Use of Lower Limb Exoskeletons as an Assessment Tool for Human Motor Performance: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
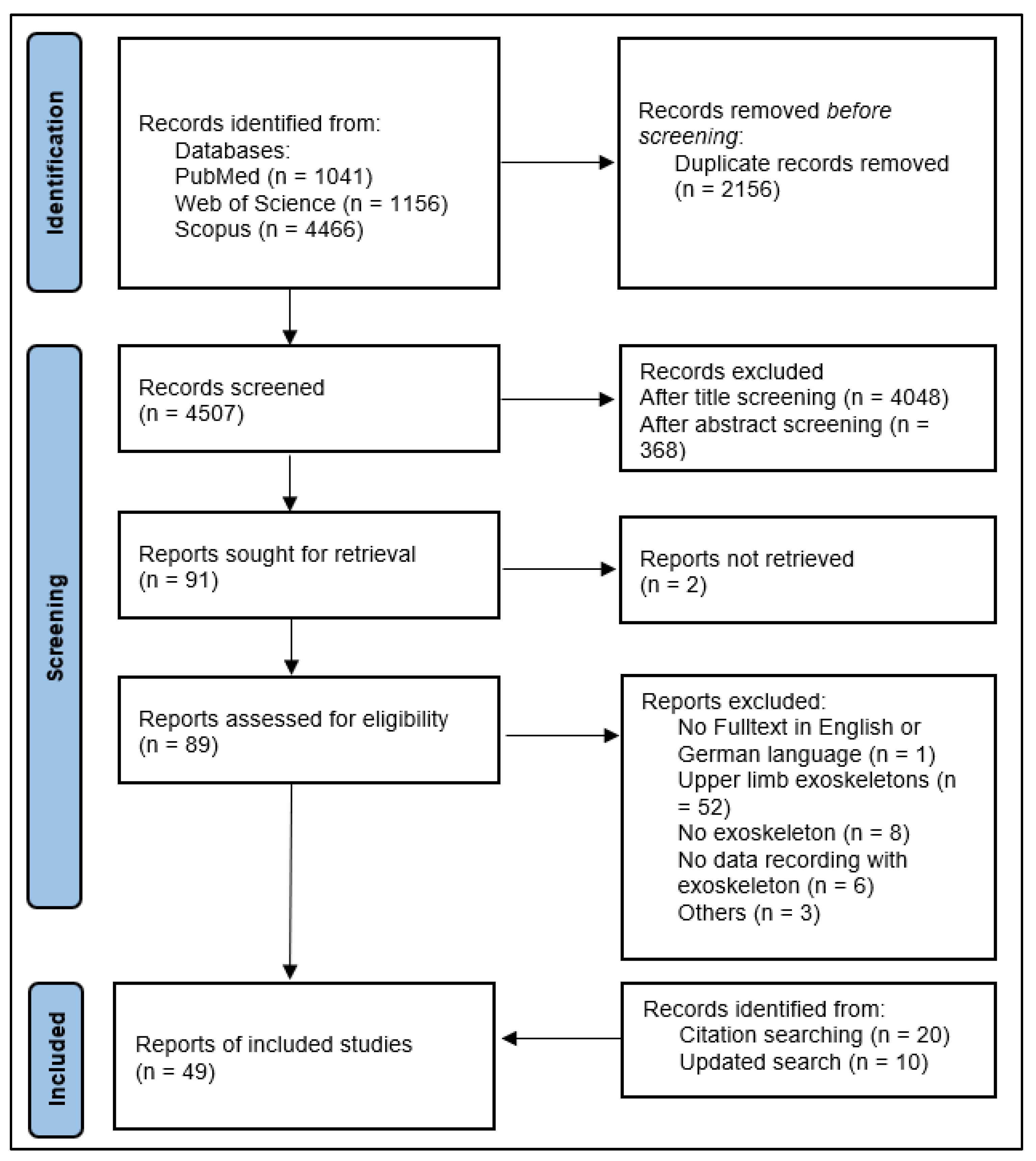
2.4. Selection Progress
2.5. Data Collection Process and Data Items
2.6. Study Risk of Bias Assessment
3. Results
| Author | Exoskeleton | Motor Performance Parameter | Sensors | Task | VS | RS |
|---|---|---|---|---|---|---|
| Joint Angles | ||||||
| Lunenburger et al. [36] | Lokomat (full leg; stationary) | Hip and knee angle | Position sensors (potentiometers) | n.d. | X | X |
| Chaparro-Rico et al. [37] | X | X | ||||
| Hu et al. [38] | Lower extremity exoskeleton (full leg; stationary) | Hip, knee and ankle angle | Encoder (hip), three degree-of-freedom (3-DOF) magnetic sensor/a pantographic exoskeleton sensor | Sit to Stand exercise | ✓ | X |
| Bryan et al. [39] | Exoskeleton emulator system (full leg; stationary) | Hip, knee and ankle angle | Magnetic rotary encoders | Walking on a treadmill | X | X |
| Agrawal et al. [40] | Gravity balancing orthosis (hip and knee; stationary) | Hip and knee angle | Optical joint encoders (USDigital, 2500 CPR, 1 kHz, Vancouver, WA, USA) | n.d. | X | X |
| Banala et al. [41] | X | X | ||||
| Veneman et al. [42] | LOPES Exoskeleton (hip and knee; stationary) | Hip and knee angle | n.d. | Sagittal walking on a treadmill | ✓ | X |
| Fan and Yin [43] | Standing bed exoskeleton (hip and knee; stationary) | Hip and knee angle | Angular encoder (200 Hz) | n.d. | X | X |
| Koginov et al. [44] | Myosuit (hip and knee, mobile) | Thigh angle | 5 IMUs (2 on each shank and thigh, 1 at the back, 100 Hz) | n.d. | ✓ | X |
| Zhang et al. [45] | Single-joint robotic hip exoskeleton (hip; mobile) | Thigh angle | IMU (50 Hz) | n.d. | X | X |
| Molinaro et al. [46] | Robotic hip exoskeleton (hip; mobile) | Hip angle | Absolute magnetic encoders (Orbis, Renishaw, Wotton-under-Edge, UK; 100 Hz) | n.d. | X | X |
| d’Elia et al. [47] | Active pelvis orthosis (APO) (hip; mobile) | Hip angle | 2 absolute 17- bit Rotary Electric Encoder™ units (DS-37 + DS-25 Netzer Precision Motion Sensors Ltd., Misgav, Israel) | Walking on a treadmill | ✓ | X |
| Buesing et al. [48] | Honda Stride Management Assist (hip; mobile) | Hip angle | Angular sensors | n.d. | X | X |
