Effect of a Patient-Specific Structural Prior Mask on Electrical Impedance Tomography Image Reconstructions
Abstract
1. Introduction
2. Methods
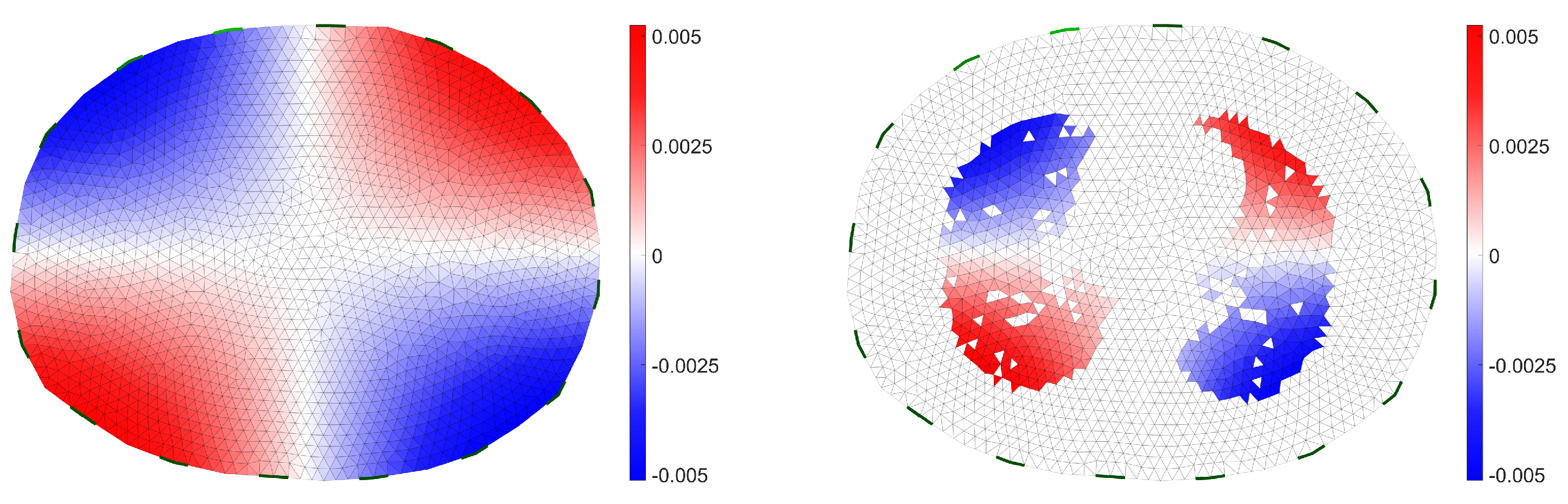
2.1. Structural Prior Mask in EIT Reconstruction Algorithm
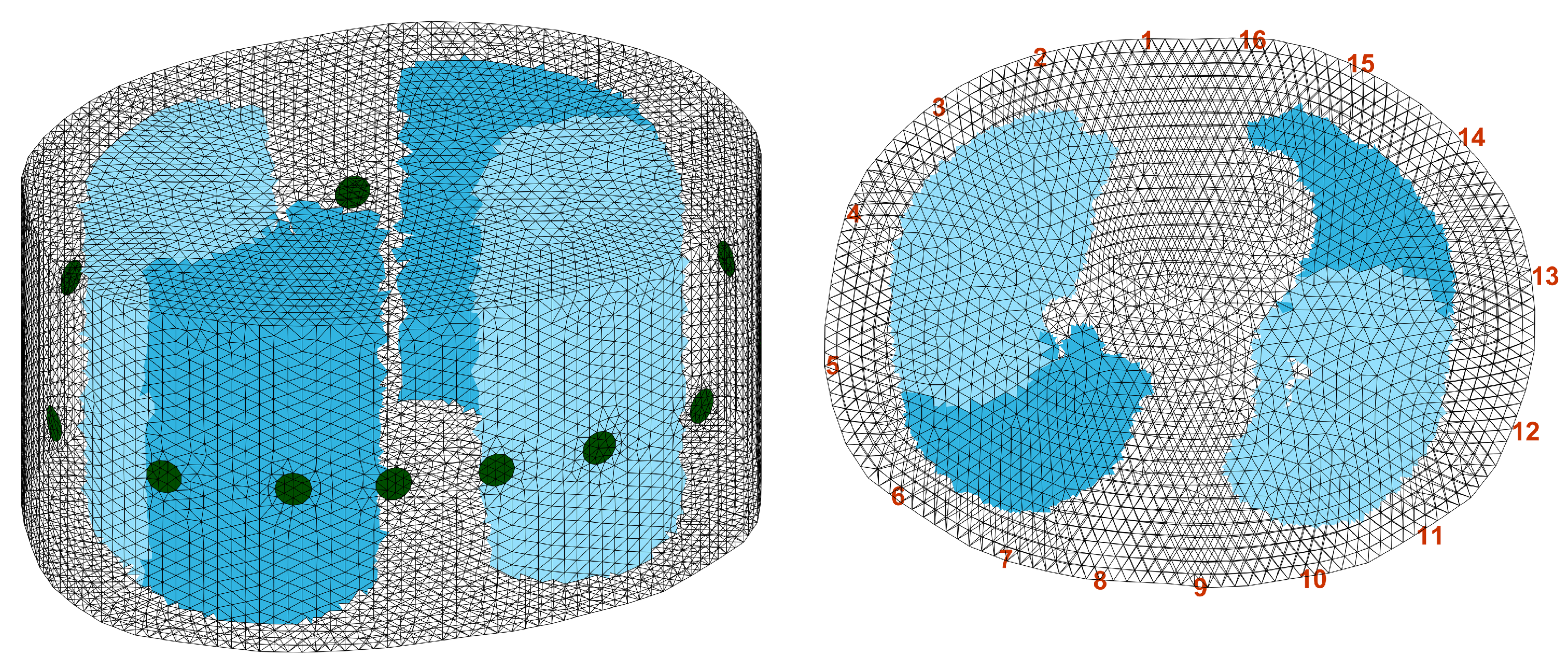
2.2. Simulation Data
- No ventilation in the dorsal right lung
- No ventilation in the dorsal parts of both lungs
- No ventilation in the ventral left lung and the dorsal right lung
- No ventilation in the most ventral and most dorsal parts of both lungs.
