DNA Sensing Platforms: Novel Insights into Molecular Grafting Using Low Perturbative AFM Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. AFM Gold Substrate Preparation
2.2. Methods
2.2.1. Nanoshaving and Nanografting
2.2.2. Atomic Force Microscopy
3. Results and Discussion
| 2 Hz | 8 Hz | |
|---|---|---|
| S/A = 0.5 | 180 | 230 |
| S/A = 1 | 120 | 200 |
| S/A = 2 | 100 | 110 |
| S/A = 4 | 80 | 100 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maduraiveeran, G. Bionanomaterial-based electrochemical biosensing platforms for biomedical applications. Anal. Methods 2020, 12, 1688–1701. [Google Scholar] [CrossRef]
- Flores-Contreras, E.A.; González-González, R.B.; Rodríguez-Sánchez, I.P.; León, J.F.Y.-D.; Iqbal, H.M.N.; González-González, E. Microfluidics-Based Biosensing Platforms: Emerging Frontiers in Point-of-Care Testing SARS-CoV-2 and Seroprevalence. Biosensors 2022, 12, 179. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Alapan, Y.; ElKabbash, M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; De Luca, A.; Strangi, G. Extreme sensitivity biosensing platform based on hyperbolic metamaterials. Nat. Mater. 2016, 15, 621–627. [Google Scholar] [CrossRef]
- Qu, J.-H.; Dillen, A.; Saeys, W.; Lammertyn, J.; Spasic, D. Advancements in SPR biosensing technology: An overview of recent trends in smart layers design, multiplexing concepts, continuous monitoring and in vivo sensing. Anal. Chim. Acta 2019, 1104, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Souto, D.E.; Volpe, J.; Gonçalves, C.D.C.; Ramos, C.H.; Kubota, L.T. A brief review on the strategy of developing SPR-based biosensors for application to the diagnosis of neglected tropical diseases. Talanta 2020, 205, 120122. [Google Scholar] [CrossRef] [PubMed]
- Pollet, J.; Delport, F.; Janssen, K.P.; Jans, K.; Maes, G.; Pfeiffer, H.; Wevers, M.; Lammertyn, J. Fiber optic SPR biosensing of DNA hybridization and DNA–protein interactions. Biosens. Bioelectron. 2009, 25, 864–869. [Google Scholar] [CrossRef]
- Pinto, G.; Dante, S.; Rotondi, S.M.C.; Canepa, P.; Cavalleri, O.; Canepa, M. Spectroscopic Ellipsometry Investigation of a Sensing Functional Interface: DNA SAMs Hybridization. Adv. Mater. Interfaces 2022, 9, 2200364. [Google Scholar] [CrossRef]
- Al Rubaye, A.; Nabok, A.; Catanante, G.; Marty, J.-L.; Takacs, E.; Szekacs, A. Detection of ochratoxin A in aptamer assay using total internal reflection ellipsometry. Sensors Actuators Chem. 2018, 263, 248–251. [Google Scholar] [CrossRef]
