Surface Plasmon Resonance Sensor Based on Core-Shell Fe3O4@SiO2@Au Nanoparticles Amplification Effect for Detection of T-2 Toxin
Abstract
1. Introduction
2. Experimental Procedure
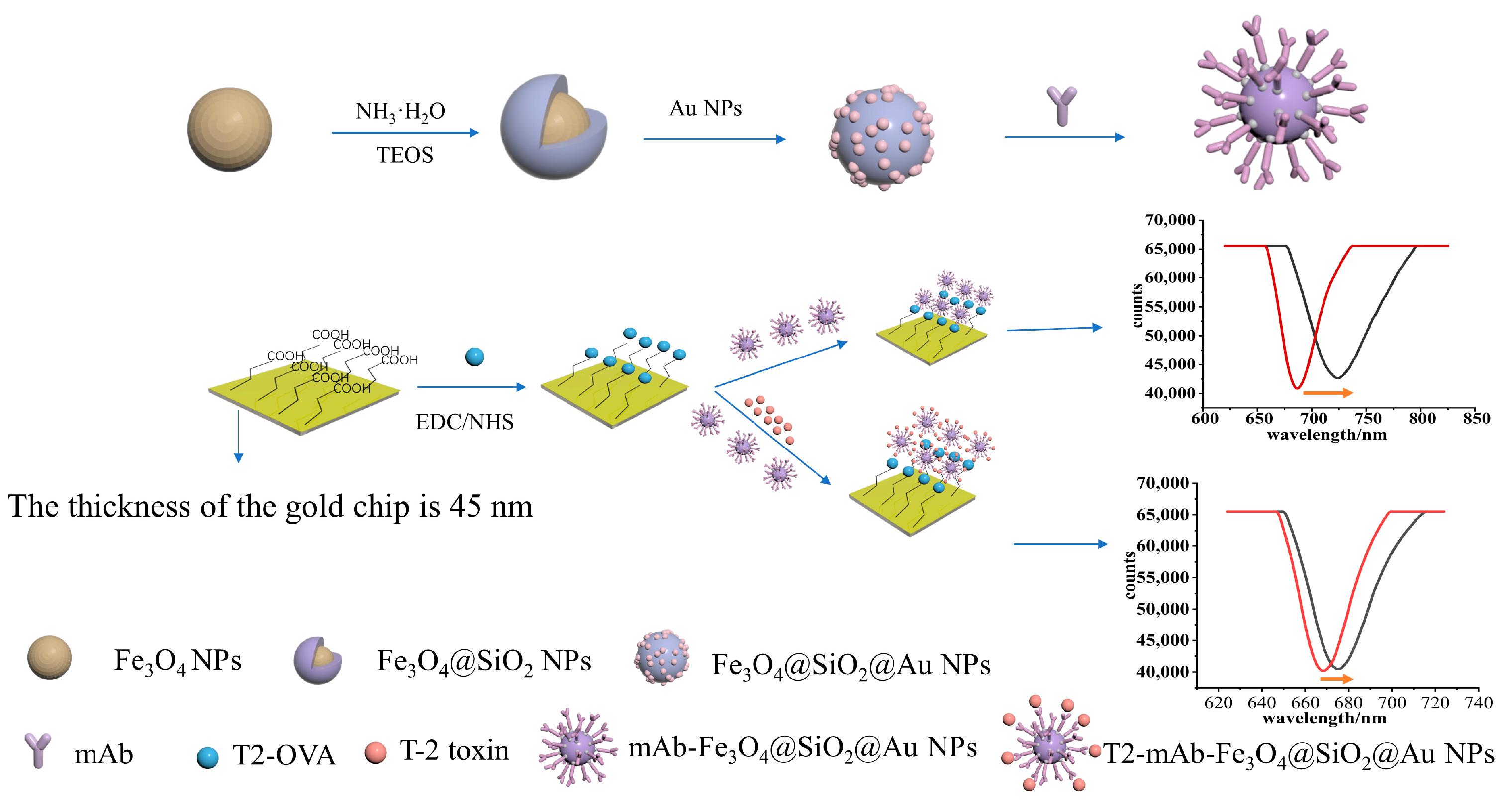
2.1. Synthesis of Fe3O4@SiO2@Au
2.2. Functionalization of the Sensing Chip
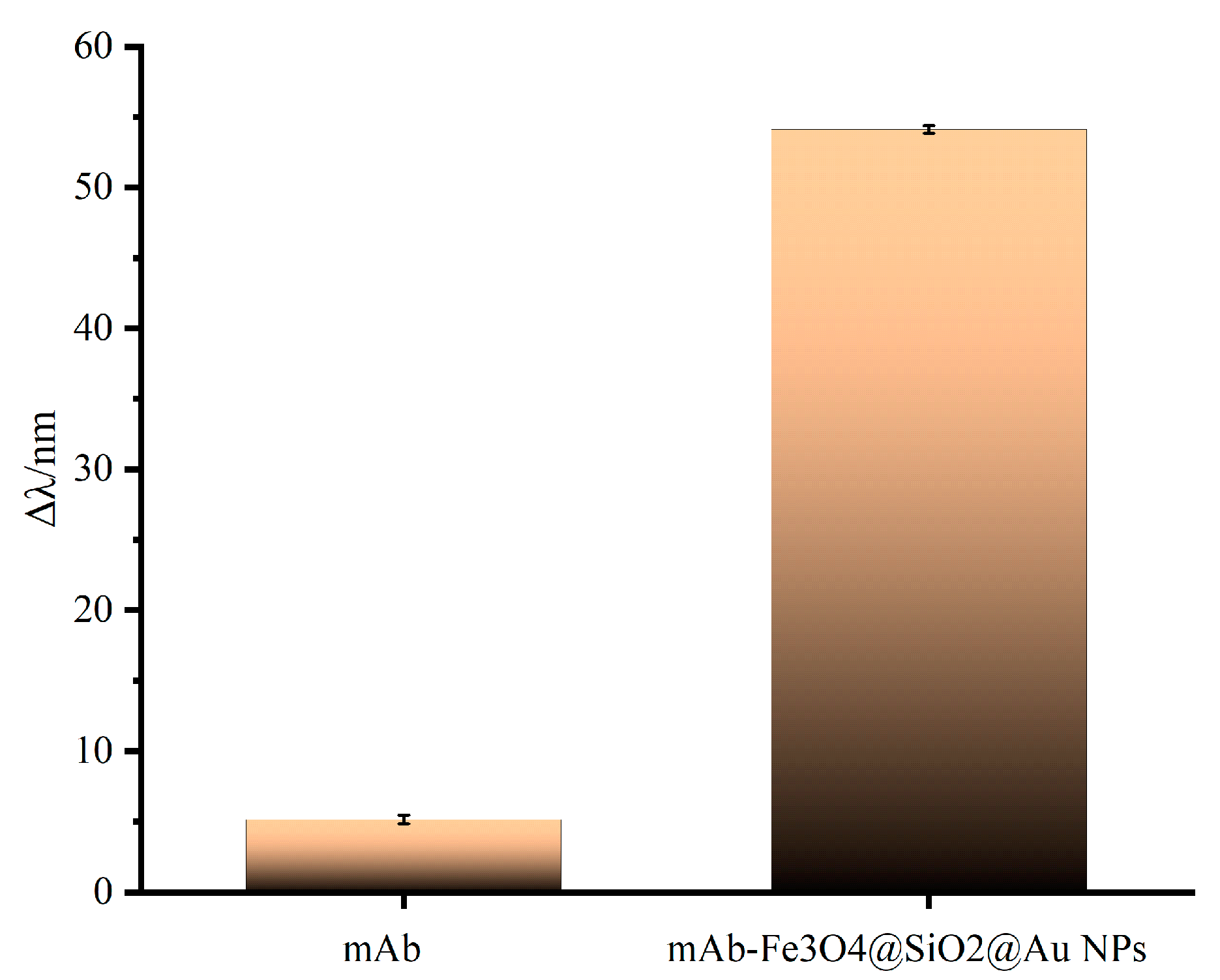
2.3. Preparation of mAb-Fe3O4@SiO2@AuNPs
2.4. Immunoassay
2.5. Specificity and Repeatability
2.6. Analysis of Actual Samples
3. Results and Discussion
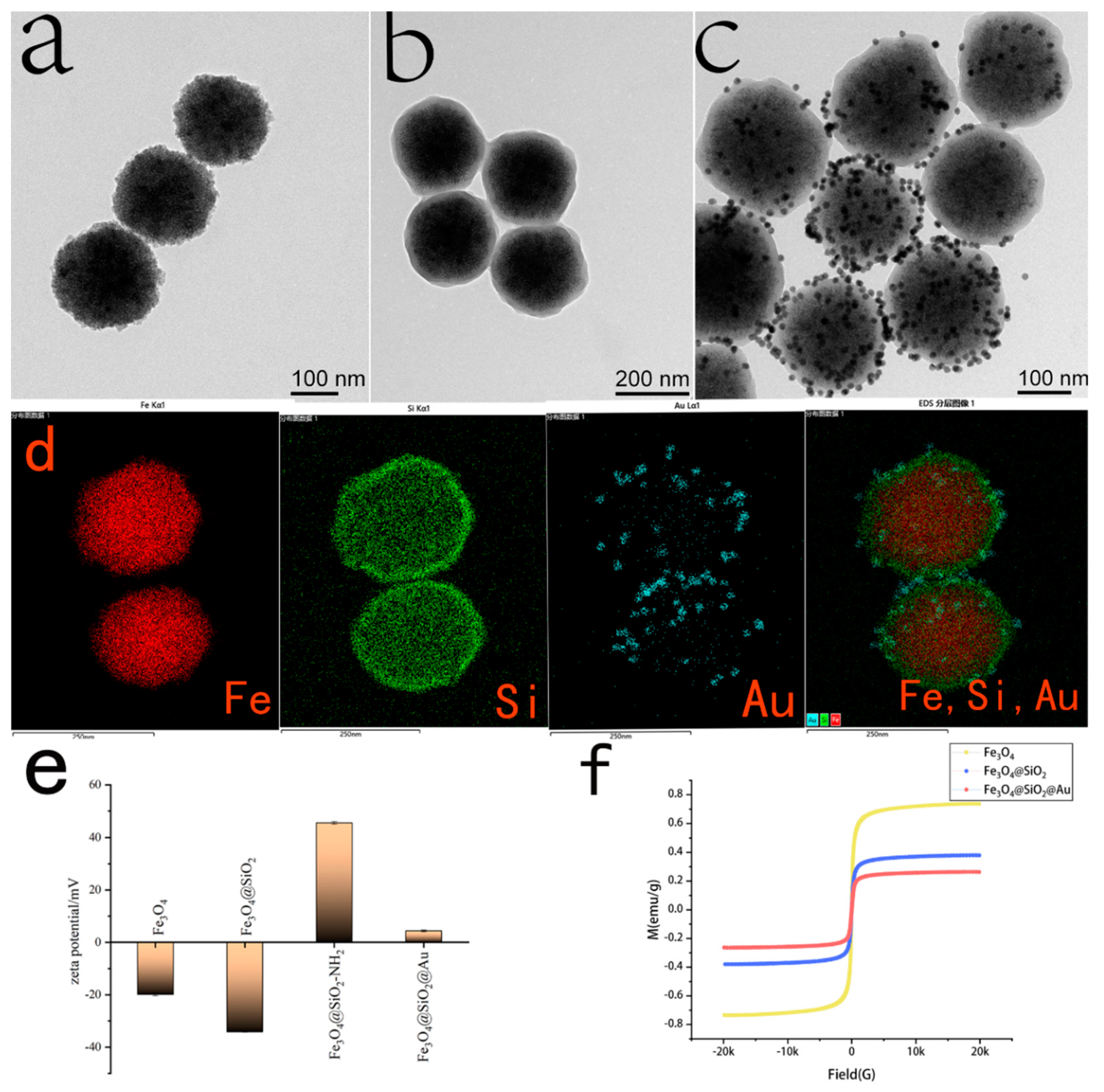
3.1. Characterization of Fe3O4@SiO2@AuMNPs
3.2. Functionalization of the Sensing Chip
3.3. T-2 Toxin Detection
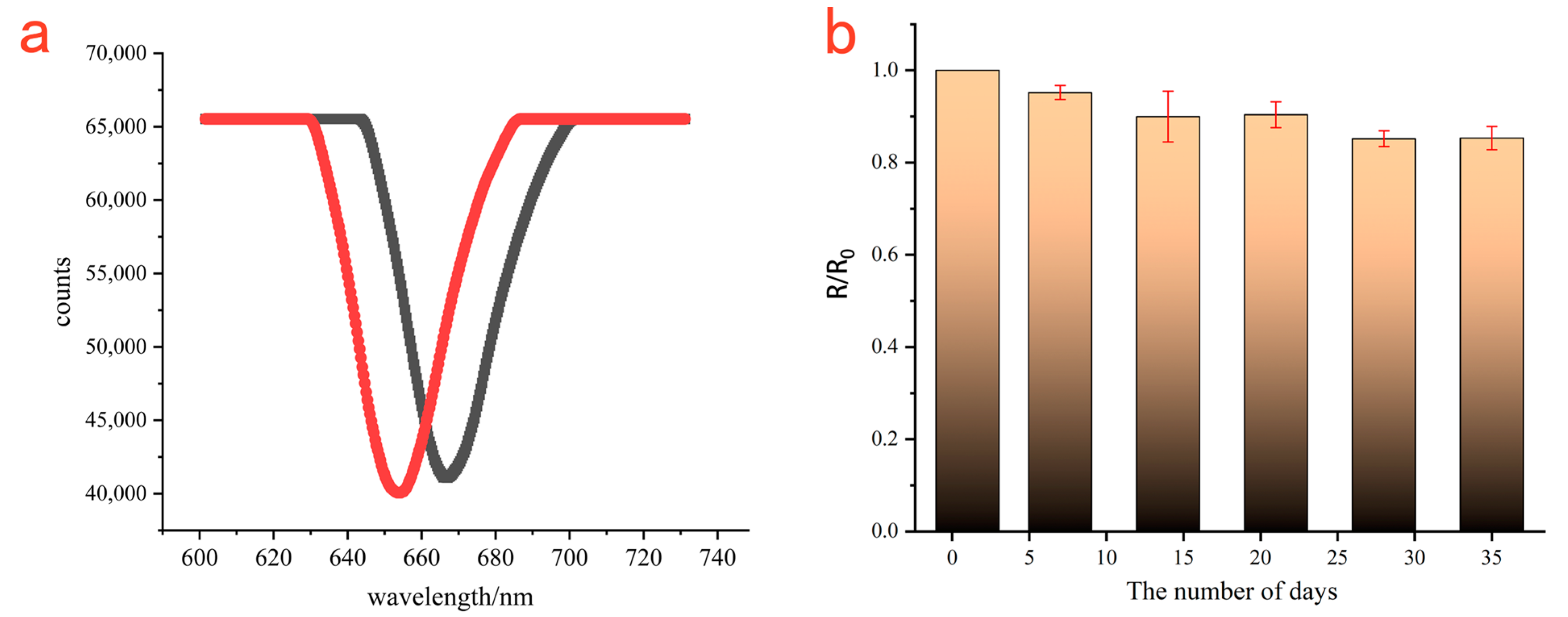
3.4. Specificity and Repeatability of the Strategy
3.5. Analysis of Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thariani, R.; Yager, P. Imaging of surfaces by concurrent surface plasmon resonance and surface plasmon resonance-enhanced fluorescence. PLoS ONE 2010, 5, e9833. [Google Scholar] [CrossRef] [PubMed]
- Kamal Eddin, F.B.; Fen, Y.W.; Omar, N.A.S.; Liew, J.Y.C.; Daniyal, W. Femtomolar detection of dopamine using surface plasmon resonance sensor based on chitosan/graphene quantum dots thin film. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 263, 120202. [Google Scholar] [CrossRef] [PubMed]
- Alagdar, M.; Yousif, B.; Areed, N.F.; Elzalabani, M. Improved the quality factor and sensitivity of a surface plasmon resonance sensor with transition metal dichalcogenide 2D nanomaterials. J. Nanoparticle Res. 2020, 22, 189. [Google Scholar] [CrossRef]
- Bo Liedberg, C.N.; Lundstrom, I. Surface Plasmon resonance for gas detection and biosensing. Sens. Actuators 1983, 4, 299–304. [Google Scholar] [CrossRef]
