/health-58032842 (accessed on 4 July 2022).
- Hinkelbein, J.; Genzwuerker, H.V.; Sogl, R.; Fiedler, F. Effect of nail polish on oxygen saturation determined by pulse oximetry in critically ill patients. Resuscitation 2007, 72, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ries, A.L.; Prewitt, L.M.; Johnson, J.J. Skin color and ear oximetry. Chest 1989, 96, 287–290. [Google Scholar] [CrossRef]
- Zeballos, R.J.; Weisman, I.M. Reliability of noninvasive oximetry in black subjects during exercise and hypoxia. Am. Rev. Respir. Dis. 1991, 144, 1240–1244. [Google Scholar] [CrossRef]
- Jubran, A.; Tobin, M.J. Reliability of pulse oximetry in titrating supplemental oxygen therapy in ventilator-dependent patients. Chest 1990, 97, 1420–1425. [Google Scholar] [CrossRef]
- Young, K. A Study of the Accuracy of Pulse Oximetry among Varying Skin Pigmentation Populations [Abstract]. Ph.D. Thesis, California State University, Long Beach, CA, USA, 1994. [Google Scholar]
- Coté, C.J.; Goldstein, E.A.; Fuchsman, W.H.; Hoaglin, D.C. The effect of nail polish on pulse oximetry. Anesth. Analg. 1988, 67, 683–686. [Google Scholar]
- Hakverdioğlu Yönt, G.; Akin Korhan, E.; Dizer, B. The effect of nail polish on pulse oximetry readings. Intensive Crit. Care Nurs. 2013, 30, 111–115. [Google Scholar] [CrossRef]
- Shi, C.; Goodall, M.; Dumville, J.; Hill, J.; Norman, G.; Hamer, O.; Clegg, A.; Watkins, C.L.; Georgiou, G.; Hodkinson, A.; et al. The accuracy of pulse oximetry in measuring oxygen saturation by levels of skin pigmentation: A systematic review and meta-analysis. BMC Med. 2022, 20, 267. [Google Scholar] [CrossRef] [PubMed]
- Bickler, P.E.; Feiner, J.R.; Severinghaus, J.W. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology 2005, 102, 715–719. [Google Scholar] [CrossRef]
- Adler, J.N.; Hughes, L.A.; Vivilecchia, R.; Camargo, C.A., Jr. Effect of skin pigmentation on pulse oximetry accuracy in the emergency department. Acad. Emerg. Med. 1998, 5, 965–970. [Google Scholar] [CrossRef]
- Holder, A.L.; Wong, A.K.I. The Big Consequences of Small Discrepancies: Why Racial Differences in Pulse Oximetry Errors Matter. Crit. Care Med. 2022, 50, 335–337. [Google Scholar] [CrossRef]
- Wong, A.K.I.; Charpignon, M.; Kim, H.; Josef, C.; de Hond, A.A.H.; Fojas, J.J.; Tabaie, A.; Liu, X.; Mireles-Cabodevila, E.; Carvalho, L.; et al. Analysis of Discrepancies Between Pulse Oximetry and Arterial Oxygen Saturation Measurements by Race and Ethnicity and Association with Organ Dysfunction and Mortality. JAMA Netw. Open 2021, 4, e2131674. [Google Scholar] [CrossRef]
- Mahgoub, M.T.A.; Khalifa, O.O.; Sidek, K.A.; Khan, S. Health monitoring system using Pulse Oximeter with remote alert. In Proceedings of the 2015 International Conference on Computing, Control, Networking, Electronics and Embedded Systems Engineering (ICCNEEE), Khartoum, Sudan, 7–9 September 2015; pp. 357–361. [Google Scholar]
- Chugh, S.; Kaur, J. Low cost calibration free Pulse oximeter. In Proceedings of the 2015 Annual IEEE India Conference (INDICON), New Delhi, India, 17–20 December 2015; pp. 1–5. [Google Scholar]
- Naeem, Z.H.; Youseffi, M.; Sefat, F.; Khaghani, S.A.; Raja, T.I.; Patel, A.; Javid, F.; Jamil, M.M.A.; Wahab, M.H.A. Design and Development of a Low Cost Pulse Oximeter. J. Phys. Conf. Ser. 2021, 1793, 012068. [Google Scholar] [CrossRef]
- Al Rasyid, M.U.H.; Lee, B.H.; Sudarsono, A. Implementation of body temperature and pulseoximeter sensors for wireless body area network. Sens. Mater. 2015, 27, 727–732. [Google Scholar]
- Nwibor, C.; Haxha, S.; Ali, M.M.; Sakel, M.; Haxha, A.R.; Saunders, K.; Nabakooza, S. Remote Health Monitoring System for the Estimation of Blood Pressure, Heart Rate, and Blood Oxygen Saturation Level. IEEE Sens. J. 2023, 23, 5401–5411. [Google Scholar] [CrossRef]
- HomeCare Magazine. Understanding SpO2 and Normal Oxygen Levels. 2022. Available online: https://www.homecaremag.com/understanding-SpO2-and-normal-oxygen-levels#:~:text=SpO2%2C%20also%20known%20as%20oxygen,will%20not%20function%20as%20efficiently (accessed on 2 February 2022).
