Involvement of Human Volunteers in the Development and Evaluation of Wearable Devices Designed to Improve Medication Adherence: A Scoping Review
Abstract
1. Introduction
2. Method
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Sources and Search Period
2.5. Study Selection
2.6. Data Extraction
2.7. Data Synthesis and Analysis
- Idea validation: also called the ideation phase, during which ideas are collected that serve to answer questions on the challenges identified and possible solutions. Concept validation or idea chaining can entail different research approaches, including interviews, observations, and behavioral mapping of potential users [21];
- Prototyping validation: also called the prototyping phase. This aims to provide physical means for experimentation and encourages early failure/success in the form of a test product at a reduced cost. It also serves as an object of transition during interdisciplinary collaboration and communication, as well as emphasizing the importance of the ability to visualize/manipulate solutions [22];
- Product validation: testing the product throughout the development phase reduces, or even eliminates, the chances of error and problems in the product under development [23]. In the case of medical devices, product testing that involves any investigation with humans aimed at discovering or verifying clinical effects is called a clinical trial [16].
- Smartwatches: these are digital watches that offer features such as heart rate monitoring, activity tracking, and providing reminders [24]. These watches rely on a compatible smartphone to deliver data over a Bluetooth® connection and radio technology that provides solutions to meet specific connectivity needs [25]. As smartwatch apps can issue visual, verbal, audible, and vibrational alerts and reminders to wearers, they are useful for promoting medication adherence [26,27,28];
- Patches: these are thin, flexible, adhesive patch-like medical devices that use integrated circuits and nanomaterials to detect small amounts of toxins, proteins, DNA, or chemicals through the skin [29]. These wearable adhesive sensors can detect and record medication intake and emit vibrating signals at scheduled times for medication administration [30,31];
- Wristbands: these are equipped with sensors that can be used to monitor physical activity and the user’s heart rate and issue alerts for scheduled tasks. The bands also provide users with recommendations for health, fitness, and other warnings and can be programmed, for example, to receive reminders and notify the user when it is time to remove drugs to be administered from the bottles [32,33];
- Neckwear: these are devices that capture signs of swallowing and medication ingestion in the form of a necklace. They can also pair with mobile devices that receive and store data [11].
3. Results