- Pawula, M.; Altintas, Z.; Tothill, I.E. SPR detection of cardiac troponin T for acute myocardial infarction. Talanta 2016, 146, 823–830. [Google Scholar] [CrossRef]
- Shankaran, D.R.; Gobiand, K.V.; Miura, N. Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food and environmental interest. Sens. Actuators B Chem. 2007, 121, 158–177. [Google Scholar] [CrossRef]
- Guo, X. Fe3O4@Au nanoparticles enhanced surface plasmon resonance for ultrasensitive immunoassay. Sens. Actuators B Chem. 2014, 205, 276–280. [Google Scholar] [CrossRef]
- Philip, A.; Kumar, A.R. The performance enhancement of surface plasmon resonance optical sensors using nanomaterials: A review. Coord. Chem. Rev. 2022, 458, 214424. [Google Scholar] [CrossRef]
- Klestova, Z.; Voronina, A.; Yushchenko, A.; Vatlitsova, O.; Dorozinsky, G.; Ushenin, Y.; Maslov, V.; Doroshenko, T.; Kravchenko, S. Aspects of “antigen-antibody” interaction of chicken infectious bronchitis virus determined by surface plasmon resonance. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120236. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Chang, Y.-F.; Lee, Y.-Y.; Su, L.-C.; Yu, C.-J.; Lin, Y.-H.; Chou, C.; Chiu, N.-F.; Lai, H.-C.; Liu, K.-C. Application of an OLED integrated with BEF and giant birefringent optical (GBO) film in a SPR biosensor. Sens. Actuators B Chem. 2014, 198, 424–430. [Google Scholar] [CrossRef]
- Jang, D.-H.; Choi, Y.; Choi, Y.-S.; Kim, S.-M.; Kwak, H.; Shin, S.-H.; Hong, S. Sensitive and selective analysis of a wide concentration range of IGFBP7 using a surface plasmon resonance biosensor. Colloids Surf. B Biointerfaces 2014, 123, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, J.; Ryu, S.; Kwon, Y.; Kim, K.-H.; Jeong, D.H.; Paik, S.R.; Kim, B.-S. Gold Nanoparticle/Graphene Oxide Hybrid Sheets Attached on Mesenchymal Stem Cells for Effective Photothermal Cancer Therapy. Chem. Mater. 2017, 29, 3461–3476. [Google Scholar] [CrossRef]