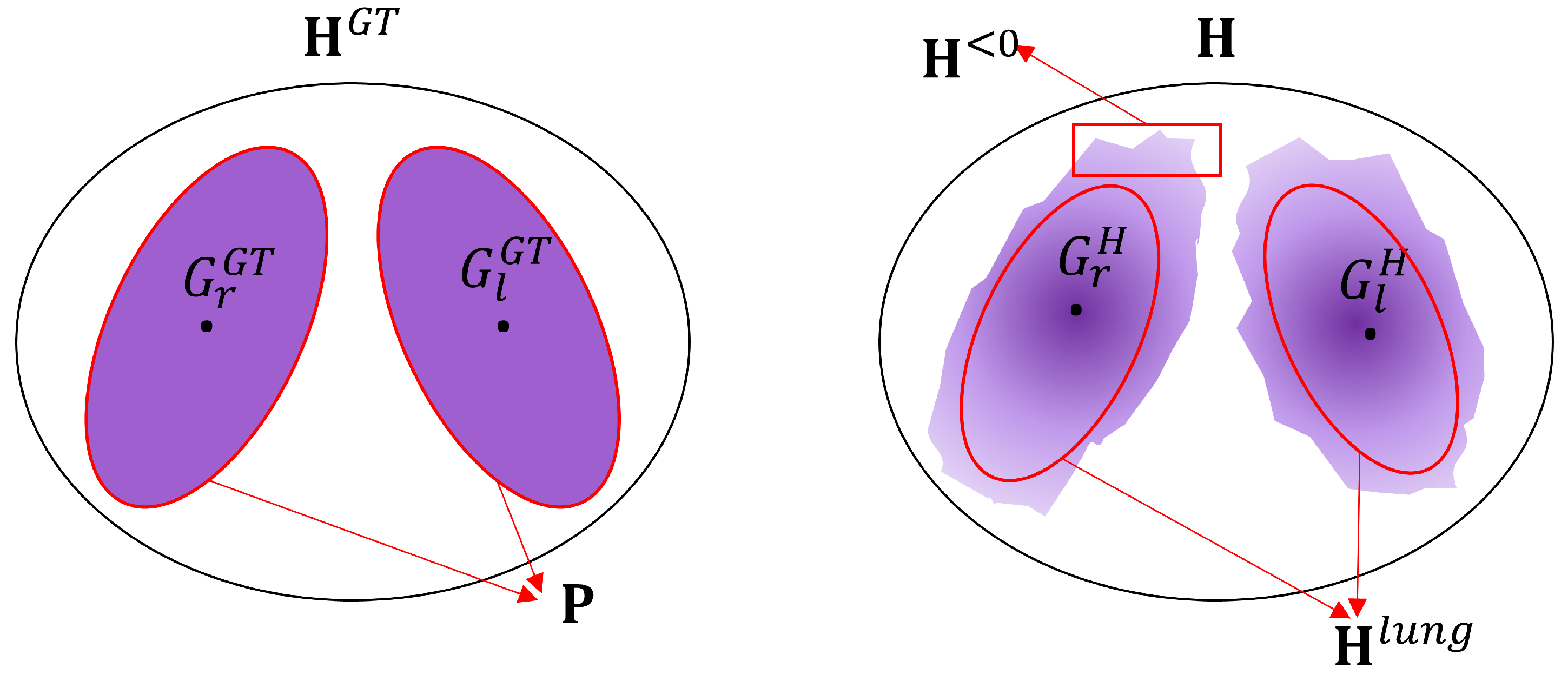
2.3. Evaluation of the Reconstruction
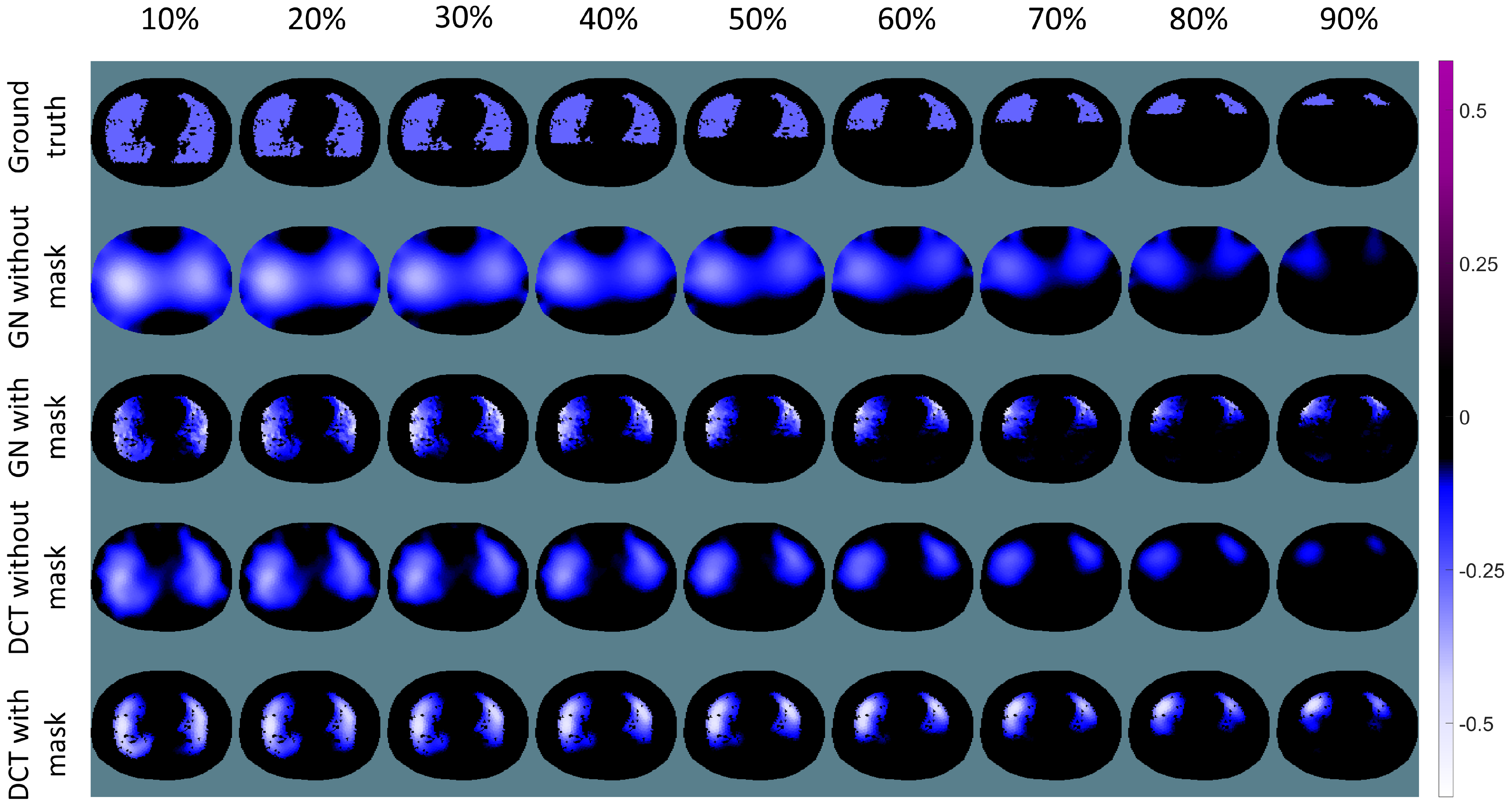
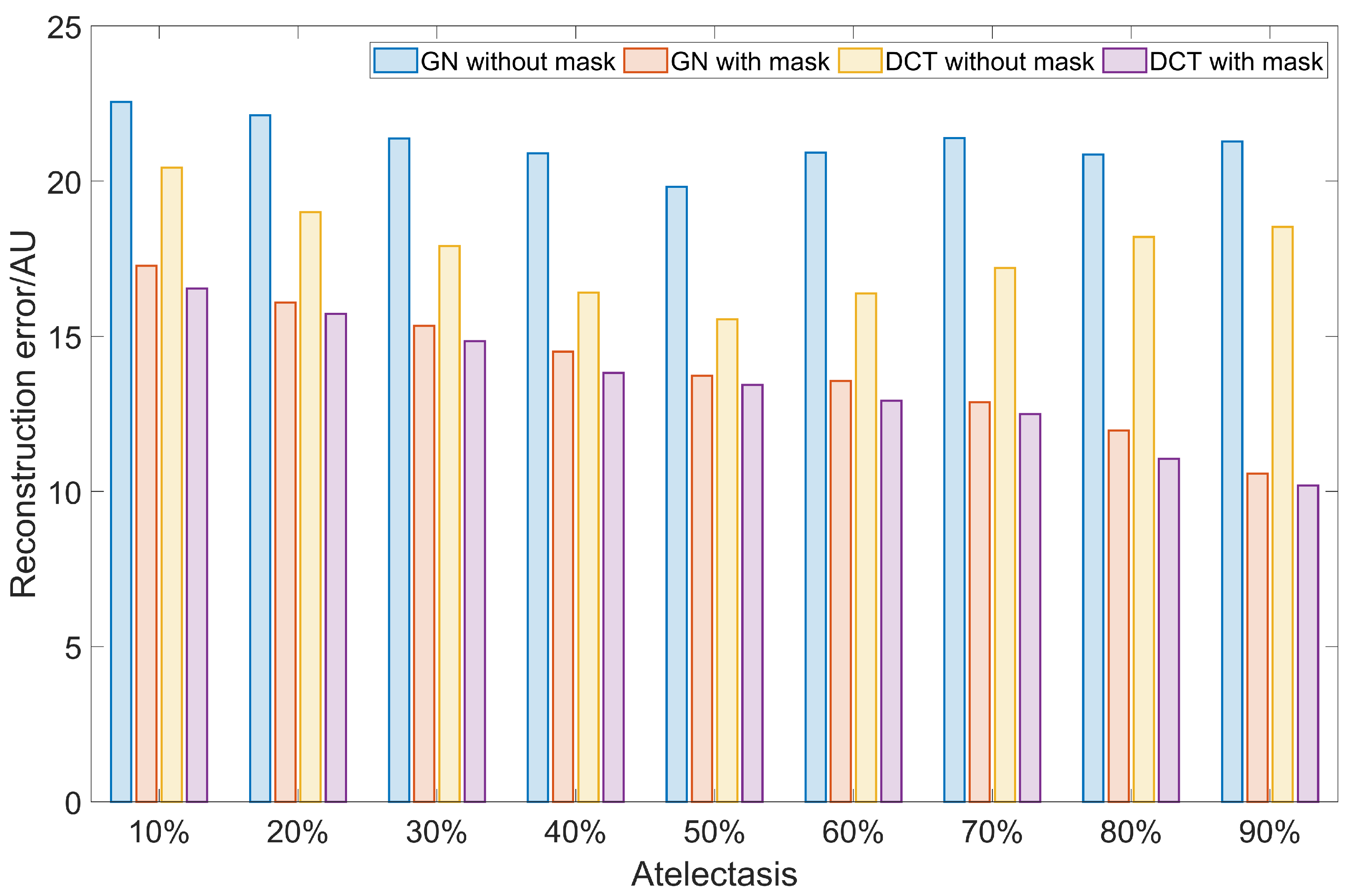
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EIT | Electrical Impedance Tomography |
| DCT | Discrete Cosine Transform |
| ICU | Intensive Care Unit |
| ARDS | Acute Respiratory Distress Syndrome |
| PEEP | Positive End-Expiratory Pressure |
| VILI | Ventilator-Induced Lung Injury |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| FEM | Finite Element Model |
| RE | Reconstruction Error |
Appendix A
References
- Gong, B.; Krueger-Ziolek, S.; Moeller, K.; Schullcke, B.; Zhao, Z. Electrical Impedance Tomography: Functional Lung Imaging on Its Way to Clinical Practice? Expert Rev. Respir. Med. 2015, 9, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I.; Amato, M.B.P.; van Kaam, A.H.; Tingay, D.G.; Zhao, Z.; Grychtol, B.; Bodenstein, M.; Gagnon, H.; Bohm, S.H.; Teschner, E.; et al. Chest Electrical Impedance Tomography Examination, Data Analysis, Terminology, Clinical Use and Recommendations: Consensus Statement of the TRanslational EIT developmeNt stuDy Group. Thorax 2017, 72, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I.; Achtzehn, U.; Pechmann, A.; Pulletz, S.; Schmidt, E.W.; Quintel, M.; Weiler, N. High-Frequency Oscillatory Ventilation in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. J. Crit. Care 2012, 27, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I. Electrical Impedance Tomography (EIT) in Applications Related to Lung and Ventilation: A Review of Experimental and Clinical Activities. Physiol. Meas. 2000, 21, R1. [Google Scholar] [CrossRef]