| Pinheiro et al. [49] | Ankle–foot exoskeleton (ankle; mobile) | Ankle angle | Potentiometer (resolution: 0.5°; 100 Hz), four strain gauges, FSR (toe and heel) | Walking at 1 km/h | X | X |
| Bolus et al. [50] | Instrumented ankle–foot orthosis (ankle; mobile) | Ankle angle | Optical encoder (S4T, US Digital, 1 kHz, Vancouver, WA, USA), 4 IMUs (MTw Series, XSense, 50 Hz, Enschede, The Netherlands) | Walking on a treadmill at 1 m/s | X | X |
| Satici et al. [51] | SUkorpion AR (ankle; mobile) | Ankle angle | Angular encoder | n.d. | X | X |
| Park et al. [52] | Active Soft Orthotic Device (ankle; mobile) | Ankle angle | Custom-built strain sensor; IMUs | n.d. | ✓ | X |
| Aíin et al. [53] | MAFO (motorized ankle foot orthosis) (ankle; mobile) | Ankle joint angular position | n.d. | n.d. | X | X |
| Durandau et al. [54] | Symbitron Exoskeleton (only ankle modules) (ankle; mobile) | Ankle angle | Rotational encoder (16 b MHM, IC Haus, Bodenheim, Germany) | n.d. | X | X |
| Dambreville et al. [55] | Electrohydraulic robotized ankle–foot orthosis (ankle; mobile) | Sagittal plane ankle angle | Optical encoder | n.d. | X | X |
| Proprioception | ||||||
| Chisholm et al. [56] | Lokomat (full leg; stationary) | Proprioception (hip and knee) | Angular encoder/potentiometer |
| ✓ | ✓ |
| Domingo et al. [57] | X | X | ||||
| Domingo and Lam [58] | ✓ | ✓ | ||||
| Dambreville et al. [55] | Electrohydraulic robotized ankle–foot orthosis (ankle; mobile) | Proprioception (ankle) | Optical encoder, load cell | Walking on a treadmill, pushing a button if perturbation is remarked | X | ✓ |
| Gait Phase, Spatio-temporal Gait Parameters and Walking Ability | ||||||
| Maggioni et al. [59] | Lokomat (full leg; stationary) | Walking ability (based on the required amount of support) | force sensors, potentiometers (hip and knee angles) | n.d. | X | X |
| Lonini et al. [60] | ReWalk (full leg; mobile) | Walking ability score (step frequency, standard deviation of the frontal angle, approximated energy expenditure, number of steps) | Accelerometer (Actigraph, ActiGraph LLC, Pensacola, FL, USA) on exoskeleton (mid-sagittal position, 20 cm above hip) | 6MWT | (✓) | X |
