Validation of Commercial Activity Trackers in Everyday Life of People with Parkinson’s Disease
Abstract
1. Introduction
- To examine whether the ATs can detect day-to-day fluctuations accurately and adequately rank days with high and low step counts when compared with the research-grade device in PD versus the HC.
- To explore the correlations with other gait and balance capacity measures in PD.
- To examine the compliance and user-friendliness of the tracking devices in PD.
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.3. Procedure
2.4. Statistical Analysis
3. Results
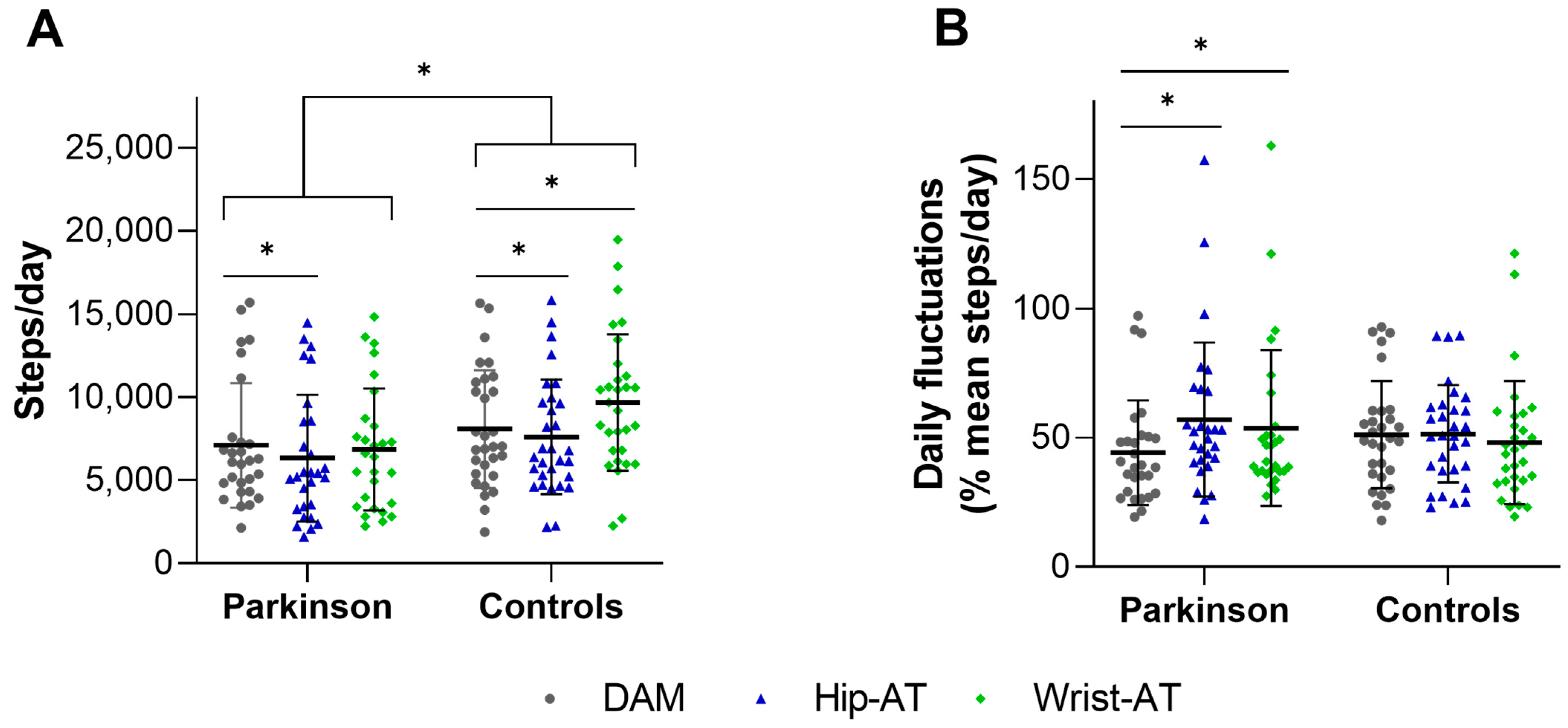
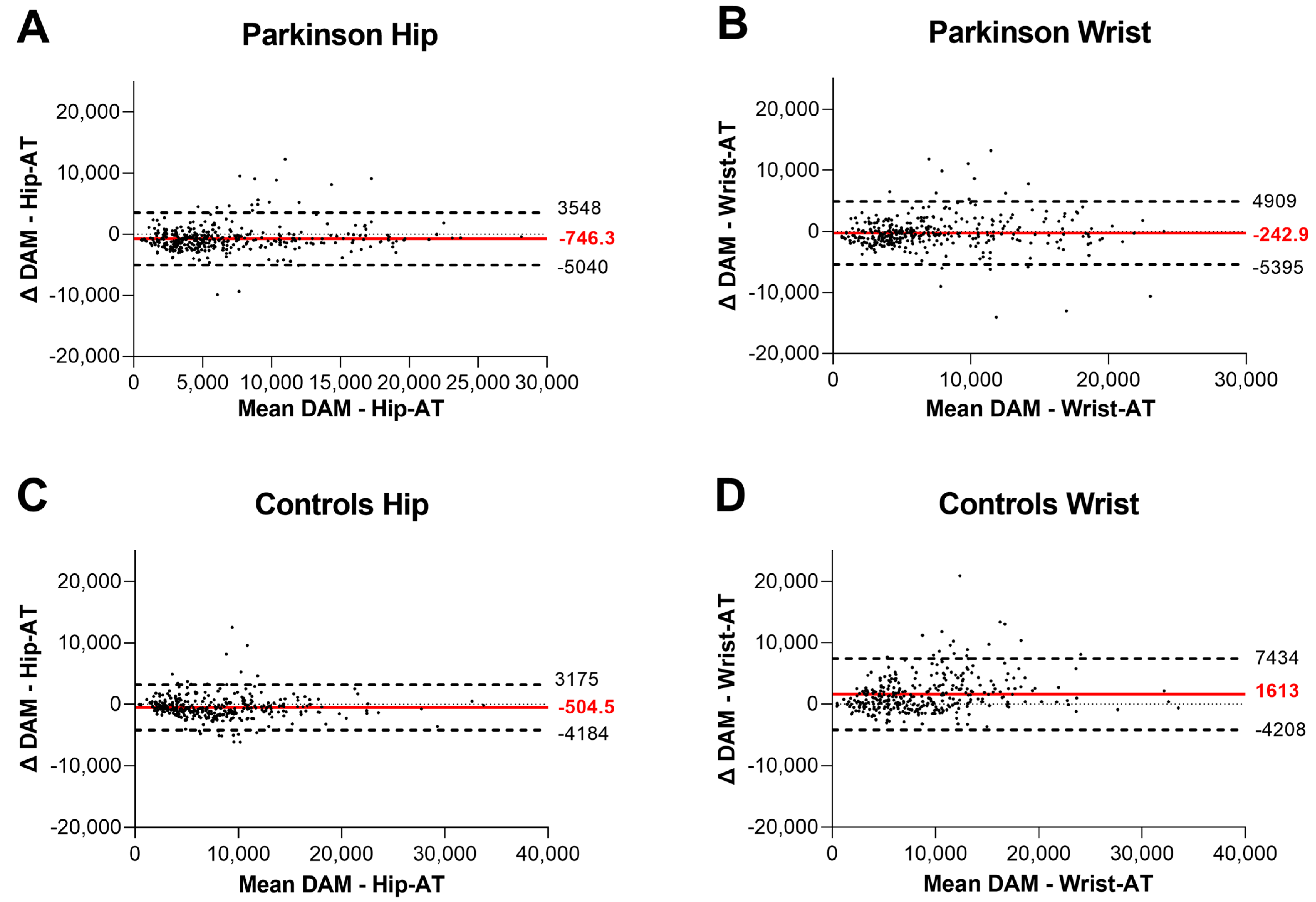
3.1. Criterion Validity
3.2. Detection of Daily Fluctuations
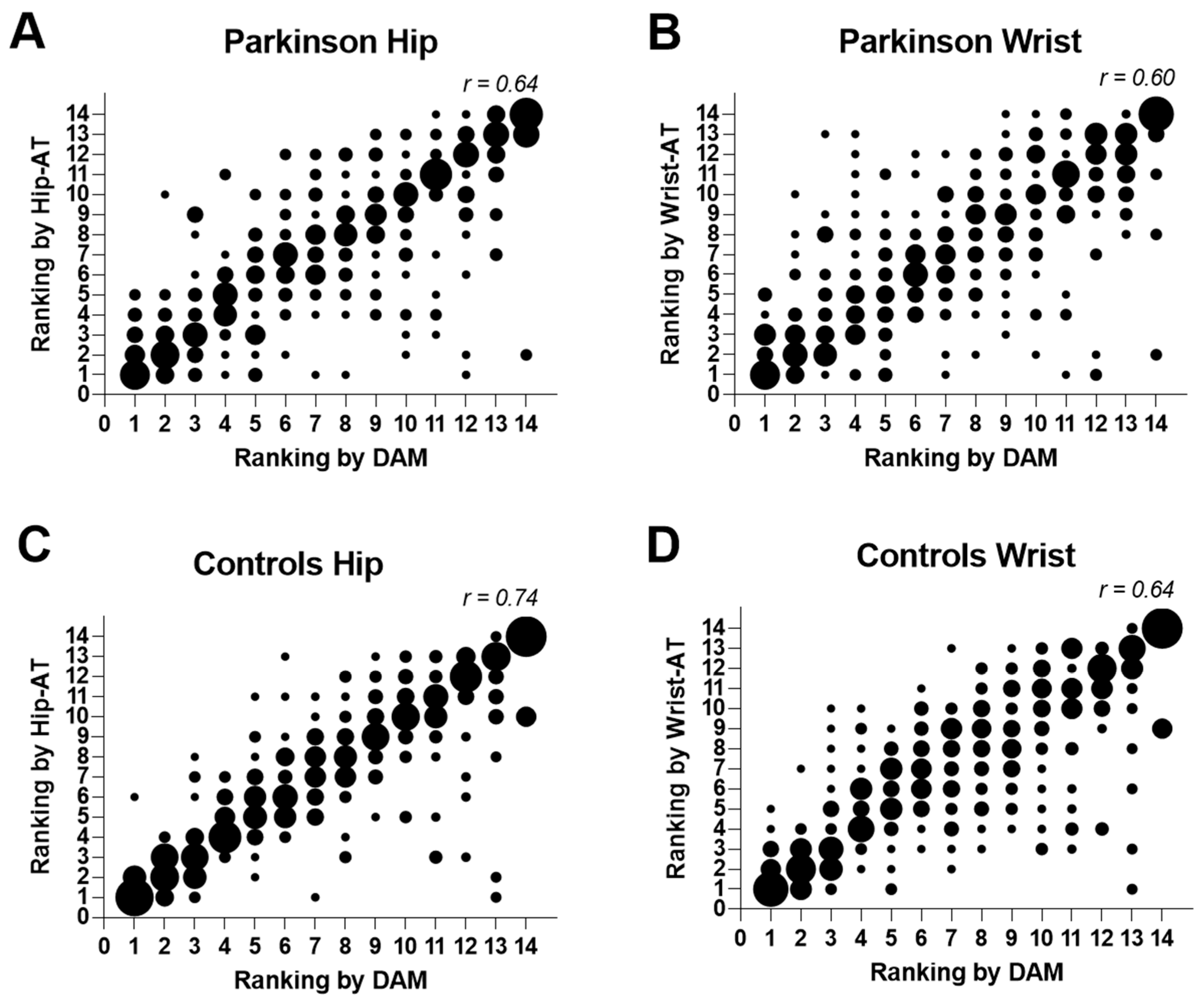
3.3. Concurrent Validity
3.4. User Experiences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schootemeijer, S.; van der Kolk, N.M.; Bloem, B.R.; de Vries, N.M. Current Perspectives on Aerobic Exercise in People with Parkinson’s Disease. Neurotherapeutics 2020, 17, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.K.; Wong-Yu, I.S.; Shen, X.; Chung, C.L. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat. Rev. Neurol. 2017, 13, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Keus, S.H.; Munneke, M.; Graziano, M.; Paltamaa, J.; Pelosin, E.; Domingos, J.; Brühlmann, S.; Ramaswamy, B.; Prins, J.; Struiksma, C.; et al. European Physiotherapy Guideline for Parkinson’s Disease; KNGF/ParkinsonNet: The Netherlands, 2014; p. 191. Available online: https://www.parkinsonnet.nl/app/uploads/sites/3/2019/11/eu_guideline_parkinson_guideline_for_pt_s1.pdf (accessed on 23 December 2022).