- Gao, W.; Li, P.; Qin, S.; Huang, Z.; Cao, Y.; Liu, X. A highly sensitive tetracycline sensor based on a combination of magnetic molecularly imprinted polymer nanoparticles and surface plasmon resonance detection. Mikrochim. Acta 2019, 186, 637. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Peng, Y.; Bai, J.; Zhang, X.; Cui, Y.; Ning, B.; Cui, J.; Gao, Z. Magnetic nanoparticle enhanced surface plasmon resonance sensor for estradiol analysis. Sens. Actuators B Chem. 2018, 254, 629–635. [Google Scholar] [CrossRef]
- Yao, G.-H.; Liang, R.-P.; Huang, C.-F.; Wang, Y.; Qiu, J.-D. Surface plasmon resonance sensor based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal. Chem. 2013, 85, 11944–11951. [Google Scholar] [CrossRef]
- Yakes, B.J.; Kanyuck, K.M.; DeGrasse, S.L. First report of a direct surface plasmon resonance immunosensor for a small molecule seafood toxin. Anal. Chem. 2014, 86, 9251–9255. [Google Scholar] [CrossRef]
- Bi, N.; Chen, Y.; Qi, H.; Zheng, X.; Chen, Y.; Liao, X.; Zhang, H.; Tian, Y. A sensitive localized surface plasmon resonance sensor for determining mercury(II) ion using noble metal nanoparticles as probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 95, 276–281. [Google Scholar] [CrossRef]
- Chen, H.; Qi, F.; Zhou, H.; Jia, S.; Gao, Y.; Koh, K.; Yin, Y. Fe3O4@Au nanoparticles as a means of signal enhancement in surface plasmon resonance spectroscopy for thrombin detection. Sens. Actuators B Chem. 2015, 212, 505–511. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Jiang, S.Z.; Li, C.H.; Xu, S.C.; Yu, J.; Wang, M.H.; Liu, A.H.; Man, B.Y. U-bent fiber optic SPR sensor based on graphene/AgNPs. Sens. Actuators B Chem. 2017, 251, 127–133. [Google Scholar] [CrossRef]
