EMG-Based Estimation of Lower Limb Joint Angles and Moments Using Long Short-Term Memory Network
Abstract
1. Introduction
2. Related Works
3. Methodology
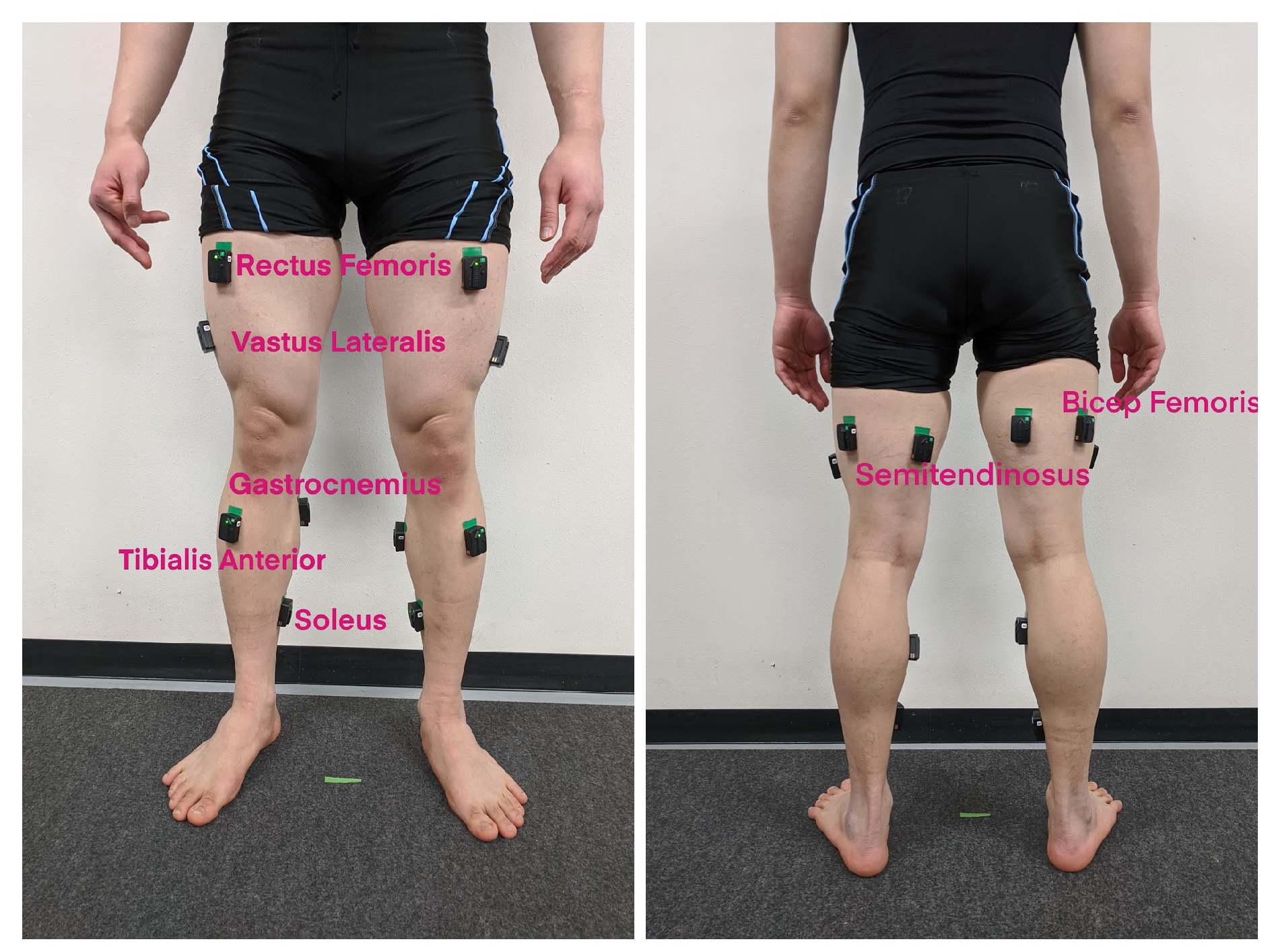