- Zhao, Z.; Steinmann, D.; Frerichs, I.; Guttmann, J.; Möller, K. PEEP Titration Guided by Ventilation Homogeneity: A Feasibility Study Using Electrical Impedance Tomography. Crit. Care 2010, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fischer, R.; Frerichs, I.; Müller-Lisse, U.; Möller, K. Regional Ventilation in Cystic Fibrosis Measured by Electrical Impedance Tomography. J. Cyst. Fibros. 2012, 11, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Hinz, J.; Neumann, P.; Dudykevych, T.; Andersson, L.G.; Wrigge, H.; Burchardi, H.; Hedenstierna, G. Regional Ventilation by Electrical Impedance Tomography: A Comparison with Ventilation Scintigraphy in Pigs. Chest 2003, 124, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, S.; Lachmann, B. Electrical Impedance Tomography: The Holy Grail of Ventilation and Perfusion Monitoring? Intensive Care Med. 2012, 38, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Gaburro, R.; Lionheart, W. Electrical Impedance Tomography. In Handbook of Mathematical Methods in Imaging; Scherzer, O., Ed.; Springer: New York, NY, USA, 2015; pp. 701–762. [Google Scholar] [CrossRef]
- Gong, B.; Schullcke, B.; Krueger-Ziolek, S.; Vauhkonen, M.; Wolf, G.; Mueller-Lisse, U.; Moeller, K. EIT Imaging Regularization Based on Spectral Graph Wavelets. IEEE Trans. Med. Imaging 2017, 36, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Schullcke, B.; Gong, B.; Krueger-Ziolek, S.; Soleimani, M.; Mueller-Lisse, U.; Moeller, K. Structural-Functional Lung Imaging Using a Combined CT-EIT and a Discrete Cosine Transformation Reconstruction Method. Sci. Rep. 2016, 6, 25951. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Moeller, K. Redistribution Index—Detection of an Outdated Prior Information in the Discrete Cosine Transformation-based EIT Algorithm. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Mexico City, Mexico, 1–5 November 2021; pp. 3693–3696. [Google Scholar] [CrossRef]
- Chen, R.; Rupitsch, S.J.; Moeller, K. Influence of Hyperparameter on the Untrue Prior Detection in Discrete Transformation-based EIT Algorithm. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 580–583. [Google Scholar] [CrossRef]
- Liu, S.; Jia, J.; Zhang, Y.D.; Yang, Y. Image Reconstruction in Electrical Impedance Tomography Based on Structure-Aware Sparse Bayesian Learning. IEEE Trans. Med. Imaging 2018, 37, 2090–2102. [Google Scholar] [CrossRef]
- Liu, S.; Wu, H.; Huang, Y.; Yang, Y.; Jia, J. Accelerated Structure-Aware Sparse Bayesian Learning for Three-Dimensional Electrical Impedance Tomography. IEEE Trans. Ind. Inform. 2019, 15, 5033–5041. [Google Scholar] [CrossRef]