- Lin, K.; Lu, Y.; Chen, J.; Zheng, R.; Wang, P.; Ming, H. Surface plasmon resonance hydrogen sensor based on metallic grating with high sensitivity. Opt. Express 2008, 16, 18599–18604. [Google Scholar] [CrossRef]
- Beccati, D.; Halkes, K.M.; Batema, G.D.; Guillena, G.; de Souza, A.C.; van Koten, G.; Kamerling, J.P. SPR studies of carbohydrate-protein interactions: Signal enhancement of low-molecular-mass analytes by organoplatinum(II)-labeling. Chembiochem 2005, 6, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Z.; Munir, A.; Zhou, H.S. Fe3O4 nanoparticles-enhanced SPR sensing for ultrasensitive sandwich bio-assay. Talanta 2011, 84, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, Y.; Wang, J.; Zhang, J.; Zhang, H.; Zhou, H.; Song, D. Preparation and application of novel nanocomposites of magnetic-Au nanorod in SPR biosensor. Biosens. Bioelectron. 2012, 34, 137–143. [Google Scholar] [CrossRef]
- Grześkowiak, B.F.; Tuśnio, K.; Woźniak, A.; Szalata, M.; Lipiński, D.; Jurga, S.; Słomski, R. Transgenic Plant Detection Using an AuNPs Based SPR Biosensor. Biosensors 2019, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Deymehkar, E.; Taher, M.A.; Karami, C.; Arman, A. Synthesis of SPR Nanosensor using Gold Nanoparticles and its Application to Copper (II) Determination. Silicon 2017, 10, 1329–1336. [Google Scholar] [CrossRef]
- Liang, R.-P.; Yao, G.-H.; Fan, L.-X.; Qiu, J.-D. Magnetic Fe3O4@Au composite-enhanced surface plasmon resonance for ultrasensitive detection of magnetic nanoparticle-enriched alpha-fetoprotein. Anal. Chim. Acta 2012, 737, 22–28. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Beier, R.C.; Shen, J.; De Smet, D.; De Saeger, S.; Zhang, S. T-2 toxin, a trichothecene mycotoxin: Review of toxicity, metabolism, and analytical methods. J. Agric. Food Chem. 2011, 59, 3441–3453. [Google Scholar] [CrossRef]