- Wenmackers, S.; Pop, S.D.; Roodenko, K.; Vermeeren, V.; Williams, O.A.; Daenen, M.; Douhéret, O.; D’Haen, J.; Hardy, A.; Van Bael, M.; et al. Structural and Optical Properties of DNA Layers Covalently Attached to Diamond Surfaces. Langmuir 2008, 24, 7269–7277. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, Z.; Zhou, H.S. Review—Measurement and Analysis of Cancer Biomarkers Based on Electrochemical Biosensors. J. Electrochem. Soc. 2019, 167, 037525. [Google Scholar] [CrossRef]
- Hasan, M.; Ahommed, M.; Daizy, M.; Bacchu, M.; Ali, M.; Al-Mamun, M.; Aly, M.S.; Khan, M.; Hossain, S. Recent development in electrochemical biosensors for cancer biomarkers detection. Biosens. Bioelectron. X 2021, 8, 100075. [Google Scholar] [CrossRef]
- Kikkeri, K.; Wu, D.; Voldman, J. A sample-to-answer electrochemical biosensor system for biomarker detection. Lab A Chip 2021, 22, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.L.D.; Câmara, I.M.; Silva, P.P.; Wanderley, N.R.S.; Alves, M.B.C.; Morais, C.L.M.; Martin, F.L.; Lajus, T.B.P.; Lima, K.M.G. Spectrochemical analysis of liquid biopsy harnessed to multivariate analysis towards breast cancer screening. Sci. Rep. 2020, 10, 12818. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidi, M.; Morais, C.L.M.; Lima, K.M.G.; Snowden, J.S.; Saxon, J.A.; Richardson, A.M.T.; Jones, M.; Mann, D.M.A.; Allsop, D.; Martin-Hirsch, P.L.; et al. Differential diagnosis of Alzheimer’s disease using spectrochemical analysis of blood. Proc. Natl. Acad. Sci. USA 2017, 114, E7929–E7938. [Google Scholar] [CrossRef]
- Alves, M.V.S.; Maciel, L.I.L.; Passos, J.O.S.; Morais, C.L.M.; dos Santos, M.C.D.; Lima, L.A.S.; Vaz, B.G.; Pegado, R.; Lima, K.M.G. Spectrochemical approach combined with symptoms data to diagnose fibromyalgia through paper spray ionization mass spectrometry (PSI-MS) and multivariate classification. Sci. Rep. 2023, 13, 4658. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.O.S.; Alves, M.V.D.S.; Morais, C.L.M.; Martin, F.L.; Cavalcante, A.F.; Lemos, T.M.A.M.; Moura, S.; Freitas, D.L.D.; Mariz, J.V.M.; Carvalho, J.L.; et al. Spectrochemical analysis in blood plasma combined with subsequent chemometrics for fibromyalgia detection. Sci. Rep. 2020, 10, 11769. [Google Scholar] [CrossRef]
- Lim, H.J.; Saha, T.; Tey, B.T.; Tan, W.S.; Ooi, C.W. Quartz crystal microbalance-based biosensors as rapid diagnostic devices for infectious diseases. Biosens. Bioelectron. 2020, 168, 112513. [Google Scholar] [CrossRef]
