Volatile-Based Diagnosis for Pathogenic Wood-Rot Fungus Fulvifomes siamensis by Electronic Nose (E-Nose) and Solid-Phase Microextraction/Gas Chromatography/Mass Spectrometry
Abstract
1. Introduction
1.1. Wood Rot Disease in Local Urban Trees
1.2. VOC Detection with E-Nose and SPME GC-MS
1.2.1. SPME GC-MS
1.2.2. E-Nose System
1.2.3. Sampling Algorithm: Canonical Discriminant Analysis (CDA)
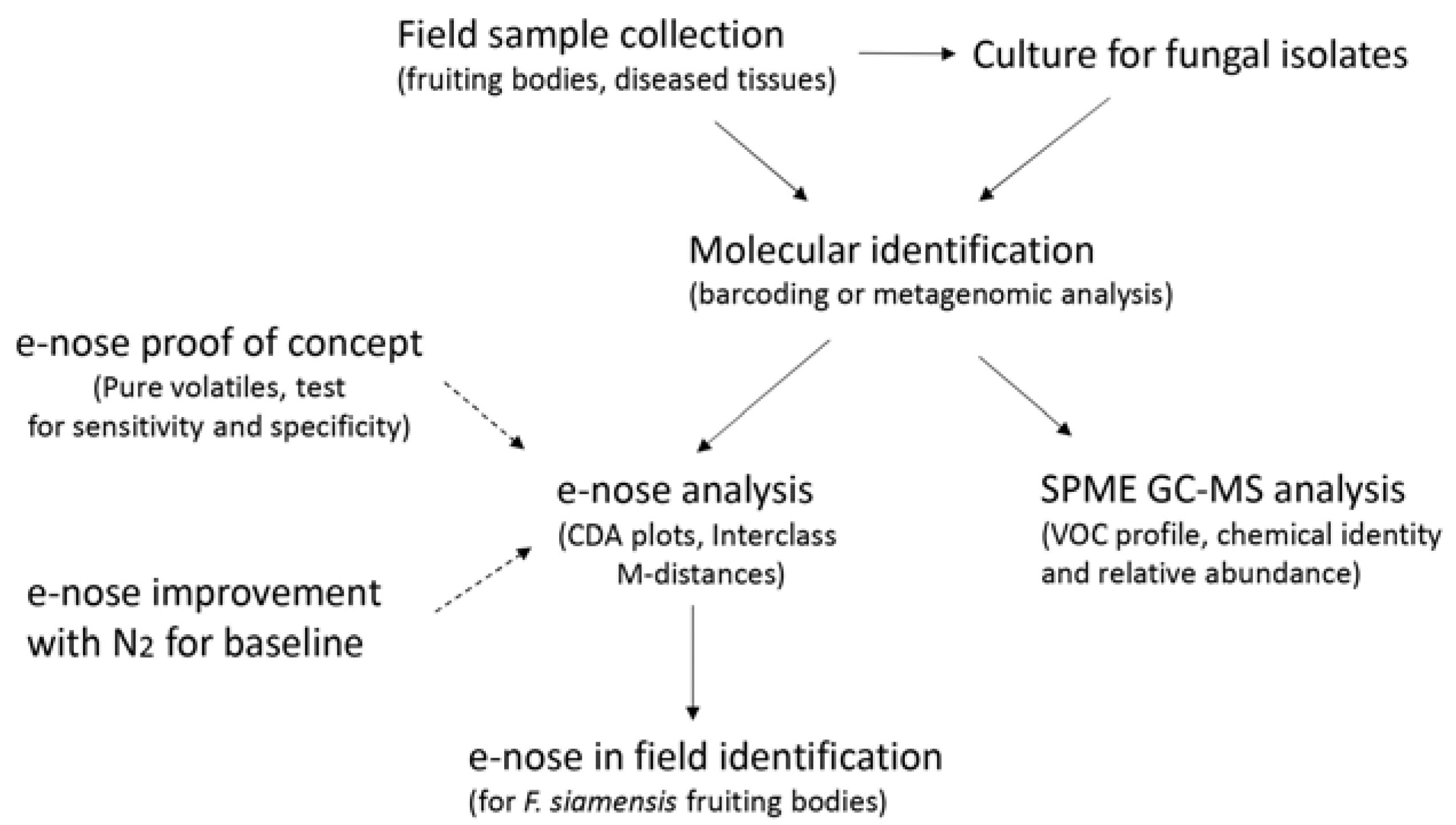
2. Objectives
3. Methodology
3.1. Field Sample Collection, DNA Isolation, Metagenomic Survey, and Sample Barcoding
3.2. Culture for Pure Fungal Isolates
3.3. Cyranose 320 E-Nose Method Settings
3.4. E-Nose Tests
3.4.1. Wood Rot Fungi and Plant-Related Volatiles: Turpentine, Farnesene, and Acetic Acid
3.4.2. Fungal Mycelia Volatiles
3.4.3. E-Nose Field Identification of F. siamensis Fruiting Bodies
3.5. SPME GC-MS Procedures
4. Results
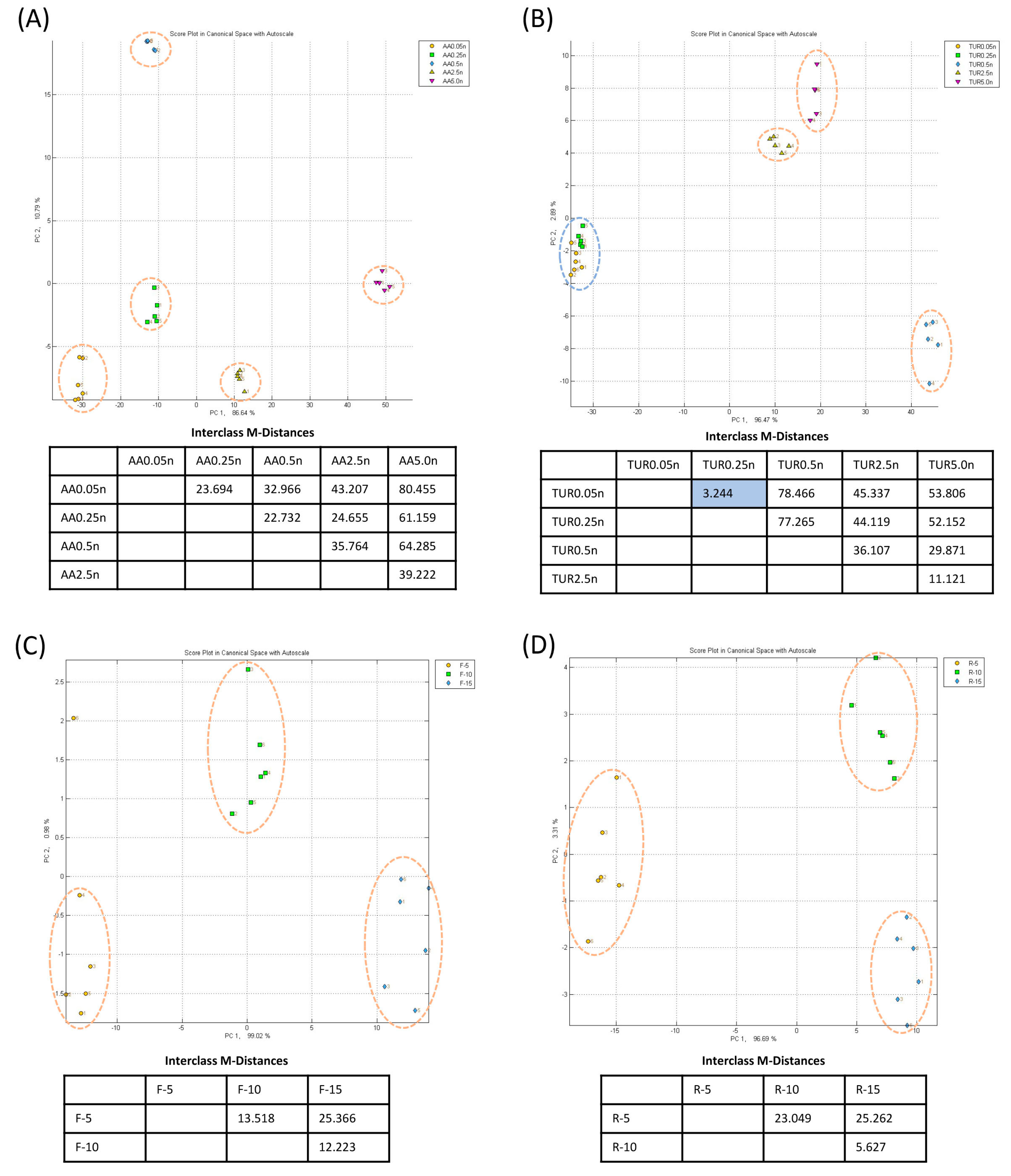
4.1. Sensitivity and Specificity of E-Nose with Nitrogen Gas for Baseline
4.1.1. Multiple VOC Differentiation by E-Nose
4.1.2. F. siamensis Culture, Fruiting Body, and Diseased Tissue Differentiation by E-Nose
4.2. Volatile Profiles of Samples Identified by SPME GC-MS
4.3. Field Identification of F. siamensis Fruiting Bodies with the E-Nose
4.4. Sensitivity of E-Nose to Changes in VOC Concentration
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. The garden city in Singapore. In The Culture of Nature in the History of Design; Fallan, K., Ed.; Routledge: London, UK, 2019; pp. 89–102. [Google Scholar]
- Aldous, D.E. Planning Green Open Spaces for South East Asian Capital Cities. Park.—Enhancing Liveability Cities 2011, 3, 10–15. [Google Scholar] [CrossRef]
- Tan, A. Not a Concrete Jungle: Singapore Beats 16 Cities in Green Urban Areas. The Straits Times, 24 February 2017. [Google Scholar]
- Downer, A.J.; Perry, E.J. Wood Decay Fungi in Landscape Trees. Available online: http://ipm.ucanr.edu/PMG/PESTNOTES/pn74109.html (accessed on 31 March 2023).
- Gauthier, N.; Fountain, W.E.; Missun, T. Tree Wounds—Invitations to Wood Decay Fungi; University of Kentucky: Lexington, KY, USA, 2015. [Google Scholar]