- Holland, K. Is My Blood Oxygen Level Normal? 2022. Available online: https://www.healthline.com/health/normal-blood-oxygen-level#:~:text=Normal%3A%20A%20normal%20ABG%20oxygen,between%2095%20and%20100%20percent (accessed on 23 March 2022).
- Shi, C.; Goodall, M.; Dumville, J.; Hill, J.; Norman, G.; Hamer, O.; Clegg, A.; Watkins, C.L.; Georgiou, G.; Hodkinson, A.; et al. The effects of skin pigmentation on the accuracy of pulse oximetry in measuring oxygen saturation: A systematic review and meta-analysis. medRxiv 2022. [Google Scholar] [CrossRef]
- Al-Halawani, R.; Charlton, P.H.; Qassem, M.; Kyriacou, P.A. A review of the effect of skin pigmentation on pulse oximeter accuracy. Physiol. Meas. 2023, 44, 05TR01. [Google Scholar] [CrossRef]
- Cabanas, A.M.; Fuentes-Guajardo, M.; Latorre, K.; León, D.; Martín-Escudero, P. Skin Pigmentation Influence on Pulse Oximetry Accuracy: A Systematic Review and Bibliometric Analysis. Sensors 2022, 22, 3402. [Google Scholar] [CrossRef]
- Elgendi, M. On the analysis of fingertip photoplethysmogram signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef]
- Visualdictionaryonline.com. HUMAN BEING:: SENSE ORGANS:: TOUCH:: FINGER Image—Visual Dictionary Online. 2022. Available online: http://www.visualdictionaryonline.com/human-being/sense-organs/touch/finger.php (accessed on 18 September 2018).
- Wang, C.; Li, Z.; Wei, X. Monitoring heart and respiratory rates at radial artery based on PPG. Optik 2013, 124, 3954–3956. [Google Scholar] [CrossRef]
- Tremper, K.K.; Barker, S.J. Pulse oximetry. Anesthesiology 1989, 70, 98–108. [Google Scholar] [CrossRef]
- Sinex, J.E. Pulse oximetry: Principles and limitations. Am. J. Emerg. Med. 1999, 17, 59–66. [Google Scholar] [CrossRef]
- Shi, P. Photoplethysmography in Noninvasive Cardiovascular Assessment. Ph.D. Thesis, Loughborough University, Loughborough, UK, 2009. [Google Scholar]
- Baker, W.B.; Parthasarathy, A.B.; Busch, D.R.; Mesquita, R.C.; Greenberg, J.H.; Yodh, A.G. Modified Beer-Lambert law for blood flow. Biomed. Opt. Express 2014, 5, 4053–4075. [Google Scholar] [CrossRef]
- Ralston, A.C.; Webb, R.K.; Runciman, W.B. Potential errors in pulse oximetry. III: Effects of interferences, dyes, dyshaemoglobins and other pigments. Anaesthesia 1991, 46, 291–295. [Google Scholar] [CrossRef]
- Hampson, N.B.; Scott, K.L.; Zmaeff, J.L. Carboxyhemoglobin measurement by hospitals: Implications for the diagnosis of carbon monoxide poisoning. J. Emerg. Med. 2006, 31, 13–16. [Google Scholar] [CrossRef]
- Haxha, S.; Jhoja, J. Optical Based Noninvasive Glucose Monitoring Sensor Prototype. IEEE Photonics J. 2016, 8, 6805911. [Google Scholar] [CrossRef]
- Solà, J.; Castoldi, S.; Chételat, O.; Correvon, M.; Dasen, S.; Droz, S.; Jacob, N.; Kormann, R.; Neumann, V.; Perrenoud, A.; et al. SpO2 sensor embedded in a finger ring: Design and implementation. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 2006, 4295–4298. [Google Scholar]
- Zhang, Q.; Xi, S.; Xing, B. Design of noninvasive blood oxygen measurement system based on S3C2440A. Appl. Electron. Tech. 2013, 6, 67–69+73. [Google Scholar]
- Zhi, W.; Feng, L.L. Study on multiParameter monitoring instrument based on embedded system. Appl. Electron. Tech. 2009, 11, 71–74+78. [Google Scholar]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med. Devices 2014, 7, 231–239. [Google Scholar] [CrossRef]