| Gambon et al. [61] | EksoGT exoskeleton (full leg; mobile) | Stride time + length, gait speed + events | Resistive force sensor (heel and toe), motor encoder | Level ground walking at self-selected speed | X | X |
| Li et al. [62] | Unilateral rehabilitation exoskeleton robot (full leg; mobile) | Gait phase | Infrared Distance Sensors | Level ground walking at self-selected speed | ✓ | X |
| Xia et al. [63] | Passive lower limb weight-bearing exoskeleton (full leg; mobile) | Gait phase | IMU (thigh and shank, 2000 Hz) | Treadmill walking | ✓ | X |
| Kang et al. [64] | Powered hip exoskeleton (hip; mobile) | Gait phase, walking speed | Angular encoder (hip), IMU (Micro USB, Yost Lab, Portsmouth, OH, USA) (trunk + thigh) | Level ground walking at self-selected speed (between 0.8 m/s and 1.2 m/s) | ✓ | X |
| Kang et al. [65] | Gait Enhancing and Motivating System (hip; mobile) | Gait phase | FSR (only [65]), Angular encoder (hip), IMU (Micro USB, Yost Lab) (trunk + thigh); sampling rate: 100 Hz [65]; 200 Hz [66] | n.d. | X | X |
| Kang et al. [66] | X | X | ||||
| Zhang et al. [45] | Single-joint robotic hip exoskeleton (hip; mobile) | Gait phase estimation (based on thigh angle and thigh acceleration) | IMU (50 Hz) | Different walking tasks for validation study (treadmill; level ground) | ✓ | X |
| Zhang et al. [67] | Hip joint lower limb exoskeleton (hip; mobile) | Gait phase | IMU (thigh) | Level ground walking, Stair walking (up and down) | X | X |
| Crea et al. [68] | Active pelvis orthosis (APO) (hip; mobile) | Gait phase | Capacitive pressure sensors | Treadmill walking at different speed | ✓ | X |
| Cao et al. [69] | Soft lower limb exoskeleton (hip; mobile) | Gait Phase | IMU (1000 Hz) | n.d. | X | X |
| Yu et al. [70] | Portable knee exoskeleton (knee; mobile) | Gait phase | IMUs (HI219M, HiPNUC Technology, 200 Hz) | Treadmill walking at different speed | ✓ | X |
| Pinheiro et al. [49] | Ankle–foot exoskeleton (ankle; mobile) | Gait phase | Potentiometer (resolution: 0.5°; 100 Hz), four strain gauges (resolution: 1 Nm, 100 Hz), FSR (toe and heel, 100 Hz) | Walking at 1 km/h | X | X |