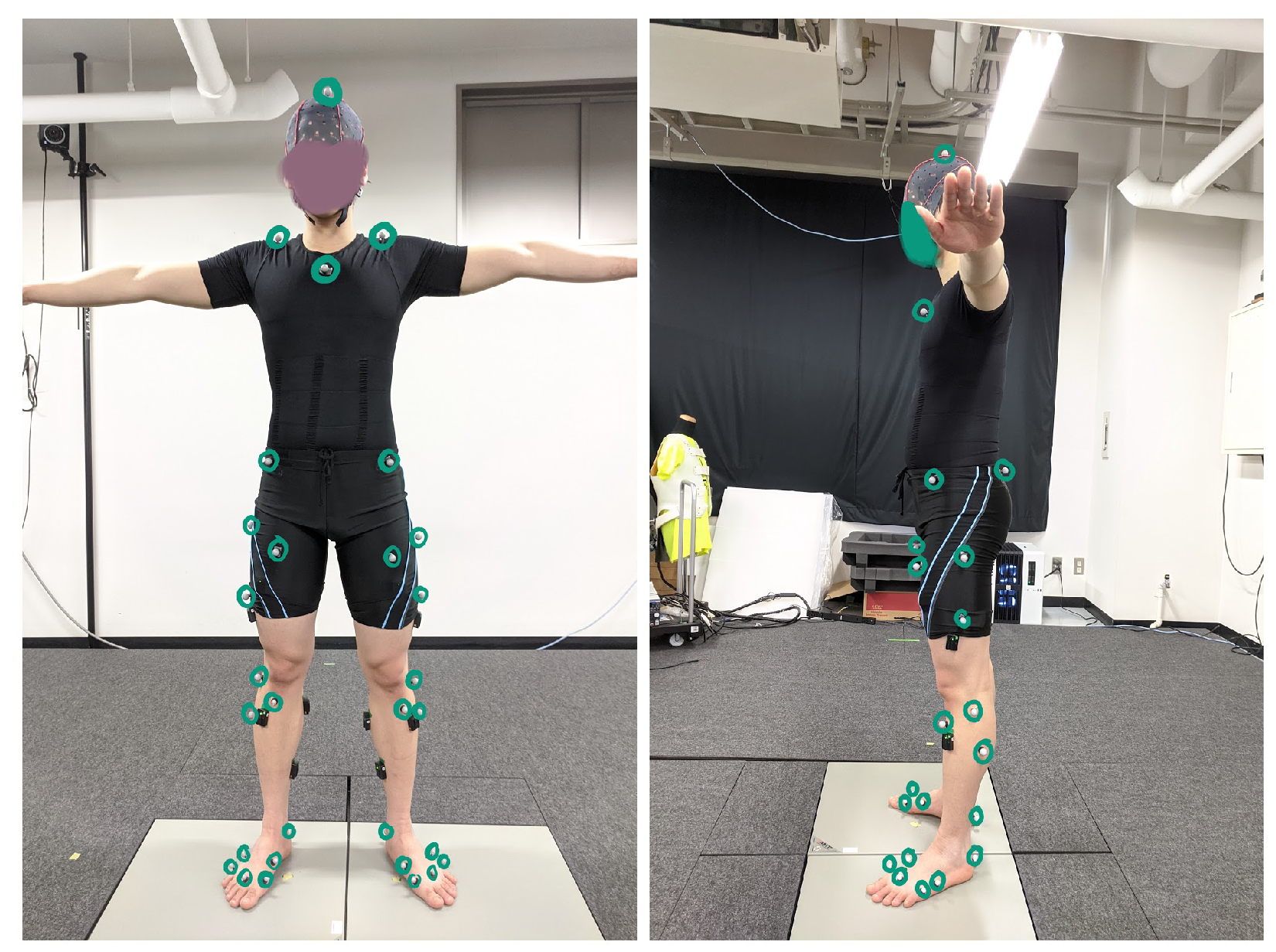
3.1. Data Acquisition Process
3.2. Experiment
3.3. Data Processing
- Passing raw signals through a fourth-order bandpass filter with a frequency range from 20 to 450 Hz [28].