- Xi, X.; Niyonshuti, I.I.; Yu, N.; Yao, L.; Fu, Y.; Chen, J.; Li, Y. Label-Free Quartz Crystal Microbalance Biosensor Based on Aptamer-Capped Gold Nanocages Loaded with Polyamidoamine for Thrombin Detection. ACS Appl. Nano Mater. 2021, 4, 10047–10054. [Google Scholar] [CrossRef]
- Stachiv, I.; Kuo, C.-Y.; Li, W. Protein adsorption by nanomechanical mass spectrometry: Beyond the real-time molecular weighting. Front. Mol. Biosci. 2023, 9, 1058441. [Google Scholar] [CrossRef]
- Zhou, D.; Sinniah, K.; Abell, C.; Rayment, T. Label-Free Detection of DNA Hybridization at the Nanoscale: A Highly Sensitive and Selective Approach Using Atomic-Force Microscopy. Angew. Chem. Int. Ed. 2003, 42, 4934–4937. [Google Scholar] [CrossRef]
- Liu, M.; Liu, G.-Y. Hybridization with Nanostructures of Single-Stranded DNA. Langmuir 2005, 21, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Bano, F.; Fruk, L.; Sanavio, B.; Glettenberg, M.; Casalis, L.; Niemeyer, C.M.; Scoles, G. Toward Multiprotein Nanoarrays Using Nanografting and DNA Directed Immobilization of Proteins. Nano Lett. 2009, 9, 2614–2618. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, E.; Paoletti, P.; Bosco, A.; Parisse, P.; Scaini, D.; Tagliabue, E.; de Marco, A.; Casalis, L. Quantification of Circulating Cancer Biomarkers via Sensitive Topographic Measurements on Single Binder Nanoarrays. ACS Omega 2017, 2, 2618–2629. [Google Scholar] [CrossRef]
- Olulana, A.F.A.; Soler, M.A.; Lotteri, M.; Vondracek, H.; Casalis, L.; Marasco, D.; Castronovo, M.; Fortuna, S. Computational Evolution of Beta-2-Microglobulin Binding Peptides for Nanopatterned Surface Sensors. Int. J. Mol. Sci. 2021, 22, 812. [Google Scholar] [CrossRef]
- Bordi, F.; Prato, M.; Cavalleri, O.; Cametti, C.; Canepa, M.; Gliozzi, A. Azurin Self-Assembled Monolayers Characterized by Coupling Electrical Impedance Spectroscopy and Spectroscopic Ellipsometry. J. Phys. Chem. B 2004, 108, 20263–20272. [Google Scholar] [CrossRef]
- Solano, I.; Parisse, P.; Gramazio, F.; Cavalleri, O.; Bracco, G.; Castronovo, M.; Casalis, L.; Canepa, M. Spectroscopic ellipsometry meets AFM nanolithography: About hydration of bio-inert oligo(ethylene glycol)-terminated self assembled monolayers on gold. Phys. Chem. Chem. Phys. 2015, 17, 28774–28781. [Google Scholar] [CrossRef] [PubMed]
- Solano, I.; Parisse, P.; Gramazio, F.; Ianeselli, L.; Medagli, B.; Cavalleri, O.; Casalis, L.; Canepa, M. Atomic Force Microscopy and Spectroscopic Ellipsometry combined analysis of Small Ubiquitin-like Modifier adsorption on functional monolayers. Appl. Surf. Sci. 2017, 421, 722–727. [Google Scholar] [CrossRef]