- Osborne, J.A.; Botkin, R.; Colon-Semenza, C.; DeAngelis, T.R.; Gallardo, O.G.; Kosakowski, H.; Martello, J.; Pradhan, S.; Rafferty, M.; Readinger, J.L.; et al. Physical Therapist Management of Parkinson Disease: A Clinical Practice Guideline From the American Physical Therapy Association. Phys. Ther. 2022, 102, pzab302. [Google Scholar] [CrossRef]
- Tsukita, K.; Sakamaki-Tsukita, H.; Takahashi, R. Long-term Effect of Regular Physical Activity and Exercise Habits in Patients with Early Parkinson Disease. Neurology 2022, 98, e859–e871. [Google Scholar] [CrossRef]
- Janssen Daalen, J.M.; Schootemeijer, S.; Richard, E.; Darweesh, S.K.L.; Bloem, B.R. Lifestyle Interventions for the Prevention of Parkinson Disease: A Recipe for Action. Neurology 2022, 99 (Suppl. S1), 42–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Trolle Lagerros, Y.; Bellocco, R.; Adami, H.O.; Fang, F.; Pedersen, N.L.; Wirdefeldt, K. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain 2015, 138 Pt 2, 269–275. [Google Scholar] [CrossRef]
- Paul, K.C.; Chuang, Y.H.; Shih, I.F.; Keener, A.; Bordelon, Y.; Bronstein, J.M.; Ritz, B. The association between lifestyle factors and Parkinson’s disease progression and mortality. Mov. Disord. 2019, 34, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Godfrey, A.; Galna, B.; Mhiripiri, D.; Burn, D.; Rochester, L. Ambulatory activity in incident Parkinson’s: More than meets the eye? J. Neurol. 2013, 260, 2964–2972. [Google Scholar] [CrossRef]
- Benka Wallen, M.; Franzen, E.; Nero, H.; Hagstromer, M. Levels and Patterns of Physical Activity and Sedentary Behavior in Elderly People with Mild to Moderate Parkinson Disease. Phys. Ther. 2015, 95, 1135–1141. [Google Scholar] [CrossRef]
- Schootemeijer, S.; van der Kolk, N.M.; Ellis, T.; Mirelman, A.; Nieuwboer, A.; Nieuwhof, F.; Schwarzschild, M.A.; de Vries, N.M.; Bloem, B.R. Barriers and Motivators to Engage in Exercise for Persons with Parkinson’s Disease. J. Parkinsons Dis. 2020, 10, 1293–1299. [Google Scholar] [CrossRef]
- Ginis, P.; Nieuwboer, A.; Dorfman, M.; Ferrari, A.; Gazit, E.; Canning, C.G.; Rocchi, L.; Chiari, L.; Hausdorff, J.M.; Mirelman, A. Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson’s disease: A pilot randomized controlled trial. Parkinsonism Relat. Disord. 2016, 22, 28–34. [Google Scholar] [CrossRef]
- Ellis, T.D.; Cavanaugh, J.T.; DeAngelis, T.; Hendron, K.; Thomas, C.A.; Saint-Hilaire, M.; Pencina, K.; Latham, N.K. Comparative Effectiveness of mHealth-Supported Exercise Compared with Exercise Alone for People with Parkinson Disease: Randomized Controlled Pilot Study. Phys. Ther. 2019, 99, 203–216. [Google Scholar] [CrossRef]
- Lamont, R.M.; Daniel, H.L.; Payne, C.L.; Brauer, S.G. Accuracy of wearable physical activity trackers in people with Parkinson’s disease. Gait Posture 2018, 63, 104–108. [Google Scholar] [CrossRef]
- Wendel, N.; Macpherson, C.E.; Webber, K.; Hendron, K.; DeAngelis, T.; Colon-Semenza, C.; Ellis, T. Accuracy of Activity Trackers in Parkinson Disease: Should We Prescribe Them? Phys. Ther. 2018, 98, 705–714. [Google Scholar] [CrossRef]
- Pradhan, S.; Kelly, V.E. Quantifying physical activity in early Parkinson disease using a commercial activity monitor. Parkinsonism Relat. Disord. 2019, 66, 171–175. [Google Scholar] [CrossRef]
- Lai, B.; Sasaki, J.E.; Jeng, B.; Cederberg, K.L.; Bamman, M.M.; Motl, R.W. Accuracy and Precision of Three Consumer-Grade Motion Sensors During Overground and Treadmill Walking in People with Parkinson Disease: Cross-Sectional Comparative Study. JMIR Rehabil. Assist. Technol. 2020, 7, e14059. [Google Scholar] [CrossRef]