- Shortle, W.C.; Dudzik, K.R. Wood Decay in Living and Dead Trees: A Pictorial Overview; U.S. Department of Agriculture, Forest Service, Northern Research Station: Washington, DC, USA, 2012.
- Ann, P.-J.; Chang, T.-T.; Ko, W.-H. Phellinus noxius Brown Root Rot of Fruit and Ornamental Trees in Taiwan. Plant Dis. 2002, 86, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Sahashi, N.; Akiba, M.; Ishihara, M.; Abe, Y.; Morita, S. First report of the brown root rot disease caused by Phellinus noxius, its distribution and newly recorded host plants in the Amami Islands, southern Japan. For. Pathol. 2007, 37, 167–173. [Google Scholar] [CrossRef]
- Oghenekaro, A.O.; Miettinen, O.; Omorusi, V.I.; Evueh, G.A.; Farid, M.A.; Gazis, R.; Asiegbu, F.O. Molecular phylogeny of Rigidoporus microporus isolates associated with white rot disease of rubber trees (Hevea brasiliensis). Fungal Biol. 2014, 118, 495–506. [Google Scholar] [CrossRef]
- Chang, T.-T. Survival of Phellinus noxius in soil and in the roots of dead host plants. Phytopathology 1996, 86, 272–276. [Google Scholar] [CrossRef]
- Ding, S.; Hu, H.; Gu, J.-D. Diversity, Abundance, and Distribution of Wood-Decay Fungi in Major Parks of Hong Kong. Forests 2020, 11, 1030. [Google Scholar] [CrossRef]
- Sakayaroj, J.; Preedanon, S.; Suetrong, S.; Klaysuban, A.; Jones, E.B.G.; Hattori, T. Molecular characterization of basidiomycetes associated with the decayed mangrove tree Xylocarpus granatum in Thailand. Fungal Divers. 2012, 56, 145–156. [Google Scholar] [CrossRef]
- Hong, Y.; Tan, J.Y.; Xue, H.; Chow, M.L.; Ali, M.; Ng, A.; Leong, A.; Yeo, J.; Koh, S.M.; Tang, S.Y.M.; et al. A metagenomic survey of wood decay fungi in the urban trees of Singapore. J. Fungi 2023, 9, 460. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2014, 35, 1–25. [Google Scholar] [CrossRef]