- Passing signals through a second-order infinite impulse response (IIR) notch digital filter with a cutoff frequency of 50 Hz [28].
- Differentiating the signals to make them more stationary than the original [29].
- Normalizing the signals concerning the maximum value to maintain the signal between 0 and 1 [30].
3.4. OpenSim Simulation
3.5. Dataset
3.6. Evaluation Metrics
- (1)
- Coefficient of determination (R2) to determine how well the model fits the variance. However, it does not account significantly for the offset.
- (2)
- Root mean square error (RMSE) is affected by the offset between the actual and estimated values. The main drawback of using RMSE is that comparing results between different subjects and joints will result in misleading information because subjects and joints will have distinct ranges depending on the subject’s anthropometrics and other factors, such as the subject’s patterns, to achieve the tasks.
- (3)
- Normalized root mean square error (NRMSE) to make results’ intrasubject and intersubject links more meaningful.
3.7. LSTM Model
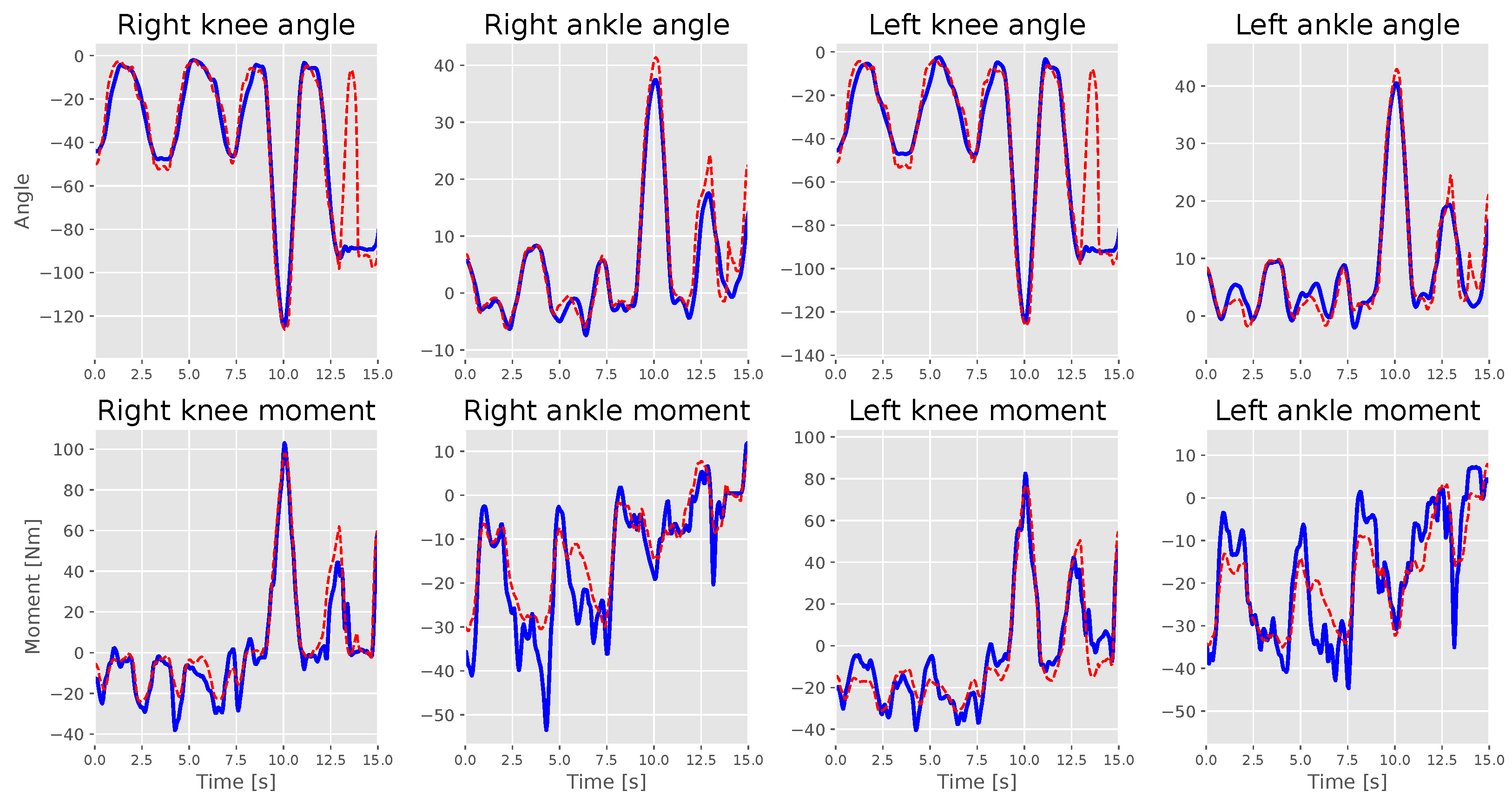
4. Results and Discussion
4.1. Experimental Motion Analysis
4.1.1. Squat
4.1.2. Pick Up an Object
4.1.3. Sit Stand
4.2. Estimation Performance with Personalized Intrasubject Models
5. Conclusions
- We assumed that all the output measurements from the motion capture system, force plates, and OpenSim calculations were accurate and reliable.
- sEMG signals are susceptible to external factors such as sweat or motion artifacts, which are inherent issues when opting for the surface type. These factors may affect the quality of the signal.
- The number of movements is still limited. This system does not cover walking, jumping, or running activities, which are usually in high demand.
- The number of investigated joints is also insufficient. The hip joint was excluded from this study because the sEMG signals of the muscles of interest contributing to hip joints are challenging to access stably for the measurement.
- Results are not always consistent from one subject to another (intersubject variability).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Resnik, L.; Klinger, S.L.; Etter, K. The DEKA Arm: Its features, functionality, and evolution during the veterans affairs study to optimize the DEKA Arm. Prosthet. Orthot. Int. 2014, 38, 492–504. [Google Scholar] [CrossRef]
- Cipriani, C.; Controzzi, M.; Carrozza, M.C. The SmartHand transradial prosthesis. J. Neuroeng. Rehabil. 2011, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Au, A.T.; Kirsch, R.F. EMG-based prediction of shoulder and elbow kinematics in able-bodied and spinal cord injured individuals. IEEE Trans. Rehabil. Eng. 2000, 8, 471–480. [Google Scholar] [CrossRef]