- Hillel, I.; Gazit, E.; Nieuwboer, A.; Avanzino, L.; Rochester, L.; Cereatti, A.; Croce, U.D.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; et al. Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. Eur. Rev. Aging Phys. Act. 2019, 16, 6. [Google Scholar] [CrossRef]
- Blondeel, A.; Demeyer, H.; Janssens, W.; Troosters, T. Accuracy of consumer-based activity trackers as measuring tool and coaching device in patients with COPD and healthy controls. PLoS ONE 2020, 15, e0236676. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Dijkstra, B.; Zijlstra, W.; Scherder, E.; Kamsma, Y. Detection of walking periods and number of steps in older adults and patients with Parkinson’s disease: Accuracy of a pedometer and an accelerometry-based method. Age Ageing 2008, 37, 436–441. [Google Scholar] [CrossRef]
- Godinho, C.; Domingos, J.; Cunha, G.; Santos, A.T.; Fernandes, R.M.; Abreu, D.; Goncalves, N.; Matthews, H.; Isaacs, T.; Duffen, J.; et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson’s disease. J. Neuroeng. Rehabil. 2016, 13, 24. [Google Scholar] [CrossRef]
- Mikolaizak, A.S.; Rochester, L.; Maetzler, W.; Sharrack, B.; Demeyer, H.; Mazza, C.; Caulfield, B.; Garcia-Aymerich, J.; Vereijken, B.; Arnera, V.; et al. Connecting real-world digital mobility assessment to clinical outcomes for regulatory and clinical endorsement-the Mobilise-D study protocol. PLoS ONE 2022, 17, e0269615. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Mak, M.K.Y.; Wong-Yu, I.S.K. Six-Month Community-Based Brisk Walking and Balance Exercise Alleviates Motor Symptoms and Promotes Functions in People with Parkinson’s Disease: A Randomized Controlled Trial. J. Parkinsons Dis. 2021, 11, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Warmerdam, E.; Hausdorff, J.M.; Atrsaei, A.; Zhou, Y.; Mirelman, A.; Aminian, K.; Espay, A.J.; Hansen, C.; Evers, L.J.W.; Keller, A.; et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol. 2020, 19, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Silva de Lima, A.L.; Hahn, T.; Evers, L.J.W.; de Vries, N.M.; Cohen, E.; Afek, M.; Bataille, L.; Daeschler, M.; Claes, K.; Boroojerdi, B.; et al. Feasibility of large-scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS ONE 2017, 12, e0189161. [Google Scholar] [CrossRef]
- Chen, M.D.; Kuo, C.C.; Pellegrini, C.A.; Hsu, M.J. Accuracy of Wristband Activity Monitors during Ambulation and Activities. Med. Sci. Sports Exerc. 2016, 48, 1942–1949. [Google Scholar] [CrossRef]
- Vanbellingen, T.; Nyffeler, T.; Nef, T.; Kwakkel, G.; Bohlhalter, S.; van Wegen, E.E. Reliability and validity of a new dexterity questionnaire (DextQ-24) in Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 33, 78–83. [Google Scholar] [CrossRef]
- Cederberg, K.L.J.; Jeng, B.; Sasaki, J.E.; Lai, B.; Bamman, M.; Motl, R.W. Accuracy and precision of wrist-worn actigraphy for measuring steps taken during over-ground and treadmill walking in adults with Parkinson’s disease. Parkinsonism Relat. Disord. 2021, 88, 102–107. [Google Scholar] [CrossRef]
- Mazza, C.; Alcock, L.; Aminian, K.; Becker, C.; Bertuletti, S.; Bonci, T.; Brown, P.; Brozgol, M.; Buckley, E.; Carsin, A.E.; et al. Technical validation of real-world monitoring of gait: A multicentric observational study. BMJ Open 2021, 11, e050785. [Google Scholar] [CrossRef]
| Parkinson Disease | Healthy Controls | Significance | |||
|---|---|---|---|---|---|
| (n = 28) | (n = 30) | ||||
| Age (years) | 66. | (8) | 64 | (8) | p = 0.39 |