| Bolus et al. [50] | Instrumented ankle–foot orthosis (ankle; mobile) | Gait phase | Optical encoder (S4T, US Digital, 1 kHz), 4 IMUs (MTw Series, XSense, 50 Hz), strain gauge-based reaction torque sensor (TFF350, Futek, 1 kHz), FSR (model 42, Interlink Elect., 75 Hz), pressure-sensitive capacitive films (Pedar and Pliance, Novel, 50 Hz). | Walking on a treadmill at 1 m/s | X | X |
| Joint Torque and Strength | ||||||
| Galen et al. [71] | Lokomat (full leg; stationary) | Maximum voluntary isometric Hip, Knee and ankle (only [72]) torque/strength | Force transducers (integrated in every joint actuator), potentiometer | Maximal isometric contraction against the exoskeleton | ✓ | X |
| Cherni et al. [73] | ✓ | ✓ | ||||
| Chaparro-Rico et al. [37] | X | X | ||||
| Lunenburger et al. [36] | X | X | ||||
| Tan and Dhaher [72] | X | X | ||||
| Bolliger et al. [74] | X | ✓ | ||||
| Cruz and Dhaher [75] | Motorized, instrumented exoskeletal orthosis (full leg; stationary) | Hip and knee torque | Load cells (thigh, proximal shank, and distal shank; sample rate: 1 kHz) | Maximal isometric contraction against the exoskeleton in fixed position | X | X |
| Agrawal et al. [40] | Gravity balancing orthosis (hip and knee; stationary) | Hip and knee torque | Optical joint encoders (USDigital, 1 kHz), two built-in force-torque sensors (ATI, 1 kHz) | n.d. | X | X |
| Banala et al. [41] | X | X | ||||
| Fan and Yin [43] | Standing bed exoskeleton (hip and knee; stationary) | Muscular strength (isometric and isokinetic) | Angular encoder (200 Hz); Force sensor (air pressure sensor; 200 Hz) | n.d. | X | X |
| Rea et al. [76] | X1 exoskeleton (full leg; mobile) | Isokinetic, isotonic, and isometric muscle strength, torque, rate of change of torque, | n.d. | n.d. | (✓) | (✓) |
| Naghavi et al. [77] | FUM HEXA-I (hip; mobile) | Strength index for hip extension/flexion | Beam-type load-cells, 16-bit incremental angular encoder | Treadmill walking (self-selected speed) | X | X |
| Molinaro et al. [46] | Robotic hip exoskeleton (hip; mobile) | Hip torque | Absolute magnetic encoders (Orbis, Renishaw, UK; 100 Hz), IMU sensors (100 Hz) | Walking on the ground/ascending ramp/descending ramp | ✓ | X |
| Aíin et al. [53] | MAFO (motorized ankle foot orthosis) (ankle; mobile) | Ankle joint torque | n.d. | n.d. | X | X |
| Satici et al. [51] | SUkorpion AR (ankle; mobile) | Ankle torque | Angular encoder | n.d. | X | X |