- Fragkiadaki, K.; Levine, S.; Felsen, P.; Malik, J. Recurrent network models for human dynamics. In Proceedings of the IEEE International Conference on Computer Vision, Santiago, Chile, 7–13 December 2015. [Google Scholar] [CrossRef]
- Chao, Y.W.; Yang, J.; Price, B.; Cohen, S.; Deng, J. Forecasting human dynamics from static images. In Proceedings of the 30th IEEE Conference on Computer Vision and Pattern Recognition (CVPR 2017), Honolulu, HI, USA, 21–26 July 2017. [Google Scholar] [CrossRef]
- Li, Z.; Hayashibe, M.; Fattal, C.; Guiraud, D. Muscle fatigue tracking with evoked EMG via recurrent neural network: Toward personalized neuroprosthetics. IEEE Comput. Intell. Mag. 2014, 9, 38–46. [Google Scholar] [CrossRef]
- Sakamoto, S.; Owaki, D.; Hayashibe, M. Ground Reaction Force Estimation from EMG Using Recurrent Neural Network. Proc. JSME Annu. Conf. Robot. Mechatron. (Robomec) 2019, 55, 38–46. [Google Scholar] [CrossRef]
- Atzori, M.; Cognolato, M.; Müller, H. Deep learning with convolutional neural networks applied to electromyography data: A resource for the classification of movements for prosthetic hands. Front. Neurorobot. 2016, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, S.H.; Xu, D.; Ren, Y.; Lee, S.J.; Zhang, L.Q. EMG-Based continuous and simultaneous estimation of arm kinematics in able-bodied individuals and stroke survivors. Front. Neurosci. 2017, 11, 480. [Google Scholar] [CrossRef]
- Ur Rehman, M.Z.; Waris, A.; Gilani, S.O.; Jochumsen, M.; Niazi, I.K.; Jamil, M.; Farina, D.; Kamavuako, E.N. Multiday EMG-based classification of hand motions with deep learning techniques. Sensors 2018, 18, 2497. [Google Scholar] [CrossRef]
- Jordan, M.I. Attractor dynamics and parallelism in a connectionist sequential machine. In Proceedings of the Eighth Annual Conference of the Cognitive Science Society, Amherst, MA, USA, 15–17 August 1986. [Google Scholar]
- Cleeremans, A.; Servan-Schreiber, D.; McClelland, J.L. Finite State Automata and Simple Recurrent Networks. Neural Comput. 1989, 1, 372–381. [Google Scholar] [CrossRef]
- Pearlmutter, B.A. Learning state space trajectories in recurrent neural networks. Neural Comput. 1989, 1, 263–269. [Google Scholar] [CrossRef]
- Gers, F.A.; Schmidhuber, J.; Cummins, F. Learning to forget: Continual prediction with LSTM. Neural Comput. 2000, 12, 2451–2471. [Google Scholar] [CrossRef] [PubMed]
- Staudenmann, D.; Kingma, I.; Stegeman, D.F.; Van Dieën, J.H. Towards optimal multi-channel EMG electrode configurations in muscle force estimation: A high density EMG study. J. Electromyogr. Kinesiol. 2005, 15, 1–11. [Google Scholar] [CrossRef]