| Gender (M/F) | 20/8 | 16/14 | p = 0.18 | ||
| BMI (kg/m2) | 26.39 | (3.14) | 26.57 | (3.28) | p = 0.83 |
| 6 MinWT (meters) | 466 | (100) | 642 | (69) | p < 0.001 |
| MoCA (0–30) * | 27.21 | (2.77) | 27.19 | (3.02) | p = 0.98 |
| LSA (0–120) * | 85.25 | (21.68) | 97.25 | (14.69) | p = 0.06 |
| 12-WS (0–100) * | 24.40 | (20.16) | 0.74 | (1.43) | p < 0.001 |
| Disease duration (years) | 9 | (5) | / | ||
| MDS-UPDRS III (0–132) | 34.89 | (9.60) | / | ||
| LEDD (mg/day) | 763.44 | (409.33) | / | ||
| MiniBESTest (0–28) | 21.42 | (3.57) | / | ||
| N-FOGQ (0–27) # | 14.08 | (6.56) | / | ||
| Parkinson Disease | Healthy Controls | Interaction Effect | Post-Hoc Group Effect | |||
|---|---|---|---|---|---|---|
| (n = 28) | (n = 30) | |||||
| DAM | 7187.38 | (4933.67) | 8198.52 | (5272.05) | p < 0.001 | p = 0.008 |
| Hip-AT | 6441.05 | (5200.55) | 7694.07 | (5192.60) | p < 0.001 | |
| Wrist-AT | 6944.45 | (5030.29) | 9811.70 | (5937.90) | p < 0.001 | |
| Post hoc contrast p-value DAM—Hip-AT | p < 0.001 | p < 0.001 | ||||
| ICC(2,1) DAM—Hip-AT | 0.90 | (0.86–0.92) | 0.93 | (0.91–0.95) | ||
| Post hoc contrast p-value DAM—Wrist-AT | p < 0.29 | p < 0.001 | ||||
| ICC(2,1) DAM—Wrist-AT | 0.86 | (0.83–0.88) | 0.83 | (0.68–0.89) | ||
| Parkinson Disease | Healthy Controls | Interaction Effect | Post Hoc Group Effect | |||
|---|---|---|---|---|---|---|
| (n = 28) | (n = 30) | |||||
| DAM (%) | 44.19 | (19.88) | 51.13 | (20.40) | p = 0.005 | p = 0.20 |
| Hip-AT (%) | 56.99 | (29.19) | 51.42 | (18.55) | p = 0.39 | |
| Wrist-AT (%) | 53.64 | (29.63) | 48.09 | (23.40) | p = 0.44 | |
| Post hoc contrast p-value DAM—Hip-AT | p < 0.001 | p > 0.99 | ||||
| Post hoc contrast p-value DAM—Wrist-AT | p = 0.03 | p > 0.99 | ||||
| Wrist-AT | Hip-AT | ||
|---|---|---|---|
| How pleasant was it to wear the tracker? | Pleasant | 16 (57%) | 7 (25%) |
| Neutral | 7 (25%) | 18 (64%) | |
| Not pleasant | 5 (18%) | 3 (11%) | |
| How frequently did you look at the step count values on the tracker? | Multiple times a day | 16 (57%) | 10 (36%) |
| Once a day | 6 (22%) | 5 (18%) | |
| Once or twice a week | 2 (7%) | 5 (18%) | |
| Never | 4 (14%) | 8 (28%) | |
| How long would you be willing to wear the tracker in the future as part of your clinical routine? * | A year or longer | 11 (39%) | 6 (22%) |
| Months | 5 (18%) | 5 (18%) | |
| Weeks | 4 (14%) | 3 (11%) | |
| Days | 1 (3.5%) | 0 (0%) | |
| Never | 6 (22%) | 13 (46%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginis, P.; Goris, M.; De Groef, A.; Blondeel, A.; Gilat, M.; Demeyer, H.; Troosters, T.; Nieuwboer, A. Validation of Commercial Activity Trackers in Everyday Life of People with Parkinson’s Disease. Sensors 2023, 23, 4156. https://doi.org/10.3390/s23084156
Ginis P, Goris M, De Groef A, Blondeel A, Gilat M, Demeyer H, Troosters T, Nieuwboer A. Validation of Commercial Activity Trackers in Everyday Life of People with Parkinson’s Disease. Sensors. 2023; 23(8):4156. https://doi.org/10.3390/s23084156
Chicago/Turabian StyleGinis, Pieter, Maaike Goris, An De Groef, Astrid Blondeel, Moran Gilat, Heleen Demeyer, Thierry Troosters, and Alice Nieuwboer. 2023. "Validation of Commercial Activity Trackers in Everyday Life of People with Parkinson’s Disease" Sensors 23, no. 8: 4156. https://doi.org/10.3390/s23084156
APA StyleGinis, P., Goris, M., De Groef, A., Blondeel, A., Gilat, M., Demeyer, H., Troosters, T., & Nieuwboer, A. (2023). Validation of Commercial Activity Trackers in Everyday Life of People with Parkinson’s Disease. Sensors, 23(8), 4156. https://doi.org/10.3390/s23084156