- Guglielmo, F.; Bergemann, S.E.; Gonthier, P.; Nicolotti, G.; Garbelotto, M. A multiplex PCR-based method for the detection and early identification of wood rotting fungi in standing trees. J. Appl. Microbiol. 2007, 103, 1490–1507. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Wilson, A.D.; Lester, D.G.; Oberle, C.S. Development of Conductive Polymer Analysis for the Rapid Detection and Identification of Phytopathogenic Microbes. Phytopathology 2004, 94, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Kigathi, R.N.; Weisser, W.W.; Reichelt, M.; Gershenzon, J.; Unsicker, S.B. Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol. 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The network of plants volatile organic compounds. Sci. Rep. 2017, 7, 11050. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jud, W.; Weikl, F.; Ghirardo, A.; Junker, R.; Polle, A.; Benz, J.P.; Pritsch, K.; Schnitzler, J.-P.; Rosenkranz, M. Volatile organic compound patterns predict fungal trophic mode and lifestyle. Commun. Biol. 2021, 4, 673. [Google Scholar] [CrossRef]
- Cellini, A.; Blasioli, S.; Biondi, E.; Bertaccini, A.; Braschi, I.; Spinelli, F. Potential Applications and Limitations of Electronic Nose Devices for Plant Disease Diagnosis. Sensors 2017, 17, 2596. [Google Scholar] [CrossRef]
- Gherghel, S.; Morgan, R.; Arrebola, F.; Romero-González, R.; Blackman, C.; Frenich, A.; Parkin, I. Development of a HS-SPME/GC-MS method for the analysis of volatile organic compounds from fabrics for forensic reconstruction applications. Forensic Sci. Int. 2018, 290, 207–218. [Google Scholar] [CrossRef]
- Ouyang, G.; Pawliszyn, J. A critical review in calibration methods for solid-phase microextraction. Anal. Chim. Acta 2008, 627, 184–197. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Wang, X.; Zheng, H.; Chen, Z.; Meng, C. Development of Electronic Nose for Qualitative and Quantitative Monitoring of Volatile Flammable Liquids. Sensors 2020, 20, 1817. [Google Scholar] [CrossRef]
- De Lacy Costello, B.P.J.; Evans, P.; Ewen, R.J.; Gunson, H.E.; Jones, P.R.H.; Ratcliffe, N.M.; Spencer-Phillips, P.T.N. Gas chromatography–mass spectrometry analyses of volatile organic compounds from potato tubers inoculated with Phytophthora infestans or Fusarium coeruleum. Plant Pathol. 2001, 50, 489–496. [Google Scholar] [CrossRef]