- Simon, A.M.; Stern, K.; Hargrove, L.J. A comparison of proportional control methods for pattern recognition control. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Boston, MA, USA, 30 August–3 September 2011. [Google Scholar] [CrossRef]
- Peerdeman, B. Myoelectric forearm prostheses: State of the art from a user-centered perspective. J. Rehabil. Res. Dev. 2011, 48, 719–738. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, J.; Jiang, Y.; Yokoi, H.; Huang, Q. One-channel surface electromyography decomposition for muscle force estimation. Front. Neurorobot. 2018, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Hutabarat, Y.; Owaki, D.; Hayashibe, M. Ground Reaction Force and Moment Estimation through EMG Sensing Using Long Short-Term Memory Network. Cyborg Bionic Syst. in press. 2023. [Google Scholar] [CrossRef]
- Sartori, M.; Reggiani, M.; van den Bogert, A.J.; Lloyd, D.G. Estimation of musculotendon kinematics in large musculoskeletal models using multidimensional B-splines. J. Biomech. 2012, 45, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Maculan, M.; Pizzolato, C.; Reggiani, M.; Farina, D. Modeling and simulating the neuromuscular mechanisms regulating ankle and knee joint stiffness during human locomotion. J. Neurophysiol. 2015, 114, 2509–2527. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, L.; Han, B.; Zhang, T.; Wang, Z.; Wei, P. sEMG-based continuous estimation of knee joint angle using deep learning with convolutional neural network. In Proceedings of the 2019 IEEE 15th International Conference on Automation Science and Engineering (CASE), Vancouver, BC, Canada, 22–26 August 2019; pp. 140–145. [Google Scholar]
- Kim, D.; Koh, K.; Oppizzi, G.; Baghi, R.; Lo, L.C.; Zhang, C.; Zhang, L.Q. Simultaneous Estimations of Joint Angle and Torque in Interactions with Environments using EMG. In Proceedings of the 2020 IEEE International Conference on Robotics and Automation (ICRA), Paris, France, 31 May–31 August 2020. [Google Scholar]
- Schulte, R.V.; Zondag, M.; Buurke, J.H.; Prinsen, E.C. Multi-Day EMG-Based Knee Joint Torque Estimation Using Hybrid Neuromusculoskeletal Modelling and Convolutional Neural Networks. Front. Robot. AI 2022, 9, 869476. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Hu, Y.; Smith, C.; Farewik, E.M.G.; Wang, R. Ankle Joint Torque Estimation Using an EMG-Driven Neuromusculoskeletal Model and an Artificial Neural Network Model. IEEE Trans. Autom. Sci. Eng. 2021, 18, 564–573. [Google Scholar] [CrossRef]