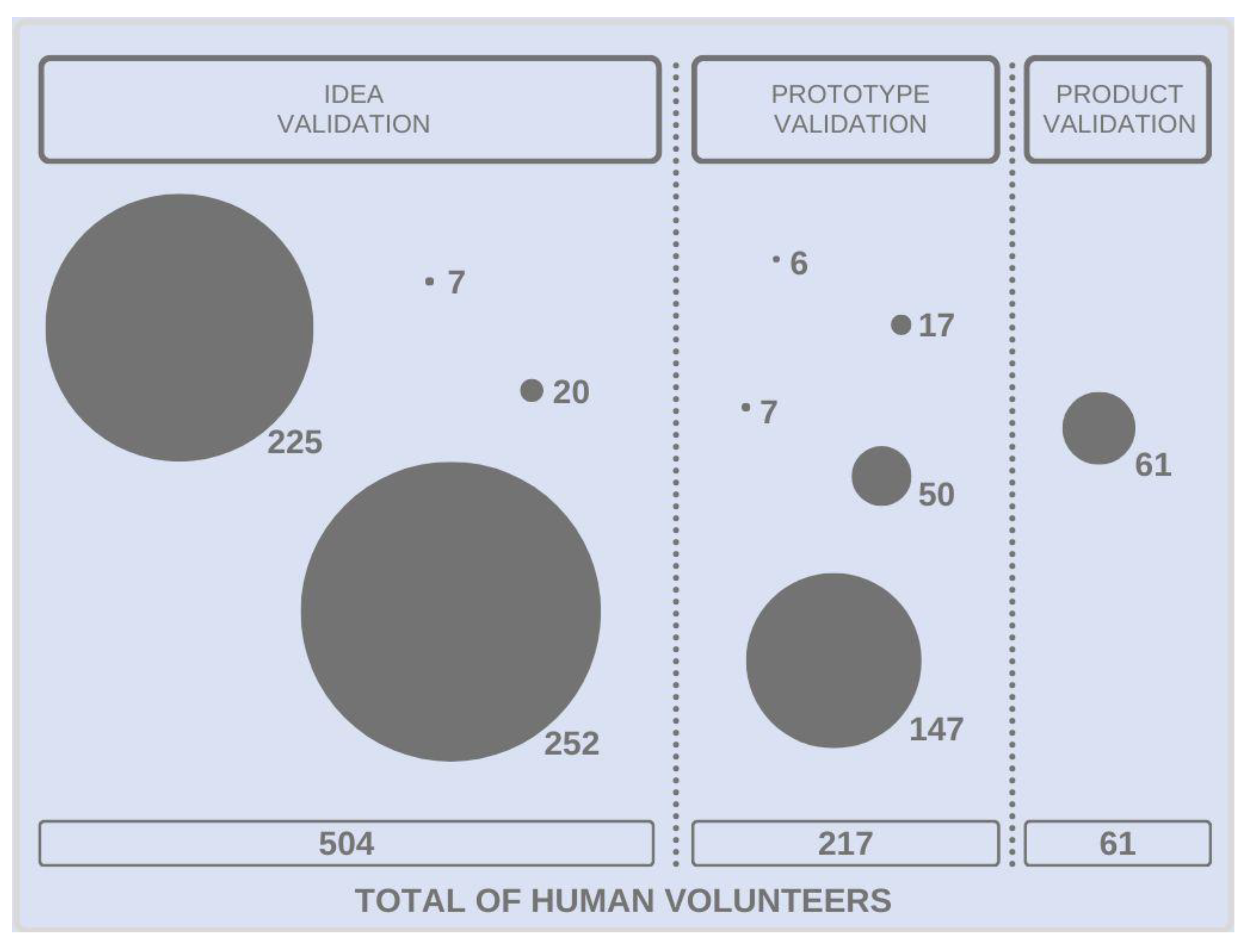
3.1. Involvement of Human Volunteers in Idea Validation
3.2. Involvement of Human Volunteers in Prototype Validation
3.3. Involvement of Human Volunteers in Product Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izmailova, E.S.; Wagner, J.A.; Perakslis, E.D. Wearable Devices in Clinical Trials: Hype and Hypothesis. Clin. Pharmacol. Ther. 2018, 104, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kalantarian, H.; Motamed, B.; Alshurafa, N.; Sarrafzadeh, M. A wearable sensor system for medication adherence prediction. Artif. Intell. Med. 2016, 69, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ometov, A.; Shubina, V.; Klus, L.; Skibińska, J.; Saafi, S.; Pascacio, P.; Flueratoru, L.; Gaibor, D.Q.; Chukhno, N.; Chukhno, O.; et al. A Survey on Wearable Technology: History, State-of-the-Art and Current Challenges. Comput. Netw. 2021, 193, 108074. [Google Scholar] [CrossRef]
- Ates, H.C.; Yetisen, A.K.; Güder, F.; Dincer, C. Wearable devices for the detection of COVID-19. Nat. Electron. 2021, 4, 13–14. [Google Scholar] [CrossRef]
- Padash, M.; Enz, C.; Carrara, S. Microfluidics by Additive Manufacturing for Wearable Biosensors: A Review. Sensors 2020, 20, 4236. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Iyawa, G.E.; Hansen, W.; Pérez, Y.V.; Badawy, S.M.; Shah, R.; Beg, U.; Heneghan, M.B. Habit Strength, Medication Adherence, and Habit-Based Mobile Health Interventions Across Chronic Medical Conditions: Systematic Review. J. Med. Internet Res. 2020, 22, e17883. [Google Scholar] [CrossRef]
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef]
- Cutler, D.M.; Everett, W. Thinking Outside the Pillbox—Medication Adherence as a Priority for Health Care Reform. N. Engl. J. Med. 2010, 362, 1553–1555. [Google Scholar] [CrossRef]
- Kvarnström, K.; Airaksinen, M.; Liira, H. Barriers and facilitators to medication adherence: A qualitative study with general practitioners. BMJ Open 2018, 8, e015332. [Google Scholar] [CrossRef]
- Choi, Y.M.; Olubanjo, T.; Farajidavar, A.; Ghovanloo, M. Potential barriers in adoption of a medication compliance neckwear by elderly population. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; Volume 12, pp. 4678–4681. [Google Scholar] [CrossRef]
- Kar, A.; Ahamad, N.; Dewani, M.; Awasthi, L.; Patil, R.; Banerjee, R. Wearable and implantable devices for drug delivery: Applications and challenges. Biomaterials 2022, 283, 121435. [Google Scholar] [CrossRef]
- Chun, J.; Dey, A.; Lee, K.; Kim, S. A qualitative study of smartwatch usage and its usability. Hum. Factors Ergon. Manuf. 2018, 28, 186–199. [Google Scholar] [CrossRef]
- Qualitylogic. Wearable Device Testing: New Technology Demands a New Approach. 2022. Available online: https://www.qualitylogic.com/2018/03/22/wearable-device-testing-new-technology-demands-new-approach/ (accessed on 14 May 2022).