- Prithiviraj, B.; Vikram, A.; Kushalappa, A.C.; Yaylayan, V. Volatile Metabolite Profiling for the Discrimination of Onion Bulbs Infected by Erwinia carotovora ssp. carotovora, Fusariumoxysporum and Botrytis allii. Eur. J. Plant Pathol. 2004, 110, 371–377. [Google Scholar] [CrossRef]
- Murray, J.M.; Delahunty, C.M.; Baxter, I.A. Descriptive sensory analysis: Past, present and future. Food Res. Int. 2001, 34, 461–471. [Google Scholar] [CrossRef]
- Falasconi, M.; Gobbi, E.; Pardo, M.; Della Torre, M.; Bresciani, A.; Sberveglieri, G. Detection of toxigenic strains of Fusarium verticillioides in corn by electronic olfactory system. Sens. Actuators B Chem. 2005, 108, 250–257. [Google Scholar] [CrossRef]
- Baietto, M.; Pozzi, L.; Wilson, A.D.; Bassi, D. Evaluation of a portable MOS electronic nose to detect root rots in shade tree species. Comput. Electron. Agric. 2013, 96, 117–125. [Google Scholar] [CrossRef]
- Markom, M.A.; Shakaff, A.Y.M.; Adom, A.H.; Ahmad, M.N.; Hidayat, W.; Abdullah, A.H.; Fikri, N.A. Intelligent electronic nose system for basal stem rot disease detection. Comput. Electron. Agric. 2009, 66, 140–146. [Google Scholar] [CrossRef]
- Baietto, M.; Wilson, A.D.; Bassi, D.; Ferrini, F. Evaluation of Three Electronic Noses for Detecting Incipient Wood Decay. Sensors 2010, 10, 1062–1092. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Hines, E.; Gardner, J.W.; Boilot, P. Bacteria classification using Cyranose 320 electronic nose. Biomed. Eng. Online 2002, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Ordukaya, E.; Karlik, B. Quality Control of Olive Oils Using Machine Learning and Electronic Nose. J. Food Qual. 2017, 2017, 9272404. [Google Scholar] [CrossRef]
- Sensigent LLC. The Cyranose® 320 eNose® User Manual; Sensigent: Baldwin Park, CA, USA, 2013; Edition 6, Revision I. [Google Scholar]
- What Is Nitrogen Purging?—Procedure and Equipment Used. Available online: https://nigen.com/what-is-nitrogen-purging-procedure-services/#:~:text=Nitrogen%20purging%20is%20an%20industrial,system%20environment%20using%20nitrogen%20gas (accessed on 15 March 2023).