- Zonios, G.; Iyer, V.K.; Shankar, U.S. Adaptive Calibration for Pulse Oximetry. U.S. Patent 6,839,580, 4 January 2005. [Google Scholar]
- Rusch, T.L.; Sankar, R.; Scharf, J.E. Signal processing methods for pulse oximetry. Comput. Biol. Med. 1996, 26, 143–159. [Google Scholar] [CrossRef]
- Salyer, J.W. Neonatal and pediatric pulse oximetry. Respir. Care 2003, 48, 386–396; discussion 397–398. [Google Scholar]
- Reddy, K.A.; George, B.; Mohan, N.M.; Kumar, V.J. A Novel Calibration-Free Method of Measurement of Oxygen Saturation in Arterial Blood. IEEE Trans. Instrum. Meas. 2009, 58, 1699–1705. [Google Scholar] [CrossRef]
- Flewelling, R. Noninvasive Optical Monitoring. In The Biomedical Engineering HandBook, 2nd ed.; Bronzino, E.J.D., Ed.; CRC Press: Boca Raton, FL, USA, 2000; Chapter 86. [Google Scholar]
- Mendelson, Y.; Kent, J. Variations in optical absorption spectra of adult and fetal haemoglobins and its effect on pulse oximetry. IEEE Trans. Biomed. Eng. 1989, 36, 844–848. [Google Scholar] [CrossRef]
- Oak, S.; Aroul, P. How to Design Peripheral Oxygen Saturation (SpO2) and Optical Heart Rate Monitoring (OHRM) Systems Using AFE4403. Application Report SLAA655. 2015. Available online: https://www.ti.com/lit/pdf/slaa655 (accessed on 17 May 2024).
- Szczepaaski, A.; Saeed, K. A Mobile Device System for Early Warning of ECG Anomalies. Sensors 2014, 14, 11031–11044. [Google Scholar] [CrossRef]
- Munsell, A.H. Atlas of the Munsell Color System; Wadsworth, Howland Co., Inc., Printers: Boston, MA, USA, 1915. [Google Scholar]
- Munsell Color System; Color Matching from Munsell Color Company. Munsell Color Notation and Color Test; Dimensions of Color Munsell Color System. 2022. Available online: https://munsell.com/about-munsell-color/how-color-notation-works (accessed on 4 July 2022).
- Zanca, J. Exploring Methods to Improve Pressure Ulcer Detection: Spectroscopic Assessment of the Blanch Response. Doctoral Dissertation, University of Pittsburgh, Pittsburgh, PA, USA, 2006. [Google Scholar]
- Muddy Colors. Thinking about Skin Tones, Chroma and Munsell. 2022. Available online: https://www.muddycolors.com/2019/07/thinking-about-skin-tones-chroma-and-munsell (accessed on 5 July 2022).
- Acurio AS-302 OLED Medical Fingertip Pulse Oximeter with FDA CE ISO Approval. 2022. Available online: https://www.medicalexpo.com/prod/shenzhen-acurio-instruments/product-301658-1127997.html (accessed on 5 July 2022).
- Braunhealthcare.com. Braun Pulse Oximeter. 2022. Available online: https://www.iacl.ie/pulse-oximeters/braun-pulse-oximeter.4100.html (accessed on 5 July 2022).
- Amazon.co.uk. 2022. Available online: https://www.amazon.co.uk/AILE-Oximeter-Approved-Monitor-Oximeter-Monitor/dp/B08TTYG32S (accessed on 5 July 2022).
- Zach, V. What Is a Bland-Altman Plot? (Definition & Example). Statology. Available online: https://www.statology.org/bland-altman-plot (accessed on 3 September 2022).
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Mizukoshi, K.; Shibasaki, M.; Amaya, F.; Mizobe, T.; Tanaka, Y. Which Finger Do You Attach Pulse Oximetry To? Index Finger or Not? Eur. J. Anaesthesiol. 2009, 26 (Suppl. S45), 3AP1–3AP5. [Google Scholar]
- Basaranoglu, G.; Bakan, M.; Umutoglu, T.; Zengin, S.U.; Idin, K.; Salihoglu, Z. Comparison of SpO2 values from different fingers of the hands. SpringerPlus 2015, 4, 561. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).