- Hamilton, S.J.; Hauptmann, A. Deep D-Bar: Real-Time Electrical Impedance Tomography Imaging With Deep Neural Networks. IEEE Trans. Med. Imaging 2018, 37, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Glidewell, M.; Ng, K.T. Anatomically Constrained Electrical Impedance Tomography for Anisotropic Bodies via a Two-Step Approach. IEEE Trans. Med. Imaging 1995, 14, 498–503. [Google Scholar] [CrossRef]
- Vauhkonen, M.; Kaipio, J.; Somersalo, E.; Karjalainen, P. Electrical Impedance Tomography with Basis Constraints. Inverse Probl. 1997, 13, 523. [Google Scholar] [CrossRef]
- Adler, A.; Arnold, J.H.; Bayford, R.; Borsic, A.; Brown, B.; Dixon, P.; Faes, T.J.; Frerichs, I.; Gagnon, H.; Garber, Y.; et al. GREIT: A Unified Approach to 2D Linear EIT Reconstruction of Lung Images. Physiol. Meas. 2009, 30, S35–S55. [Google Scholar] [CrossRef]
- Kolehmainen, V.; Ehrhardt, M.J.; Arridge, S.R. Incorporating Structural Prior Information and Sparsity into EIT Using Parallel Level Sets. Inverse Probl. Imaging 2019, 13, 285–307. [Google Scholar] [CrossRef]
- Nakanishi, R.M.; Santos, T.B.R.; Amato, M.B.P.; Lima, R.G. A Measure of Prior Information of a Pathology in an EIT Anatomical Atlas. In Proceedings of the 17th International Conference on Electrical Bioimpedance, Joinville, Brazil, 9–14 June 2020; Bertemes-Filho, P., Ed.; Springer: Singapore, 2020; pp. 173–180. [Google Scholar]
- Zhang, K.; Guo, R.; Li, M.; Yang, F.; Xu, S.; Abubakar, A. Supervised Descent Learning for Thoracic Electrical Impedance Tomography. IEEE Trans. Biomed. Eng. 2020, 68, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Lionheart, W.R.B. Uses and Abuses of EIDORS: An Extensible Software Base for EIT. Physiol. Meas. 2006, 27, S25–S42. [Google Scholar] [CrossRef] [PubMed]