- Zhao, G.; Maclean, A.L. A comparison of canonical discriminant analysis and principal component analysis for spectral transformation. Photogramm. Eng. Remote Sens. 2000, 66, 841–847. [Google Scholar]
- Farhana, A.; Bahri, A.; Thanh, T.; Zakaria, L.; Bahri, S.; Abd Razak, S.B. Morphological features of Rigidoporus microporus isolated from infected Malaysian rubber clones. Malays. J. Microsc. 2017, 13, 17–23. [Google Scholar]
- Sensigent LLC. The Practical Guide to the Cyranose®320; Sensigent: Baldwin Park, CA, USA, 2013; Revision H. [Google Scholar]
- Nord, F.F.; Vitucci, J.C. Enzyme Studies on the Mechanism of Wood Decay. Nature 1947, 160, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Munsch-Alatossava, P.; Ghafar, A.; Alatossava, T. Potential of nitrogen gas (n2) flushing to extend the shelf life of cold stored pasteurised milk. Int. J. Mol. Sci. 2013, 14, 5668–5685. [Google Scholar] [CrossRef] [PubMed]
- Hamacher, H. The effectiveness of gas purging to reduce contamination under thermal vacuum conditions. Vacuum 1985, 35, 189–194. [Google Scholar] [CrossRef]
- Pagano, T.; Biacchi, A.J.; Kenny, J.E. Nitrogen Gas Purging for the Deoxygenation of Polyaromatic Hydrocarbon Solutions in Cyclohexane for Routine Fluorescence Analysis. Appl. Spectrosc. 2008, 62, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cale, J.A.; Hussain, A.; Erbilgin, N. Exposure to Fungal Volatiles Can Influence Volatile Emissions from Other Ophiostomatoid Fungi. Front. Microbiol. 2020, 11, 567462. [Google Scholar] [CrossRef]
- Kües, U.; Navarro-González, M. How do Agaricomycetes shape their fruiting bodies? 1. Morphological aspects of development. Fungal Biol. Rev. 2015, 29, 63–97. [Google Scholar] [CrossRef]
- Kielak, A.M.; Scheublin, T.R.; Mendes, L.W.; van Veen, J.A.; Kuramae, E.E. Bacterial Community Succession in Pine-Wood Decomposition. Front. Microbiol. 2016, 7, 231. [Google Scholar] [CrossRef]




| Date of Collection | Type of Sample | Host Tree | Sample Marking | Location and Remarks | Molecular Identity/GenBank Accession No. |
|---|---|---|---|---|---|
| 11 October 2021 | fruiting body | Syzygium grande | SG145FB | 1°18′38.4″ N 103°49′58.6″ E Tree has multiple fruiting bodies at ground level, the largest fruiting body was collected | F. siamensis/OQ558845 |
| 16 December 2021 | fruiting body | Casuarina equisetifolia | CE149FB | 1°17′20.3″ N 103°46′12.6″ E | F. siamensis/OQ558844 |
| 23 December 2021 | diseased tissue | Casuarina equisetifolia | CE149DT2 | 1°17′20.3″ N 103°46′12.6″ E Decayed wood from cut open stump | ^ 79.4% F. siamensis/OQ572588^ 3.8% G. australe/OQ572592 |
| 23 December 2021 | diseased tissue | Casuarina equisetifolia | CE149DT5 | 1°17′20.3″ N 103°46′12.6″ E Decayed wood behind a F. siamensis fruiting body | ^ 90.5% F. siamensis/OQ572588 |
| 16 December 2021 | healthy tissue | Casuarina equisetifolia | CE151HT | 1°17′20.3″ N 103°46′12.6″ E Wood obtained from healthy-looking area of the root | - |
| 16 December 2021 | fruiting body | Casuarina equisetifolia | CE151FB | 1°17′20.3″ N 103°46′12.6″ E | F. siamensis/OQ558844 |