- De Angelis, C.; Drazen, J.M.; Frizelle, F.A.; Haug, C.; Hoey, J.; Horton, R.; Kotzin, S.; Laine, C.; Marusic, A.; Overbeke, A.J.P.; et al. Clinical Trial Registration: A Statement from the International Committee of Medical Journal Editors. N. Engl. J. Med. 2004, 351, 1250–1251. [Google Scholar] [CrossRef]
- ICH (International Council for Harmonisation). Adendo integrado ao ICH E6(R1): Guia de Boas Práticas Clínicas ICH E6(R2). Versão Traduzida para o Português. 2019. Available online: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/medicamentos/publicacoes-sobre-medicamentos/guia-de-boas-praticas-clinicas-ich-e6-r2/view (accessed on 1 June 2022).
- NICE (National Institute for Health and Care Excellence). Behaviour Change: Digital and Mobile Health Interventions. NICE Guideline [NG183]. 2020. Available online: https://www.nice.org.uk/guidance/ng183/chapter/Recommendations#developing-digital-and-mobile-health-interventions (accessed on 21 May 2022).
- Mück, J.E.; Ünal, B.; Butt, H.; Yetisen, A.K. Market and Patent Analyses of Wearables in Medicine. Trends Biotechnol. 2019, 37, 563–566. [Google Scholar] [CrossRef]
- Polaris Market Research. Wearable Medical Device Market Size. Global Industry Report, 2027. 2020. Available online: https://www.polarismarketresearch.com/industry-analysis/wearable-medical-devices-market (accessed on 1 June 2022).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Lorusso, L.; Lee, J.H.; Worden, E.A. Design Thinking for Healthcare: Transliterating the Creative Problem-Solving Method into Architectural Practice. HERD Health Environ. Res. Des. J. 2021, 14, 16–29. [Google Scholar] [CrossRef]
- Micheli, P.; Wilner, S.J.S.; Bhatti, S.H.; Mura, M.; Beverland, M.B. Doing Design Thinking: Conceptual Review, Synthesis, and Research Agenda. J. Prod. Innov. Manag. 2018, 36, 124–148. [Google Scholar] [CrossRef]
- Christensson, P. Smartwatch Definition. TechTerms. Sharpened Productions. 2017. Available online: https://techterms.com/definition/smartwatch (accessed on 14 May 2022).
- Bluetooth. Bluetooth Solution Areas. 2022. Available online: https://www.bluetooth.com/ (accessed on 1 March 2022).
- Espinoza, A.-N.; Garcia-Vazquez, J.P.; Rodriguez, M.D.; Andrade, G.; García-Peña, C.; Garćıa-Vázquez, J.P. Enhancing a Wearable Help Button to Support the Medication Adherence of Older Adults. In Proceedings of the 2009 Latin American Web Congress, Merida, Mexico, 9–11 November 2009; pp. 3–7. [Google Scholar] [CrossRef]
- Fozoonmayeh, D.; Le, H.V.; Wittfoth, E.; Geng, C.; Ha, N.; Wang, J.; Vasilenko, M.; Ahn, Y.; Woodbridge, D.M.-K. A Scalable Smartwatch-Based Medication Intake Detection System Using Distributed Machine Learning. J. Med. Syst. 2020, 44, 76. [Google Scholar] [CrossRef]
- Samyoun, S.; Mondol, A.S.; Emi, I.A.; Stankovic, J.A. iAdhere: A voice interactive assistant to improve adherence to medical treatments. In Proceedings of the ICCPS‘19: 10th ACM/IEEE International Conference on Cyber-Physical Systems, Montreal, QC, Canada, 16–18 April 2019; pp. 334–335. [Google Scholar] [CrossRef]
- AABME (Alliance of Advanced Biomedical Engineering). Advances in Smart Cutaneous Wearable Devices. 2020. Available online: https://aabme.asme.org/posts/advances-in-smart-cutaneous-wearable-devices (accessed on 1 June 2022).