- Schöberl, J. NETGEN An Advancing Front 2D/3D-mesh Generator Based on Abstract Rules. Comput. Vis. Sci. 1997, 1, 41–52. [Google Scholar] [CrossRef]
- Lionheart, W.R.B. EIT Reconstruction Algorithms: Pitfalls, Challenges and Recent Developments. Physiol. Meas. 2004, 25, 125. [Google Scholar] [CrossRef] [PubMed]
- Antink, C.H.; Pikkemaat, R.; Malmivuo, J.; Leonhardt, S. A Shape-Based Quality Evaluation and Reconstruction Method for Electrical Impedance Tomography. Physiol. Meas. 2015, 36, 1161. [Google Scholar] [CrossRef] [PubMed]
- Harrach, B.; Ullrich, M. Resolution Guarantees in Electrical Impedance Tomography. IEEE Trans. Med. Imaging 2015, 34, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Deibele, J.M.; Luepschen, H.; Leonhardt, S. Dynamic Separation of Pulmonary and Cardiac Changes in Electrical Impedance Tomography. Physiol. Meas. 2008, 29, S1. [Google Scholar] [CrossRef]
- Battistel, A.; Chen, R.; Hallemans, N.; Pintelon, R.; Lataire, J.; Möller, K. Harmonic Analysis for the Separation of Perfusion and Respiration in Electrical Impedance Tomography. IFAC-PapersOnLine 2021, 54, 281–286. [Google Scholar] [CrossRef]
- Kang, S.I.; Khambampati, A.K.; Jeon, M.H.; Kim, B.S.; Kim, K.Y. A Sub-Domain Based Regularization Method with Prior Information for Human Thorax Imaging Using Electrical Impedance Tomography. Meas. Sci. Technol. 2016, 27, 025703. [Google Scholar] [CrossRef]