- Zhang, L.; Soselia, D.; Wang, R.; Gutierrez-Farewik, E.M. Lower-Limb Joint Torque Prediction Using LSTM Neural Networks and Transfer Learning. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 600–609. [Google Scholar] [CrossRef]
- Hermens, H.J.; Rau, G.; Disselhorst-Klug, C.; Freriks, B. Surface Electromyography Application Areas and Parameters (SENIAM 3). In Proceedings of the Third General SENIAM Workshop, Aachen, Germany, 15–16 May 1998. [Google Scholar]
- Phinyomark, A.; Phukpattaranont, P.; Limsakul, C. The Usefulness of Wavelet Transform to Reduce Noise in the SEMG Signal. In EMG Methods for Evaluating Muscle and Nerve Function; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Phinyomark, A.; Quaine, F.; Charbonnier, S.; Serviere, C.; Tarpin-Bernard, F.; Laurillau, Y. Feature extraction of the first difference of EMG time series for EMG pattern recognition. Comput. Methods Programs Biomed. 2014, 117, 247–256. [Google Scholar] [CrossRef]
- Robertson, D.G.E.; Caldwell, G.E.; Hamill, J.; Kamen, G.; Whittlesey, S.N. Research Methods in Biomechanics; Human Kinetics Publishers: Champaign, IL, USA, 2014. [Google Scholar] [CrossRef]
- Huang, Y.; Englehart, K.B.; Hudgins, B.; Chan, A.D. A Gaussian mixture model based classification scheme for myoelectric control of powered upper limb prostheses. IEEE Trans. Biomed. Eng. 2005, 52, 1801–1811. [Google Scholar] [CrossRef]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Hicks, J.L.; Uchida, T.K.; Habib, A.; Dembia, C.L.; Dunne, J.J.; Ong, C.F.; DeMers, M.S.; Rajagopal, A.; Millard, M.; et al. OpenSim: Simulating musculoskeletal dynamics and neuromuscular control to study human and animal movement. PLoS Comput. Biol. 2018, 14, e1006223. [Google Scholar] [CrossRef] [PubMed]
- Durandau, G.; Farina, D.; Sartori, M. Robust Real-Time Musculoskeletal Modeling Driven by Electromyograms. IEEE Trans. Biomed. Eng. 2018, 65, 556–564. [Google Scholar] [CrossRef]
- Delp, S.L.; Loan, J.P.; Hoy, M.G.; Zajac, F.E.; Topp, E.L.; Rosen, J.M. An Interactive Graphics-Based Model of the Lower Extremity to Study Orthopaedic Surgical Procedures. IEEE Trans. Biomed. Eng. 1990, 37, 757–767. [Google Scholar] [CrossRef]
- D’Avella, A.; Portone, A.; Fernandez, L.; Lacquaniti, F. Control of fast-reaching movements by muscle synergy combinations. J. Neurosci. 2006, 26, 7791–7810. [Google Scholar] [CrossRef] [PubMed]



| Angle | Moment | |||||||
|---|---|---|---|---|---|---|---|---|
| Knee | Ankle | Knee | Ankle | |||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| S1 | 98.40% | 98.48% | 94.61% | 94.12% | 94.33% | 93.46% | 95.27% | 96.19% |
| S2 | 97.52% | 97.00% | 94.03% | 90.36% | 96.19% | 96.09% | 82.52% | 90.79% |