- Xu, H.; Dong, Y.; Guo, J.; Jiang, X.; Liu, J.; Xu, S.; Wang, H. Monoclonal antibody production and the development of an indirect competitive enzyme-linked immunosorbent assay for screening T-2 toxin in milk. Toxicon 2018, 156, 1–6. [Google Scholar] [CrossRef]
- Guo, T.; Wang, C.; Zhou, H.; Zhang, Y.; Ma, L.; Wang, S. A facile aptasensor based on polydopamine nanospheres for high-sensitivity sensing of T-2 toxin. Anal. Methods 2021, 13, 2654–2658. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, C.; Wen, K.; Jiang, H.; Shen, J.; Zhang, S.; Wang, Z. Comparison of Fluorescent Microspheres and Colloidal Gold as Labels in Lateral Flow Immunochromatographic Assays for the Detection of T-2 Toxin. Molecules 2015, 21, 27. [Google Scholar] [CrossRef]
- Zhai, X.; Cheng, S.; Wang, H.; Zhang, C.; Li, Y.; Dong, W. Dong, Fast preparation of Fe3O4@polydopamine/Au for highly efficient degradation of tetracycline. Chemosphere 2021, 285, 131523. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, H.; Wang, S.; Kong, N.; Xu, H.; Fu, Q.; Gu, H.; Ye, J. Multifunctional superparamagnetic nanoshells: Combining two-photon luminescence imaging, surface-enhanced Raman scattering and magnetic separation. Nanoscale 2014, 6, 14360–14370. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Liu, Y.; Rao, H.; Wang, Y.; Wang, X. Fluorescence and magnetic nanocomposite Fe3O4@SiO2@Au MNPs as peroxidase mimetics for glucose detection. Anal. Biochem. 2017, 538, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.; Gambhir, R.; Vibhute, A.; Tiwari, A.P. Tiwari, Gold Nanoparticles: Synthesis Methods, Functionalization and Biological Applications. J. Clust. Sci. 2022, 4, 1–21. [Google Scholar] [CrossRef]