- Murphy, E.K.; Mahara, A.; Wu, X.; Halter, R.J. Phantom Experiments Using Soft-Prior Regularization EIT for Breast Cancer Imaging. Physiol. Meas. 2017, 38, 1262. [Google Scholar] [CrossRef]

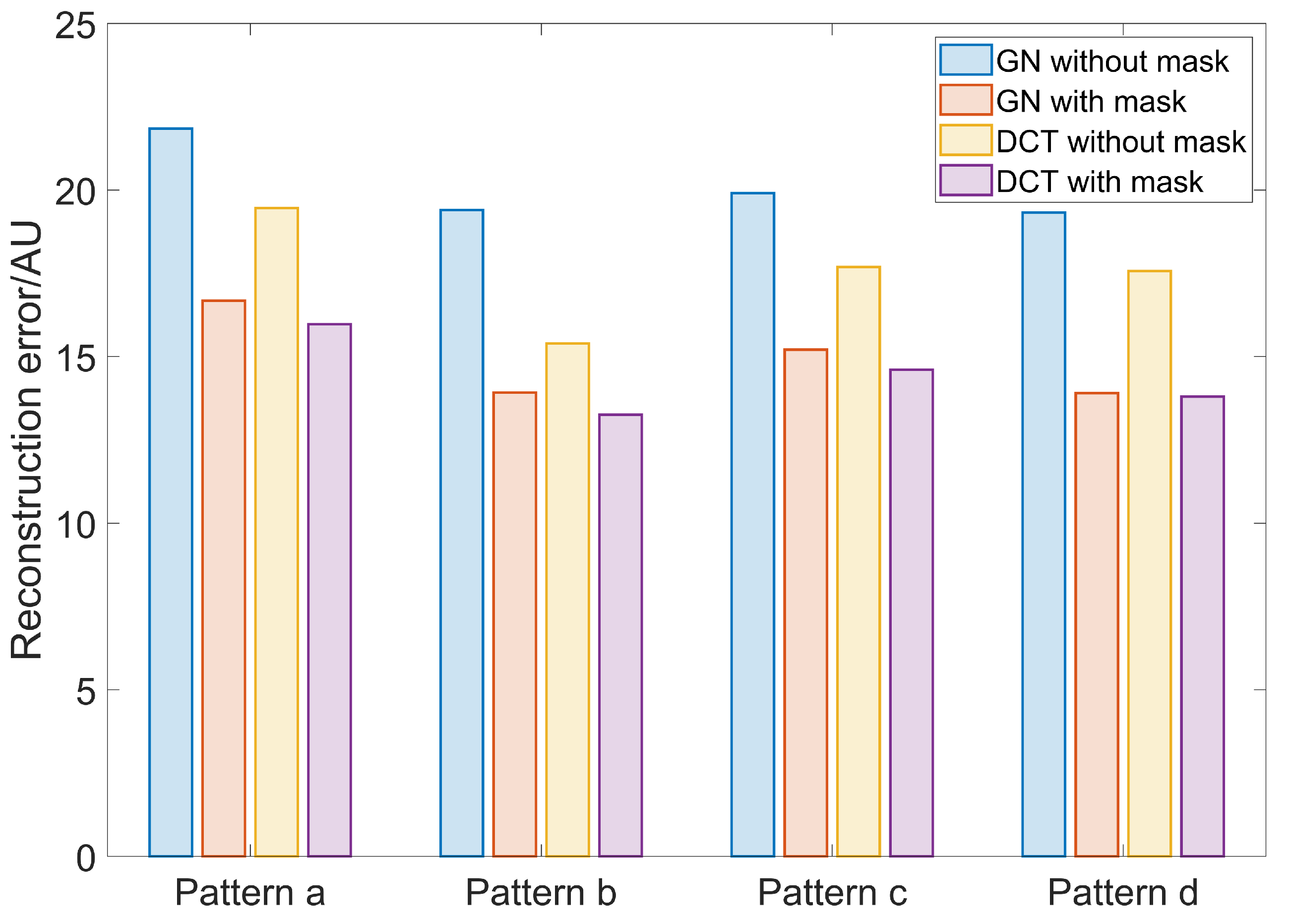
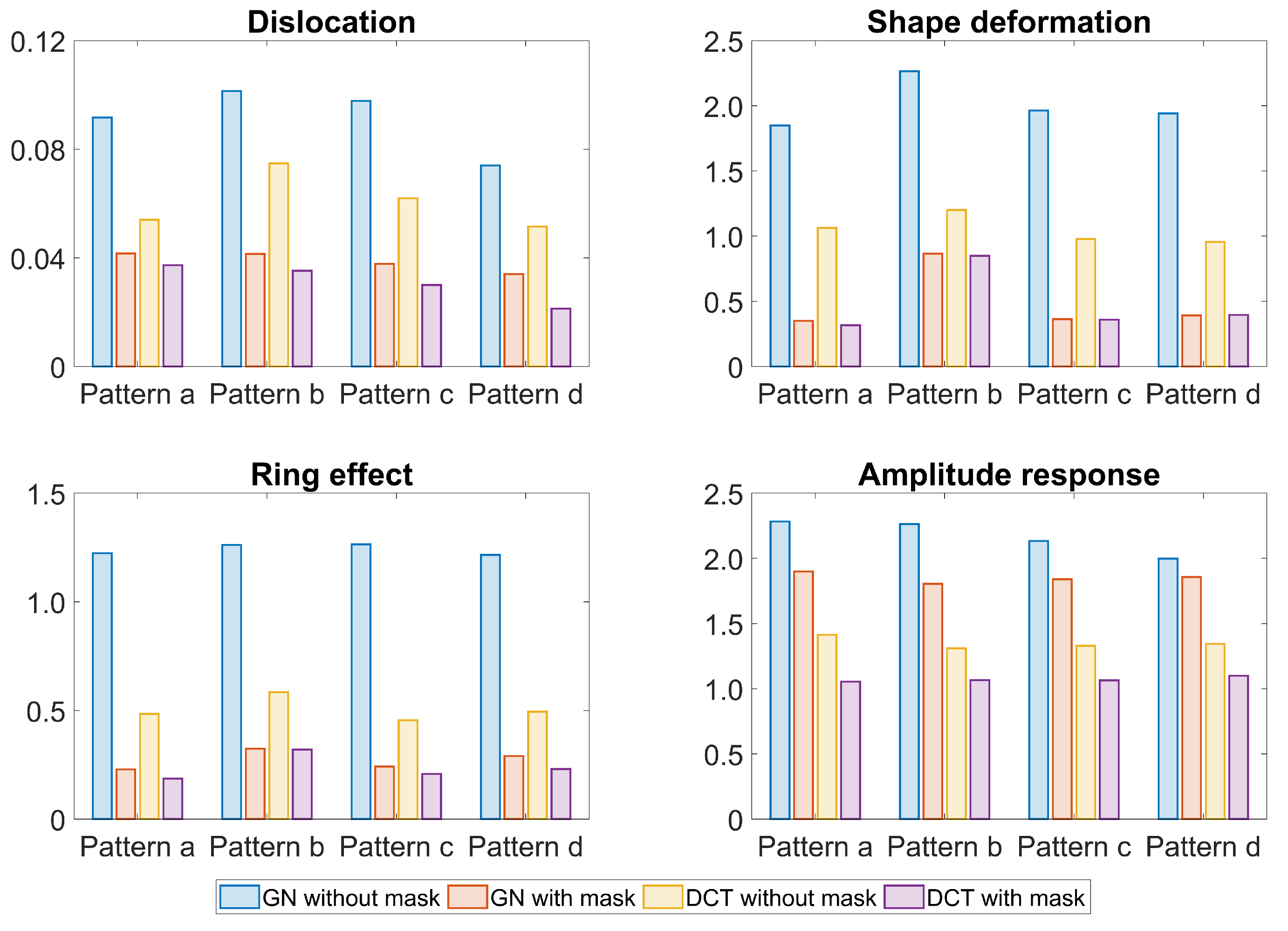
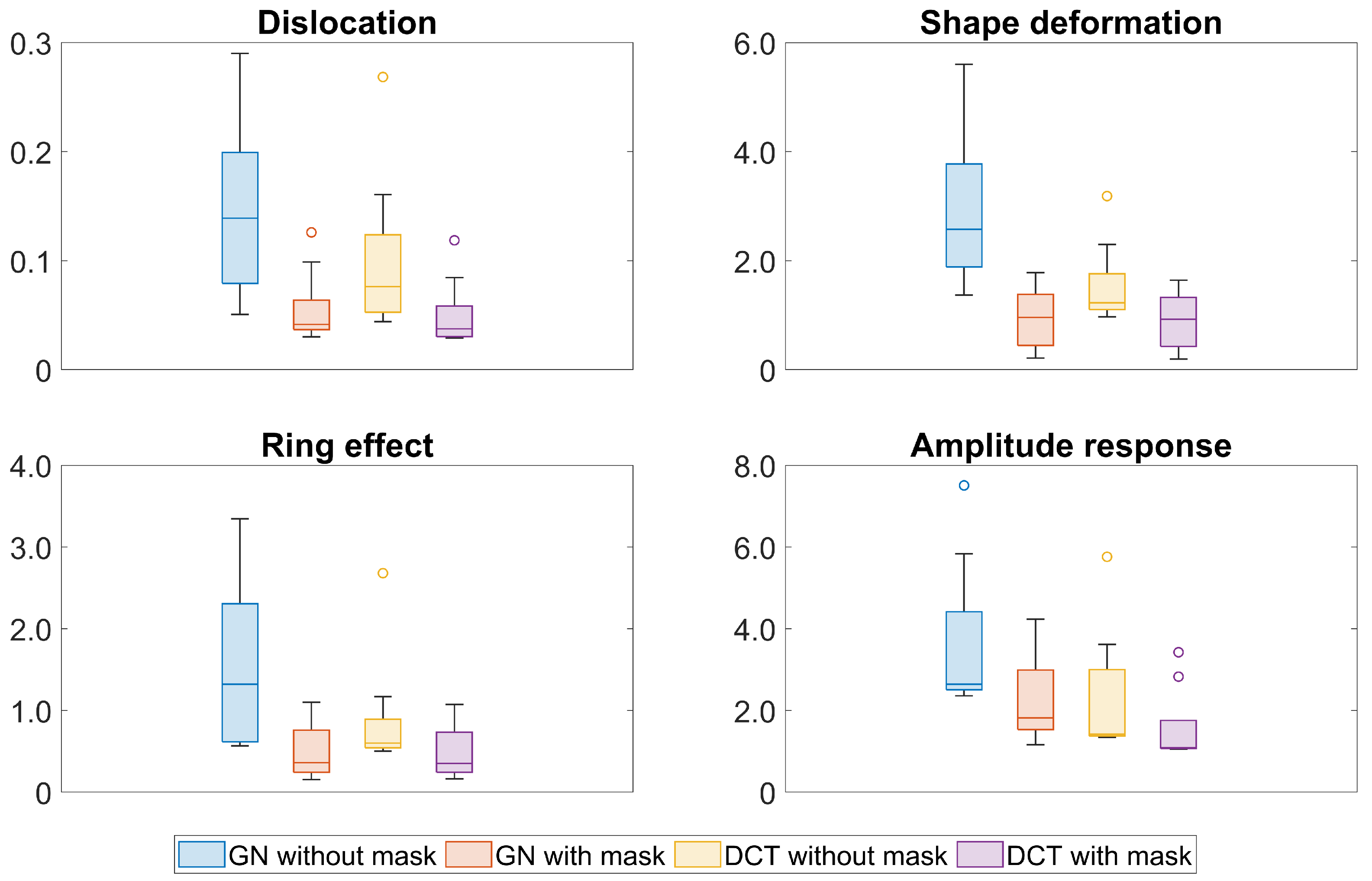
| Algorithm | GN | DCT | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Figures of Merit | D | SD | R | A | D | SD | R | A | |||||||||
| Mask | W/o | W | W/o | W | W/o | W | W/o | W | W/o | W | W/o | W | W/o | W | W/o | W | |
| Scale | 10% | 0.051 | 0.030 | 1.368 | 0.214 | 0.566 | 0.153 | 2.548 | 1.159 | 0.044 | 0.029 | 0.969 | 0.194 | 0.503 | 0.162 | 1.418 | 1.084 |
| 20% | 0.061 | 0.035 | 1.692 | 0.341 | 0.586 | 0.219 | 2.564 | 1.547 | 0.049 | 0.030 | 1.019 | 0.314 | 0.519 | 0.214 | 1.380 | 1.070 | |
| 30% | 0.085 | 0.037 | 1.948 | 0.479 | 0.627 | 0.251 | 2.371 | 1.475 | 0.054 | 0.030 | 1.130 | 0.466 | 0.551 | 0.252 | 1.370 | 1.054 | |
| 40% | 0.113 | 0.040 | 2.134 | 0.712 | 0.729 | 0.286 | 2.640 | 1.761 | 0.065 | 0.033 | 1.227 | 0.669 | 0.586 | 0.286 | 1.374 | 1.064 | |
| 50% | 0.139 | 0.041 | 2.573 | 0.960 | 1.320 | 0.361 | 2.359 | 1.818 | 0.076 | 0.037 | 1.226 | 0.922 | 0.601 | 0.352 | 1.343 | 1.069 | |
| 60% | 0.152 | 0.044 | 2.804 | 1.075 | 1.894 | 0.553 | 2.722 | 2.303 | 0.090 | 0.040 | 1.274 | 1.044 | 0.653 | 0.501 | 1.641 | 1.173 | |