| 16 December 2021 | diseased tissue | Casuarina equisetifolia | CE152DT | 1°17′20.3″ N 103°46′12.6″ E Decayed wood behind a F. siamensis fruiting body | ^ 96.0% F. siamensis/OQ572588^ 0.4% G. australe/OQ572592, OQ572594 |
| 16 December 2021 | fruiting body | Casuarina equisetifolia | CE153FB | 1°17′20.3″ N 103°46′12.6″ E | G. australe/OQ572592, OQ572594 |
| 25 February 2022 | fruiting body | Peltophorum pterocarpum | YF156FB1 | 1°19′14.8″ N 103°49′07.8″ E | F. siamensis/OQ558847 |
| 25 February 2022 | fruiting body | Peltophorum pterocarpum | YF157FB | 1°19′14.8″ N 103°49′07.8″ E | F. siamensis/OQ558848 |
| 25 February 2022 | fruiting body | Samanea saman | R159FB | 1°16′58.5″ N 103°49′53.9″ E | F. siamensis/OQ558848 |
| 25 February 2022 | fruiting body | Peltophorum pterocarpum | YF160FB | 1°16′56.7″ N 103°49′52.4″ E | F. siamensis/OQ558848 |
| 17 May 2022 | fruiting body | Sabal palmetto | P175FB1 | 1°19′02.9″ N 103°46′09.2″ E | R. microporus/OQ558868 |
| 16 September 2022 | fruiting body | Sabal palmetto | P175FB3 | 1°19′02.9″ N 103°46′09.2″ E | R. microporus/OQ558868 |
| 16 September 2022 | diseased tissue | Tabebuia rosea | TR190DT2 | 1°19′18.4″ N 103°55′31.8″ E Decayed wood behind F. siamensis fruiting body | ^ 95.8% F. siamensis/OQ572588^ 3.60% Fomitiporia bannaensis/OQ572582 |
| Time (s) | Pump Speed | |
|---|---|---|
| Baseline purge | 10 | Medium (120 mL/min) |
| Sample draw | 10 | Medium (120 mL/min) |
| Air intake purge | 5 | High (180 mL/min) |
| Sample gas purge | 30 | High (180 mL/min) |
| Samples | CAS # | FS Culture | RM Culture | SG 145FB (FS) | YF157FB (FS) | R159FB (FS) | CE149FB (FS) | CE151FB (FS) | CE153 FB (GA) | P175 FB3 (RM) | CE149DT2 (79% FS, 3% GA) ^ | CE149DT5 (90% FS) ^ | TR190DT2 (95% FS) ^ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volatiles | ||||||||||||||
| 2,4-bis(chloranyl)-1,5-dimethoxy-3-methyl-benzene | 997271-90-6 | ** | *** | |||||||||||
| 3,5-bis(chloranyl)-2,4-dimethoxy-6-methyl-phenol | 997329-33-1 | ** | * | ** | *** | ** | ** | |||||||
| Acetic acid, 2-phenylethyl ester | 000103-45-7 | ** | ||||||||||||
| Benzene, 1,2,4,5-tetrachloro-3,6-dimethoxy- | 000944-78-5 | ***** | ***** | ***** | ***** | ***** | ***** | **** | ** | ***** | ||||
| Benzene, 1,2,4-trichloro-5-nitro- | 000089-69-0 | ***** | ||||||||||||
| Benzene, 1,4-dichloro-2,5-dimethoxy- | 002675-77-6 | ** | *** | * | **** | ***** | ** | |||||||
| beta-Bisabolene | 000495-61-4 | * | * | ** | ||||||||||
| (E)-.beta.-Farnesene | 018794-84-8 | ***** | * | * | *** | |||||||||
| 2,7-dichloro-1-methoxydibenzofuran | 067061-60-3 | ** | ||||||||||||
| Methyl 2,6-Dichloro-4-methoxybenzoate | 094278-65-6 | ** | ||||||||||||
| Benzoic acid, methyl ester | 000093-58-3 | *** | ||||||||||||
| Cetrimonium Bromide | 000057-09-0 | ***** | ||||||||||||
| Disulfide, dimethyl | 000624-92-0 | **** | ||||||||||||
| Hexadecane, 1-chloro | 004860-03-1 | ** | ||||||||||||
| Phenylethyl Alcohol | 000060-12-8 | ** | ||||||||||||
| Tributylamine | 000102-82-9 | *** | ||||||||||||
| Butyrolactone | 000096-48-0 | ** | ||||||||||||
| (3aS,8aS)-6,8a-Dimethyl-3-(propan-2-ylidene)-1,2,3,3a,4,5,8,8a-octahydroazulene | 395070-76-5 | * | * | * | ** | |||||||||