- Browne, S.H.; Umlauf, A.; Tucker, A.J.; Low, J.; Moser, K.; Garcia, J.G.; Peloquin, C.A.; Blaschke, T.; Vaida, F.; Benson, C.A. Wirelessly observed therapy compared to directly observed therapy to confirm and support tuberculosis treatment adherence: A randomized controlled trial. PLoS Med. 2019, 16, e1002891. [Google Scholar] [CrossRef]
- Euliano, N.; Darmanijan, S.; Buffkin, E.; Myers, B.; Flores, G. Electronic Medication Compliance Monitoring System and Associated Methods. US Patent 2014/0309505, 17 March 2014. [Google Scholar]
- Li, J.; Peplinski, S.J.; Nia, S.M.; Farajidavar, A. An interoperable pillbox system for smart medication adherence. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1386–1389. [Google Scholar] [CrossRef]
- Phaneuf, A. Latest Trends in Medical Monitoring Devices and Wearable Health Technology. Business Insider. 2016. Available online: https://www.businessinsider.com/wearable-technology-healthcare-medical-devices#:~:text=Some%20of%20the%20simplest%20and,syncing%20to%20various%20smartphone%20apps (accessed on 14 May 2022).
- Rosner, D.; Jurba, A.-T.; Tataroiu, R.; Ilas, C.; Vasile, S.; Matei, S. Wearable Medication Reminder Architecture Enhancement: Focus Group Based Assessment and Scenario Based Testing. In Proceedings of the 2015 20th International Conference on Control Systems and Computer Science, Bucharest, Romania, 27–29 May 2015; pp. 279–284. [Google Scholar] [CrossRef]
- Stekler, J.D.; Scanlan, J.M.; Simoni, J.M.; Crane, H.M.; Fredericksen, R.J.; Marquard, J.; Saver, B.G. Predictors of Art and PrEP Adherence and Medication-Taking Practices and Preferences to Inform Development of a Wrist-Worn Adherence System. AIDS Educ. Prev. 2018, 30, 357–368. [Google Scholar] [CrossRef]
- Deutsch, M.; Burgsteiner, H. A smartwatch-based assistance system for the elderly performing fall detection, unusual inactivity recognition and medication reminding. Stud. Health Technol. Inform. 2016, 223, 259–266. [Google Scholar] [PubMed]
- Abraham, I.; Geest, W.; Geest, J.; Troy, E.; Macdonald, K. Detectability and appraisal thresholds of split pulse signals for the MemoPatch™ device, an electronic skin patch intended to deliver tactile medication reminder signals (study TS-104). In Proceedings of the IEEE Engineering in Medicine and Biology Society, Osaka, Japan, 3–7 July 2013; pp. 914–917. [Google Scholar]
- Abraham, I.; De Geest, J.; De Geest, W.; De Troy, E.; MacDonald, K. Detectability and acceptability of continuous pulse signals for the MemoPatch® device, an electronic skin patch intended to deliver tactile medication reminder signals. Med. Devices Évid. Res. 2015, 8, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Marquard, J.L.; Saver, B.; Kandaswamy, S.; Martinez, V.I.; Simoni, J.M.; Stekler, J.D.; Ganesan, D.; Scanlan, J. Designing a wrist-worn sensor to improve medication adherence: Accommodating diverse user behaviors and technology preferences. JAMIA Open 2018, 1, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Orwig, D.; Brandt, N.; Gruber-Baldini, A.L. Medication Management Assessment for Older Adults in the Community. Gerontology 2006, 46, 661–668. [Google Scholar] [CrossRef]
- Jiang, N.; Mück, J.E.; Yetisen, A.K. The Regulation of Wearable Medical Devices. Trends Biotechnol. 2020, 38, 129–133. [Google Scholar] [CrossRef]
- Van Norman, G.A. Drugs, devices, and the FDA: Part 2: An overview of approval processes: FDA approval of medical devices. J. Am. Coll. Cardiol. Basic Transl. Sci. 2016, 1, 277–287. [Google Scholar]
- Jourdan, T.; Debs, N.; Frindel, C. The Contribution of Machine Learning in the Validation of Commercial Wearable Sensors for Gait Monitoring in Patients: A Systematic Review. Sensors 2021, 21, 4808. [Google Scholar] [CrossRef]
- United Kingdom. UK Health Security Agency. Guidance. Choose Evaluation Methods: Evaluating Digital Health Products. 2021. Available online: https://www.gov.uk/guidance/choose-evaluation-methods-evaluating-digital-health-products (accessed on 2 October 2022).