| 70% | 0.191 | 0.052 | 3.446 | 1.312 | 2.143 | 0.712 | 3.943 | 2.837 | 0.112 | 0.050 | 1.583 | 1.282 | 0.802 | 0.681 | 2.788 | 1.400 | |
| 80% | 0.224 | 0.099 | 4.751 | 1.587 | 2.791 | 0.900 | 5.831 | 3.440 | 0.160 | 0.085 | 2.296 | 1.454 | 1.170 | 0.897 | 3.614 | 2.826 | |
| 90% | 0.290 | 0.126 | 5.600 | 1.779 | 3.343 | 1.099 | 7.504 | 4.236 | 0.268 | 0.119 | 3.184 | 1.639 | 2.679 | 1.071 | 5.758 | 3.423 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Krueger-Ziolek, S.; Battistel, A.; Rupitsch, S.J.; Moeller, K. Effect of a Patient-Specific Structural Prior Mask on Electrical Impedance Tomography Image Reconstructions. Sensors 2023, 23, 4551. https://doi.org/10.3390/s23094551
Chen R, Krueger-Ziolek S, Battistel A, Rupitsch SJ, Moeller K. Effect of a Patient-Specific Structural Prior Mask on Electrical Impedance Tomography Image Reconstructions. Sensors. 2023; 23(9):4551. https://doi.org/10.3390/s23094551
Chicago/Turabian StyleChen, Rongqing, Sabine Krueger-Ziolek, Alberto Battistel, Stefan J. Rupitsch, and Knut Moeller. 2023. "Effect of a Patient-Specific Structural Prior Mask on Electrical Impedance Tomography Image Reconstructions" Sensors 23, no. 9: 4551. https://doi.org/10.3390/s23094551
APA StyleChen, R., Krueger-Ziolek, S., Battistel, A., Rupitsch, S. J., & Moeller, K. (2023). Effect of a Patient-Specific Structural Prior Mask on Electrical Impedance Tomography Image Reconstructions. Sensors, 23(9), 4551. https://doi.org/10.3390/s23094551