- Bhandari, D.; Chen, F.-C.; Bridgman, R.C. Magnetic Nanoparticles Enhanced Surface Plasmon Resonance Biosensor for Rapid Detection of Salmonella Typhimurium in Romaine Lettuce. Sensors 2022, 22, 475. [Google Scholar] [CrossRef]
- Bhaskar, S.; Kowshik, N.C.S.S.; Chandran, S.P.; Ramamurthy, S.S. Femtomolar detection of spermidine using Au decorated SiO2 nanohybrid on plasmon-coupled extended cavity nanointerface: A smartphone-based fluorescence dequenching approach. Langmuir 2020, 36, 2865–2876. [Google Scholar] [CrossRef]
- Murphy, C.J.; Sau, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic Metal Nanoparticles: Synthesis, Assembly, and Optical Applications. J. Phys. Chem. B 2010, 36, 13857–13870. [Google Scholar] [CrossRef]
- Gao, X.; Cao, W.; Chen, M.; Xiong, H.; Zhang, X.; Wang, S. A High Sensitivity Electrochemical Sensor Based on Fe3+-Ion Molecularly Imprinted Film for the Detection of T-2 Toxin. Electroanalysis 2014, 26, 2739–2746. [Google Scholar] [CrossRef]
- Meneely, J.P.; Sulyok, M.; Baumgartner, S.; Krska, R.; Elliott, C.T. A rapid optical immunoassay for the screening of T-2 and HT-2 toxin in cereals and maize-based baby food. Talanta 2010, 81, 630–636. [Google Scholar] [CrossRef]



| Analytical Methods | Linear Range | Limit of Detection | Reference |
|---|---|---|---|
| Fluorescent aptasensor | 10~180 ng/mL | 7.23 ng/mL | [29] |
| Electrochemical sensor | 1.12 nM~2.12 μM | 150 ng/mL | [38] |
| SPR screening assay | 250~2000 ng/mL | 6 ng/mL | [39] |
| Lateral-flow immunochromatographic assay (LFIA) | <400 ng/mL | 230 ng/mL | [30] |
| SPR sensor based on Fe3O4@SiO2@Au nanoparticles | 1~100 ng/mL | 0.57 ng/mL | This work |