- NICE (National Institute for Health and Care Excellence). Evidence Standards Framework for Digital Health Technologies. NICE Corporate Document [ECD7]. 2022. Available online: https://www.nice.org.uk/corporate/ecd7/resources/evidence-standards-framework-for-digital-health-technologies-user-guide-9024813901/chapter/how-to-use-the-framework#deciding-which-evidence-standards-are-relevant (accessed on 2 October 2022).
- Freeman, K.; Dinnes, J.; Chuchu, N.; Takwoingi, Y.; Bayliss, S.E.; Matin, R.N.; Jain, A.; Walter, F.M.; Williams, H.C.; Deeks, J.J. Algorithm based smartphone apps to assess risk of skin cancer in adults: Systematic review of diagnostic accuracy studies. BMJ 2020, 368, m127. [Google Scholar] [CrossRef]
- Farao, J.; Malila, B.; Conrad, N.; Mutsvangwa, T.; Rangaka, M.X.; Douglas, T.S. A user-centred design framework for mHealth. PLoS ONE 2020, 15, e0237910. [Google Scholar] [CrossRef]
- Göttgens, I.; Oertelt-Prigione, S. The Application of Human-Centered Design Approaches in Health Research and Innovation: A Narrative Review of Current Practices. JMIR mHealth uHealth 2021, 9, e28102. [Google Scholar] [CrossRef]
- Smuck, M.; Odonkor, C.A.; Wilt, J.K.; Schmidt, N.; Swiernik, M.A. The emerging clinical role of wearables: Factors for successful implementation in healthcare. NPJ Digit. Med. 2021, 4, 45. [Google Scholar] [CrossRef]

| Number | Search Strategy |
|---|---|
| #1 | (wearable device) OR (wearable devices) OR (wearable electronic device) OR (wearable electronic devices) OR (wearable technologies) OR (wearable technology) OR (wearable health monitoring devices) OR (technologies wearable) OR (technology wearable) OR (device wearable) OR (devices wearable) OR (wearable wrist biosensor) OR (wearable*) OR smartwatch OR (smart watch) OR smartwatches OR (smart watches) OR wristband* OR (hearing aids) OR (hearing aid) OR (ear mold) OR (ear molds) OR (earmold) OR (earmolds) OR (electronic tattoo) OR (electronic tattoos) OR (optical tattoo) OR (optical tattoos) OR (head mounted display) OR (head mounted displays) OR (subcutaneous sensors) OR (subcutaneous sensor) OR (electronic footwear) OR (electronic textile) OR (wireless sensor) OR (body sensor) OR (body worn sensor) OR (electronic footwear) OR (electronic textiles) OR (wireless sensors) OR (body sensors) OR (body worn sensors) OR biosensor OR biosensors OR accelerometer* OR gyroscope* OR (optical sensor) OR (contact sensor) OR (optical sensors) OR (contact sensors) OR (wearable monitor) OR (wearable monitors) OR (chips diagnosis) OR (electronic skin) |
| #2 | (medication adherence) OR (medication compliance) OR (medication non adherence) OR (medication nonadherence) OR (medication non-adherence) OR (medication noncompliance) OR (medication non-compliance) OR (medication persistence) OR (therapeutic adherence) OR (therapeutic adherence and compliance) OR (treatment adherence) OR (treatment adherence and compliance) OR (compliance patient) OR (patient adherence) OR (adherence patient) OR (patient cooperation) OR (cooperation patient) OR (patient non-compliance) OR (non-compliance patient) OR (patient non compliance) OR (patient nonadherence) OR (nonadherence patient) OR (patient noncompliance) OR (noncompliance patient) OR (patient non-adherence) OR (non-adherence patient) OR (patient non adherence) OR (treatment compliance) OR (compliance treatment) OR (treatment compliances) OR (therapeutic compliance) OR (compliance therapeutic) OR (compliances therapeutic) OR (therapeutic compliances) |