- Pinto, G.; Canepa, P.; Canale, C.; Canepa, M.; Cavalleri, O. Morphological and Mechanical Characterization of DNA SAMs Combining Nanolithography with AFM and Optical Methods. Materials 2020, 13, 2888. [Google Scholar] [CrossRef]
- Larsson, C.; Rodahl, M.; Höök, F. Characterization of DNA Immobilization and Subsequent Hybridization on a 2D Arrangement of Streptavidin on a Biotin-Modified Lipid Bilayer Supported on SiO2. Anal. Chem. 2003, 75, 5080–5087. [Google Scholar] [CrossRef]
- Su, X.; Wu, Y.-J.; Knoll, W. Comparison of surface plasmon resonance spectroscopy and quartz crystal microbalance techniques for studying DNA assembly and hybridization. Biosens. Bioelectron. 2005, 21, 719–726. [Google Scholar] [CrossRef]
- Van der Meulen, S.A.J.; Dubacheva, G.V.; Dogterom, M.; Richter, R.P.; Leunissen, M.E. Quartz Crystal Microbalance with Dissipation Monitoring and Spectroscopic Ellipsometry Measurements of the Phospholipid Bilayer Anchoring Stability and Kinetics of Hydrophobically Modified DNA Oligonucleotides. Langmuir 2014, 30, 6525–6533. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; Koenig, M.; Rodenhausen, K.B.; Eichhorn, K.-J.; Oertel, U.; Schubert, M.; Stamm, M.; Uhlmann, P. Quartz crystal microbalance with coupled spectroscopic ellipsometry-study of temperature-responsive polymer brush systems. Appl. Surf. Sci. 2017, 421, 843–851. [Google Scholar] [CrossRef]
- Xu, S.; Liu, G.-Y. Nanometer-Scale Fabrication by Simultaneous Nanoshaving and Molecular Self-Assembly. Langmuir 1997, 13, 127–129. [Google Scholar] [CrossRef]
- Canepa, P.; Gonella, G.; Pinto, G.; Grachev, V.; Canepa, M.; Cavalleri, O. Anchoring of Aminophosphonates on Titanium Oxide for Biomolecular Coupling. J. Phys. Chem. C 2019, 123, 16843–16850. [Google Scholar] [CrossRef]
- Canepa, P.; Gregurec, D.; Liessi, N.; Rotondi, S.M.C.; Moya, S.E.; Millo, E.; Canepa, M.; Cavalleri, O. Biofunctionalization of Porous Titanium Oxide through Amino Acid Coupling for Biomaterial Design. Materials 2023, 16, 784. [Google Scholar] [CrossRef]
- Pinto, G.; Parisse, P.; Solano, I.; Canepa, P.; Canepa, M.; Casalis, L.; Cavalleri, O. Functionalizing gold with single strand DNA: Novel insight into optical properties via combined spectroscopic ellipsometry and nanolithography measurements. Soft Matter 2019, 15, 2463–2468. [Google Scholar] [CrossRef]