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 006846-50-0 | ** | ||||||||||||
| 3-(4-Nitro-phenylsulfanyl)-propionic acid | 997299-58-6 | **** | ||||||||||||
| 3-Methyl -6-(3-methylthiophen-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazole | 997329-32-4 | ** | ||||||||||||
| 3-Octanone | 000106-68-3 | * | * | ** | * | * | ||||||||
| Acetic acid | 000064-19-7 | *** | ** | |||||||||||
| Benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl | 000644-30-4 | * | * | * | ||||||||||
| Benzene, 1,3-dimethyl- | 000108-38-3 | * | ** | * | ||||||||||
| Decanal | 000112-31-2 | |||||||||||||
| Diethyl Phthalate | 000084-66-2 | * | **** | |||||||||||
| Docosane | 000629-97-0 | ** | ||||||||||||
| Nonanal | 000124-19-6 | * | * | * | ||||||||||
| Phenol, 2,3,5,6-tetrachloro-4-methoxy | 000484-67-3 | * | ** | * | * | |||||||||
| Toluene | 000108-88-3 | * | * | * | * | * | ** | * | ||||||
| Sample ID | Collection Date | Host Tree | Fruiting Body Weight (g) | E-Nose Diagnosis Result (Confidence) | Molecular Identity by Barcoding |
|---|---|---|---|---|---|
| R1-A | 2 March 2023 | Samanea saman | 104.50 | YF156FB1 (*****) | F. siamensis |
| R1-B | 2 March 2023 | Samanea saman | 5.33 | YF156FB1 (*****) | F. siamensis |
| YF1-A | 2 March 2023 | Peltophorum pterocarpum | 336.14 | YF156FB1 (*****) | F. siamensis |
| YF1-B | 2 March 2023 | Peltophorum pterocarpum | 12.14 | YF156FB1 (*) | F. siamensis |
| YF2-A | 2 March 2023 | Peltophorum pterocarpum | 191.17 | YF156FB1 (*****) | F. siamensis |
| YF2-B | 2 March 2023 | Peltophorum pterocarpum | 15.44 | YF156FB1 (***) | F. siamensis |
| YF2 Tree Stump | 2 March 2023 | Peltophorum pterocarpum | Negative Control | Unknown | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.Y.; Zhang, Z.; Izzah, H.J.; Fong, Y.K.; Lee, D.; Mutwil, M.; Hong, Y. Volatile-Based Diagnosis for Pathogenic Wood-Rot Fungus Fulvifomes siamensis by Electronic Nose (E-Nose) and Solid-Phase Microextraction/Gas Chromatography/Mass Spectrometry. Sensors 2023, 23, 4538. https://doi.org/10.3390/s23094538
Tan JY, Zhang Z, Izzah HJ, Fong YK, Lee D, Mutwil M, Hong Y. Volatile-Based Diagnosis for Pathogenic Wood-Rot Fungus Fulvifomes siamensis by Electronic Nose (E-Nose) and Solid-Phase Microextraction/Gas Chromatography/Mass Spectrometry. Sensors. 2023; 23(9):4538. https://doi.org/10.3390/s23094538
Chicago/Turabian StyleTan, Jhing Yein, Ziteng Zhang, Hazirah Junin Izzah, Yok King Fong, Daryl Lee, Marek Mutwil, and Yan Hong. 2023. "Volatile-Based Diagnosis for Pathogenic Wood-Rot Fungus Fulvifomes siamensis by Electronic Nose (E-Nose) and Solid-Phase Microextraction/Gas Chromatography/Mass Spectrometry" Sensors 23, no. 9: 4538. https://doi.org/10.3390/s23094538
APA StyleTan, J. Y., Zhang, Z., Izzah, H. J., Fong, Y. K., Lee, D., Mutwil, M., & Hong, Y. (2023). Volatile-Based Diagnosis for Pathogenic Wood-Rot Fungus Fulvifomes siamensis by Electronic Nose (E-Nose) and Solid-Phase Microextraction/Gas Chromatography/Mass Spectrometry. Sensors, 23(9), 4538. https://doi.org/10.3390/s23094538