| #3 | #1 AND #2 |
| Author | Country of Origin | Type of Technology | Technology Description | Study Design | Ethics Committee and Informed Consent |
|---|---|---|---|---|---|
| Idea Validation | |||||
| Choi et al., 2013 [11] | USA | Neckwear | Neckwear device with a proposed system that reminds patients when to take their medications and the proper dose of each pill and monitors medication ingestion in real-time | Survey | Not reported |
| Rosner et al., 2015 * [33] | Romania | Wristband | Development of a medication reminder system that delivers alarms effectively through a user-sensitive design to be easily integrated into patients’ and caregivers’ daily routines | Survey | Not reported |
| Stekler et al., 2018 [34] | USA | Smartwatch | Wrist worn sensor using Bluetooth technology for motion sensing and gesture recognition, tags on medication bottles, a smartphone app, and real-time adherence reminders | Survey | Yes |
| Deustch; Burgsteiner, 2019 [35] | Austria | Smartwatch | Smartwatch-based assistance system which can set medication reminders and get help from relatives at the push of a single button | Experimental study | Not reported |
| Prototype Validation | |||||
| Espinoza et al., 2009 [25] | Mexico | Smartwatch | User interface for informing (coaching) older adults on the medications and doses to take | Survey | Not reported |
| Abraham et al., 2013 [36] | USA | Patch | Electronic skin patch designed to deliver discreet tactile reminder stimuli | Experimental study | Yes |
| Abraham et al., 2015 [37] | USA | Patch | Electronic skin patch designed to deliver discreet tactile reminder stimuli | Experimental study | Yes |
| Rosner et al., 2015 * [33] | Romania | Wristband | Development of a medication reminder system that delivers alarms effectively through a user-sensitive design to be easily integrated into patients’ and caregivers’ daily routines | Survey | Not reported |
| Marquard et al., 2018 [38] | USA | Wristband | Detection of pill-taking behavior, triggering pill-taking reminders for wrist wearers | Survey | Yes |
| Product Validation | |||||
| Browne et al., 2019 [29] | USA | Patch | Small adhesive-backed detector patch worn on the torso and a paired mobile device | Randomized controlled trial | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marengo, L.L.; Barberato-Filho, S. Involvement of Human Volunteers in the Development and Evaluation of Wearable Devices Designed to Improve Medication Adherence: A Scoping Review. Sensors 2023, 23, 3597. https://doi.org/10.3390/s23073597
Marengo LL, Barberato-Filho S. Involvement of Human Volunteers in the Development and Evaluation of Wearable Devices Designed to Improve Medication Adherence: A Scoping Review. Sensors. 2023; 23(7):3597. https://doi.org/10.3390/s23073597
Chicago/Turabian StyleMarengo, Lívia Luize, and Silvio Barberato-Filho. 2023. "Involvement of Human Volunteers in the Development and Evaluation of Wearable Devices Designed to Improve Medication Adherence: A Scoping Review" Sensors 23, no. 7: 3597. https://doi.org/10.3390/s23073597
APA StyleMarengo, L. L., & Barberato-Filho, S. (2023). Involvement of Human Volunteers in the Development and Evaluation of Wearable Devices Designed to Improve Medication Adherence: A Scoping Review. Sensors, 23(7), 3597. https://doi.org/10.3390/s23073597