- Cavalleri, O.; Natale, C.; Stroppolo, M.E.; Relini, A.; Cosulich, E.; Thea, S.; Novi, M.; Gliozzi, A. Azurin immobilisation on thiol covered Au(111). Phys. Chem. Chem. Phys. 2000, 2, 4630–4635. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Xu, S.; Qian, Y. Nanofabrication of Self-Assembled Monolayers Using Scanning Probe Lithography. Acc. Chem. Res. 2000, 33, 457–466. [Google Scholar] [CrossRef]
- Liu, M.; Amro, N.A.; Chow, C.S.; Liu, G.-Y. Production of Nanostructures of DNA on Surfaces. Nano Lett. 2002, 2, 863–867. [Google Scholar] [CrossRef]
- Zhou, D.; Sinniah, K.; Abell, C.; Rayment, T. Use of Atomic Force Microscopy for Making Addresses in DNA Coatings. Langmuir 2002, 18, 8278–8281. [Google Scholar] [CrossRef]
- Mirmomtaz, E.; Castronovo, M.; Grunwald, C.; Bano, F.; Scaini, D.; Ensafi, A.A.; Scoles, G.; Casalis, L. Quantitative Study of the Effect of Coverage on the Hybridization Efficiency of Surface-Bound DNA Nanostructures. Nano Lett. 2008, 8, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Bano, F.; Parisse, P.; Casalis, L.; DeSimone, A.; Micheletti, C. Hybridization in nanostructured DNA monolayers probed by AFM: Theory versus experiment. Nanoscale 2011, 4, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Ngavouka, M.D.N.; Bosco, A.; Casalis, L.; Parisse, P. Determination of Average Internucleotide Distance in Variable Density ssDNA Nanobrushes in the Presence of Different Cations Species. Macromolecules 2014, 47, 8748–8753. [Google Scholar] [CrossRef]
- Gil, P.S.; Lacks, D.J.; Parisse, P.; Casalis, L.; Ngavouka, M.D.N. Single-stranded DNA oligomer brush structure is dominated by intramolecular interactions mediated by the ion environment. Soft Matter 2018, 14, 9675–9680. [Google Scholar] [CrossRef]
- Alsadig, A.; Abbasgholi-Na, B.; Vondracek, H.; Medagli, B.; Fortuna, S.; Posocco, P.; Parisse, P.; Cabrera, H.; Casalis, L. DNA-Directed Protein Anchoring on Oligo/Alkanethiol-Coated Gold Nanoparticles: A Versatile Platform for Biosensing Applications. Nanomaterials 2022, 13, 78. [Google Scholar] [CrossRef]
- Castronovo, M.; Radovic, S.; Grunwald, C.; Casalis, L.; Morgante, M.; Scoles, G. Control of Steric Hindrance on Restriction Enzyme Reactions with Surface-Bound DNA Nanostructures. Nano Lett. 2008, 8, 4140–4145. [Google Scholar] [CrossRef]
- Liang, J.; Castronovo, M.; Scoles, G. DNA as Invisible Ink for AFM Nanolithography. J. Am. Chem. Soc. 2011, 134, 39–42. [Google Scholar] [CrossRef]