| Theoretical Value (ng/mL) | Test (ng/mL) | RSD (%) |
|---|---|---|
| 10 | 9.88 | 0.90 |
| 50 | 50.60 | 3.97 |
| 70 | 71.27 | 2.66 |
| Sample | Spiked (ng/mL) | Test (ng/mL) | Recoveries (%) | RSD (%) |
|---|---|---|---|---|
| Milk | 25 | 24.02 | 96.07 | 13.86 |
| 10 | 9.83 | 98.26 | 3.61 | |
| 3 | 2.50 | 83.12 | 2.88 | |
| Soil | 25 | 24.89 | 99.55 | 15.38 |
| 10 | 9.74 | 97.45 | 7.87 | |
| 3 | 2.77 | 92.47 | 2.11 | |
| Rain water | 25 | 22.91 | 91.65 | 5.58 |
| 10 | 10.84 | 108.41 | 0.67 | |
| 3 | 2.79 | 92.93 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Du, B.; Pei, F.; Hu, W.; Guo, A.; Xie, Z.; Liu, B.; Tong, Z.; Mu, X.; Tan, W. Surface Plasmon Resonance Sensor Based on Core-Shell Fe3O4@SiO2@Au Nanoparticles Amplification Effect for Detection of T-2 Toxin. Sensors 2023, 23, 3078. https://doi.org/10.3390/s23063078
Fan L, Du B, Pei F, Hu W, Guo A, Xie Z, Liu B, Tong Z, Mu X, Tan W. Surface Plasmon Resonance Sensor Based on Core-Shell Fe3O4@SiO2@Au Nanoparticles Amplification Effect for Detection of T-2 Toxin. Sensors. 2023; 23(6):3078. https://doi.org/10.3390/s23063078
Chicago/Turabian StyleFan, Lirui, Bin Du, Fubin Pei, Wei Hu, Aijiao Guo, Zihao Xie, Bing Liu, Zhaoyang Tong, Xihui Mu, and Wenyuan Tan. 2023. "Surface Plasmon Resonance Sensor Based on Core-Shell Fe3O4@SiO2@Au Nanoparticles Amplification Effect for Detection of T-2 Toxin" Sensors 23, no. 6: 3078. https://doi.org/10.3390/s23063078
APA StyleFan, L., Du, B., Pei, F., Hu, W., Guo, A., Xie, Z., Liu, B., Tong, Z., Mu, X., & Tan, W. (2023). Surface Plasmon Resonance Sensor Based on Core-Shell Fe3O4@SiO2@Au Nanoparticles Amplification Effect for Detection of T-2 Toxin. Sensors, 23(6), 3078. https://doi.org/10.3390/s23063078