| S3 | 97.80% | 97.91% | 95.58% | 95.54% | 96.64% | 97.15% | 87.74% | 89.22% |
| S4 | 97.48% | 97.73% | 90.45% | 89.81% | 94.39% | 95.60% | 69.04% | 78.74% |
| S5 | 94.19% | 94.29% | 85.33% | 85.75% | 94.73% | 93.38% | 88.69% | 90.55% |
| S6 | 98.21% | 97.99% | 90.99% | 90.65% | 94.05% | 92.83% | 80.19% | 76.39% |
| Mean ± Std. | 97.25% ± 1.46% | 91.44% ± 3.48% | 94.9% ± 1.4% | 85.44% ± 8.14% | ||||
| Angle | Moment | |||||||
|---|---|---|---|---|---|---|---|---|
| Knee | Ankle | Knee | Ankle | |||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| S1 | 3.20% | 3.20% | 4.20% | 4.40% | 4.10% | 4.60% | 5.70% | 5.10% |
| S2 | 4.90% | 5.20% | 5.90% | 7.60% | 4.30% | 4.50% | 10.20% | 7.70% |
| S3 | 4.30% | 4.10% | 6.30% | 6.20% | 4.40% | 3.70% | 8.20% | 8.20% |
| S4 | 5.60% | 5.40% | 8.30% | 8.70% | 5.40% | 4.70% | 12.20% | 10.00% |
| S5 | 6.60% | 6.50% | 8.40% | 8.80% | 4.50% | 4.70% | 8.00% | 8.20% |
| S6 | 5.10% | 5.30% | 8.40% | 8.20% | 6.40% | 6.60% | 7.70% | 10.30% |
| Mean ± Std. | 4.95% ± 1.1% | 7.12% ± 1.66% | 4.83% ± 0.88% | 8.46% ± 1.98% | ||||
| Angle | Moment [Nm/Kg] | |||||||
|---|---|---|---|---|---|---|---|---|
| Knee | Ankle | Knee | Ankle | |||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| S1 | 3.76 | 3.73 | 2.23 | 2.49 | 0.073 | 0.079 | 0.065 | 0.058 |
| S2 | 6.00 | 6.21 | 2.80 | 3.05 | 0.064 | 0.066 | 0.057 | 0.053 |
| S3 | 6.73 | 5.78 | 3.12 | 3.08 | 0.074 | 0.076 | 0.060 | 0.073 |
| S4 | 5.75 | 5.66 | 2.70 | 2.72 | 0.075 | 0.063 | 0.132 | 0.096 |
| S5 | 8.35 | 8.25 | 4.88 | 4.66 | 0.102 | 0.104 | 0.073 | 0.069 |
| S6 | 5.96 | 5.90 | 3.19 | 3.21 | 0.080 | 0.080 | 0.048 | 0.062 |
| Mean ± Std. | 6.01 ± 1.40 | 3.18 ± 0.80 | 0.078 ± 0.013 | 0.071 ± 0.023 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, M.T.N.; Ali, A.E.A.; Owaki, D.; Hayashibe, M. EMG-Based Estimation of Lower Limb Joint Angles and Moments Using Long Short-Term Memory Network. Sensors 2023, 23, 3331. https://doi.org/10.3390/s23063331
Truong MTN, Ali AEA, Owaki D, Hayashibe M. EMG-Based Estimation of Lower Limb Joint Angles and Moments Using Long Short-Term Memory Network. Sensors. 2023; 23(6):3331. https://doi.org/10.3390/s23063331
Chicago/Turabian StyleTruong, Minh Tat Nhat, Amged Elsheikh Abdelgadir Ali, Dai Owaki, and Mitsuhiro Hayashibe. 2023. "EMG-Based Estimation of Lower Limb Joint Angles and Moments Using Long Short-Term Memory Network" Sensors 23, no. 6: 3331. https://doi.org/10.3390/s23063331
APA StyleTruong, M. T. N., Ali, A. E. A., Owaki, D., & Hayashibe, M. (2023). EMG-Based Estimation of Lower Limb Joint Angles and Moments Using Long Short-Term Memory Network. Sensors, 23(6), 3331. https://doi.org/10.3390/s23063331