- Olulana, A.F.A.; Choi, D.; Inverso, V.; Redhu, S.K.; Vidonis, M.; Crevatin, L.; Nicholson, A.W.; Castronovo, M. Noncanonical DNA Cleavage by BamHI Endonuclease in Laterally Confined DNA Monolayers Is a Step Function of DNA Density and Sequence. Molecules 2022, 27, 5262. [Google Scholar] [CrossRef]
- Chopinet, L.; Formosa, C.; Rols, M.-P.; Duval, R.E.; Dague, E. Imaging living cells surface and quantifying its properties at high resolution using AFM in QI™ mode. Micron 2013, 48, 26–33. [Google Scholar] [CrossRef]
- Yagi, N.; Satonaka, K.; Horio, M.; Shimogaki, H.; Tokuda, Y.; Maeda, S. The Role of DNase and EDTA on DNA Degradation in Formaldehyde Fixed Tissues. Biotech. Histochem. 1996, 71, 123–129. [Google Scholar] [CrossRef]
- Gupta, P.; Loos, K.; Korniakov, A.; Spagnoli, C.; Cowman, M.; Ulman, A. Facile Route to Ultraflat SAM-Protected Gold Surfaces by “Amphiphile Splitting”. Angew. Chem. Int. Ed. 2004, 43, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Gatto, G.J.; Stryer, L. Biochemistry, 8th ed.; W.H. Freeman & Company, a Macmillan Education Imprint: New York, NY, USA, 2015; ISBN 978-1-4641-2610-9. [Google Scholar]
- Tinland, B.; Pluen, A.; Sturm, J.; Weill, G. Persistence Length of Single-Stranded DNA. Macromolecules 1997, 30, 5763–5765. [Google Scholar] [CrossRef]
- Kimura-Suda, H.; Petrovykh, D.Y.; Tarlov, M.J.; Whitman, L.J. Base-Dependent Competitive Adsorption of Single-Stranded DNA on Gold. J. Am. Chem. Soc. 2003, 125, 9014–9015. [Google Scholar] [CrossRef] [PubMed]
- Östblom, M.; Liedberg, B.; Demers, L.M.; Mirkin, C.A. On the Structure and Desorption Dynamics of DNA Bases Adsorbed on Gold: A Temperature-Programmed Study. J. Phys. Chem. 2005, 109, 15150–15160. [Google Scholar] [CrossRef]
- Herne, T.M.; Tarlov, M.J. Characterization of DNA Probes Immobilized on Gold Surfaces. J. Am. Chem. Soc. 1997, 119, 8916–8920. [Google Scholar] [CrossRef]
- Rybenkov, V.V.; Cozzarelli, N.R.; Vologodskii, A.V. Probability of DNA knotting and the effective diameter of the DNA double helix. Proc. Natl. Acad. Sci. USA 1993, 90, 5307–5311. [Google Scholar] [CrossRef]
- Stigter, D. Interactions of highly charged colloidal cylinders with applications to double-stranded DNA. Biopolymers 1977, 16, 1435–1448. [Google Scholar] [CrossRef]
- Steel, A.B.; Herne, T.M.; Tarlov, M.J. Electrochemical Quantitation of DNA Immobilized on Gold. Anal. Chem. 1998, 70, 4670–4677. [Google Scholar] [CrossRef]
- Yao, L.; Sullivan, J.; Hower, J.; He, Y.; Jiang, S. Packing structures of single-stranded DNA and double-stranded DNA thiolates on Au(111): A molecular simulation study. J. Chem. Phys. 2007, 127, 195101. [Google Scholar] [CrossRef]
- Domínguez, C.M.; Ramos, D.; Mendieta-Moreno, J.I.; Fierro, J.L.G.; Mendieta, J.; Tamayo, J.; Calleja, M. Effect of water-DNA interactions on elastic properties of DNA self-assembled monolayers. Sci. Rep. 2017, 7, 536. [Google Scholar] [CrossRef]
- Xu, S.; Laibinis, P.E.; Liu, G. Accelerating the Kinetics of Thiol Self-Assembly on Gold—A Spatial Confinement Effect. J. Am. Chem. Soc. 1998, 120, 9356–9361. [Google Scholar] [CrossRef]
- Ryu, S.; Schatz, G.C. Nanografting: Modeling and Simulation. J. Am. Chem. Soc. 2006, 128, 11563–11573. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.W.; Heaton, R.J.; Georgiadis, R. The effect of surface probe density on DNA hybridization. Nucleic Acids Res. 2001, 29, 5163–5168. [Google Scholar] [CrossRef] [PubMed]
- Melli, M.; Scoles, G.; Lazzarino, M. Fast Detection of Biomolecules in Diffusion-Limited Regime Using Micromechanical Pillars. ACS Nano 2011, 5, 7928–7935. [Google Scholar] [CrossRef]
- Doni, G.; Ngavouka, M.D.N.; Barducci, A.; Parisse, P.; De Vita, A.; Scoles, G.; Casalis, L.; Pavan, G.M. Structural and energetic basis for hybridization limits in high-density DNA monolayers. Nanoscale 2013, 5, 9988–9993. [Google Scholar] [CrossRef] [PubMed]


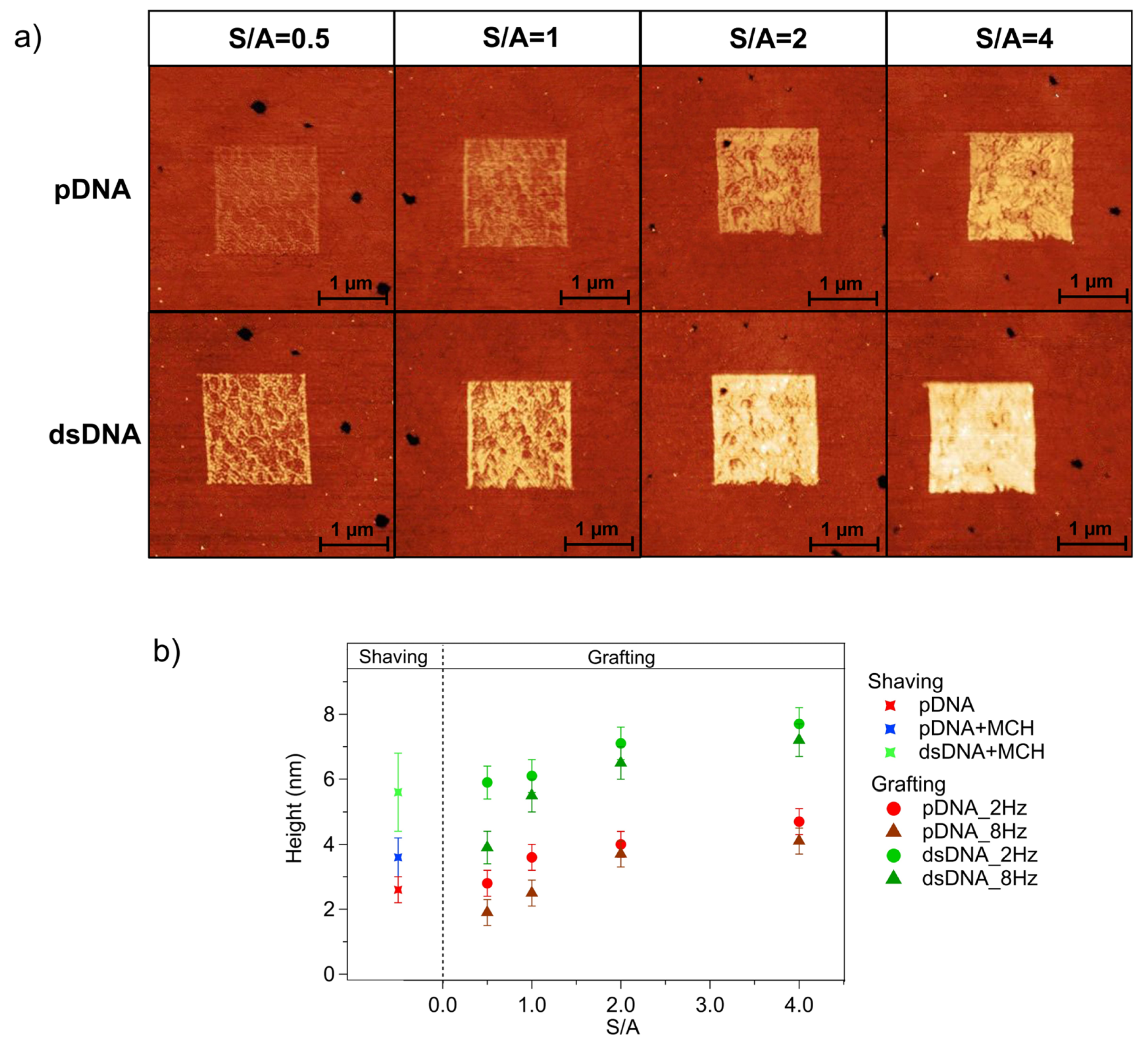
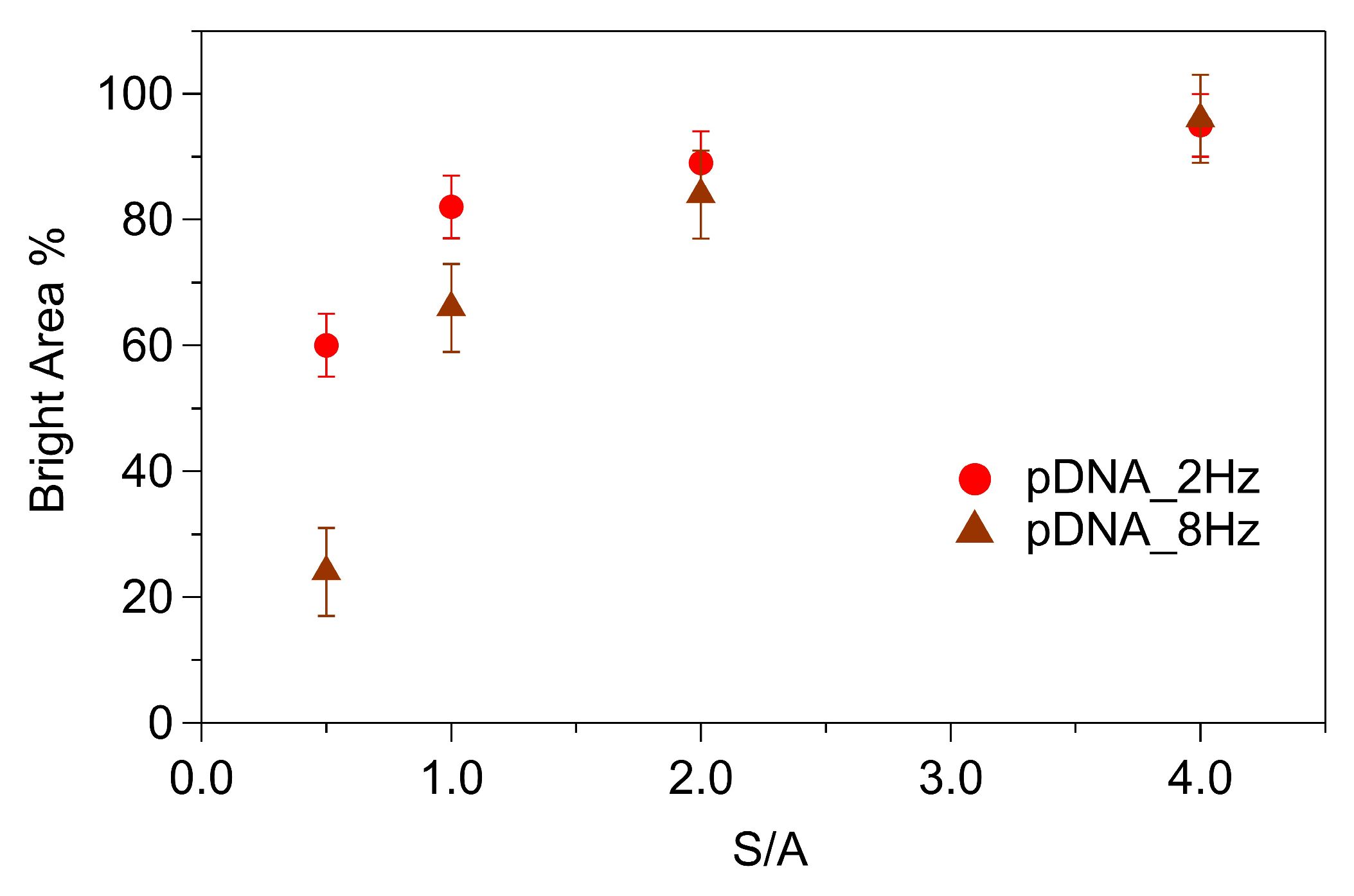

| pDNA | pDNA + MCH | dsDNA + MCH | |
|---|---|---|---|
| Thickness (nm) | 2.6 ± 0.4 | 3.6 ± 0.6 | 5.6 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotondi, S.M.C.; Canepa, P.; Angeli, E.; Canepa, M.; Cavalleri, O. DNA Sensing Platforms: Novel Insights into Molecular Grafting Using Low Perturbative AFM Imaging. Sensors 2023, 23, 4557. https://doi.org/10.3390/s23094557
Rotondi SMC, Canepa P, Angeli E, Canepa M, Cavalleri O. DNA Sensing Platforms: Novel Insights into Molecular Grafting Using Low Perturbative AFM Imaging. Sensors. 2023; 23(9):4557. https://doi.org/10.3390/s23094557
Chicago/Turabian StyleRotondi, Silvia Maria Cristina, Paolo Canepa, Elena Angeli, Maurizio Canepa, and Ornella Cavalleri. 2023. "DNA Sensing Platforms: Novel Insights into Molecular Grafting Using Low Perturbative AFM Imaging" Sensors 23, no. 9: 4557. https://doi.org/10.3390/s23094557
APA StyleRotondi, S. M. C., Canepa, P., Angeli, E., Canepa, M., & Cavalleri, O. (2023). DNA Sensing Platforms: Novel Insights into Molecular Grafting Using Low Perturbative AFM Imaging. Sensors, 23(9), 4557. https://doi.org/10.3390/s23094557