| Stiffness/Spasticity/Impedance | ||||||
| Riener et al. [78] | Lokomat (full leg; stationary) | Hip and knee spasticity | Force transducers (integrated in every joint actuator), potentiometer | Automated movement of the tested joints; participant‘s legs are 100% unloaded | X | X |
| Lunenburger et al. [36] | ✓ | X | ||||
| Chaparro-Rico et al. [37] | X | X | ||||
| Cherni et al. [79] | X | ✓ | ||||
| Koopman et al. [80] | LOPES Exoskeleton (hip and knee; stationary) | Hip and knee impedance | Potentiometers on the exoskeleton (angles; 100 Hz) and potentiometers in the SEA (torque; 100 Hz) | Two positions
| X | X |
| Mendoza-Crespo et al. [81] | H2 robotic exoskeleton (full leg; mobile) | Ankle spasticity | Force sensors | n.d. | X | X |
| Nazon et al. [82] | Torque-controllable exoskeleton (knee and ankle; mobile) | Knee impedance | SSubmicron resolution optical encoders (ATOM; Renishaw, Wotton-under-Edge, Gloucestershire, UK) | n.d. | (✓) | X |
| Roy et al. [83] | MIT’s ankle robot system (ankle; mobile) | Ankle stiffness | Linear incremental encoders (Renishaw, Chicago, IL; resolution: 5 × 10−6 m), analog current sensors (Interactive Motion Technologies; resolution: 2.59 × 10−6 Nm); | Moving the ankle in two planes (sagittal and frontal) | X | X |
| Roy et al. [84] | X | X | ||||
| Satici et al. [51] | SUkorpion AR (ankle; mobile) | Ankle impedance (joint angles + torques) | Angular encoder, | n.d. | X | X |
3.1. Study Characteristics
3.2. Risk of Bias in Studies
3.3. Results of Included Studies
3.3.1. Joint Angles
Stationary Exoskeletons
Mobile Exoskeletons or Actuated Orthoses
3.3.2. Proprioception
Stationary Exoskeletons
Mobile Exoskeletons or Actuated Orthoses
3.3.3. Gait Phase, Spatio–Temporal Gait Parameters and Walking Ability
Stationary Exoskeletons
Mobile Exoskeletons or Actuated Orthoses
3.3.4. Muscle Strength and Joint Torques
Stationary Exoskeletons
Mobile Exoskeletons or Actuated Orthoses
3.3.5. Stiffness/Spasticity/Impedance
Stationary Exoskeletons
Mobile Exoskeletons or Actuated Orthoses
4. Discussion
4.1. Studies and Devices Which Used or Tested Lower Limb Exoskeletons to Asses Motor Performance
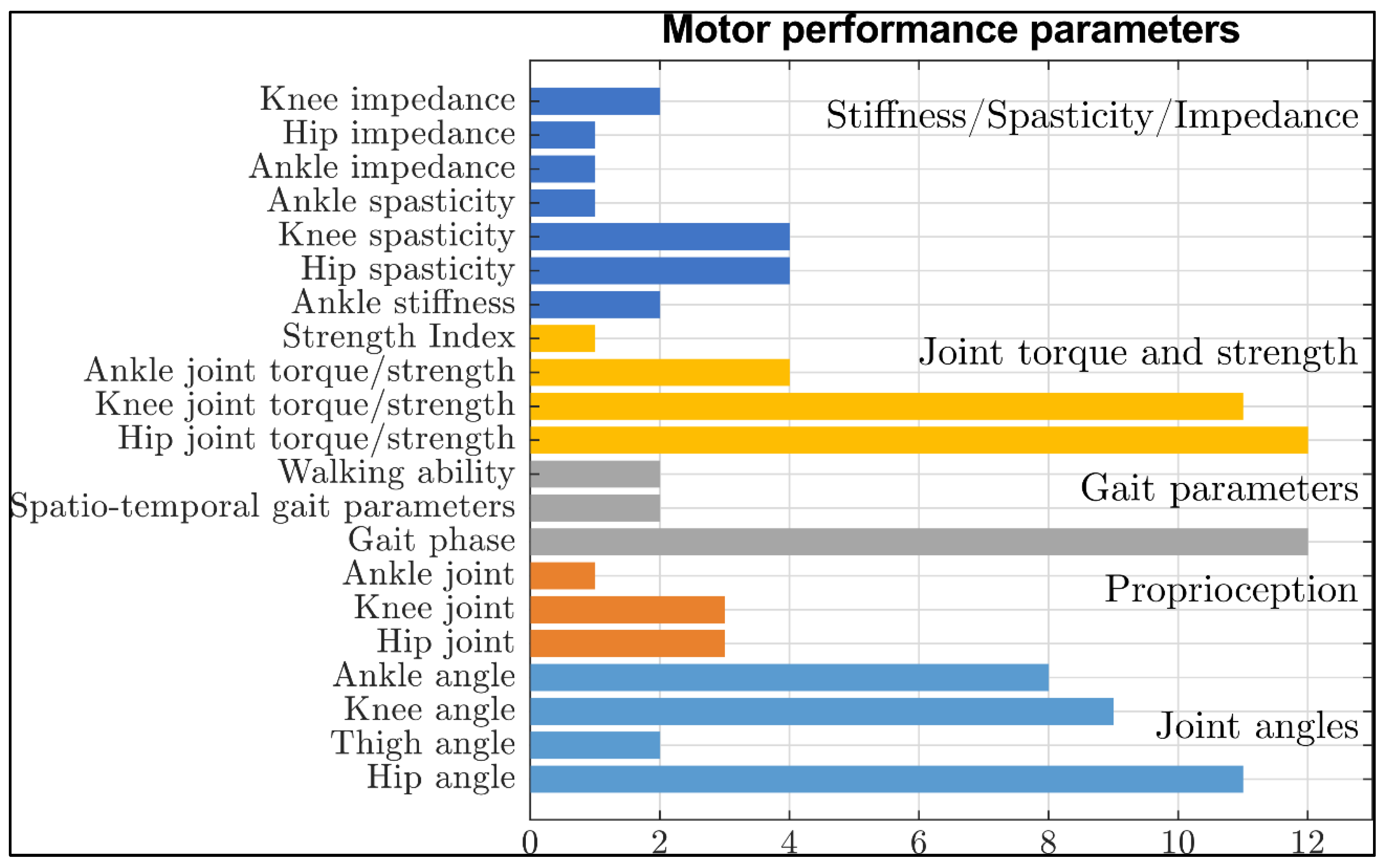
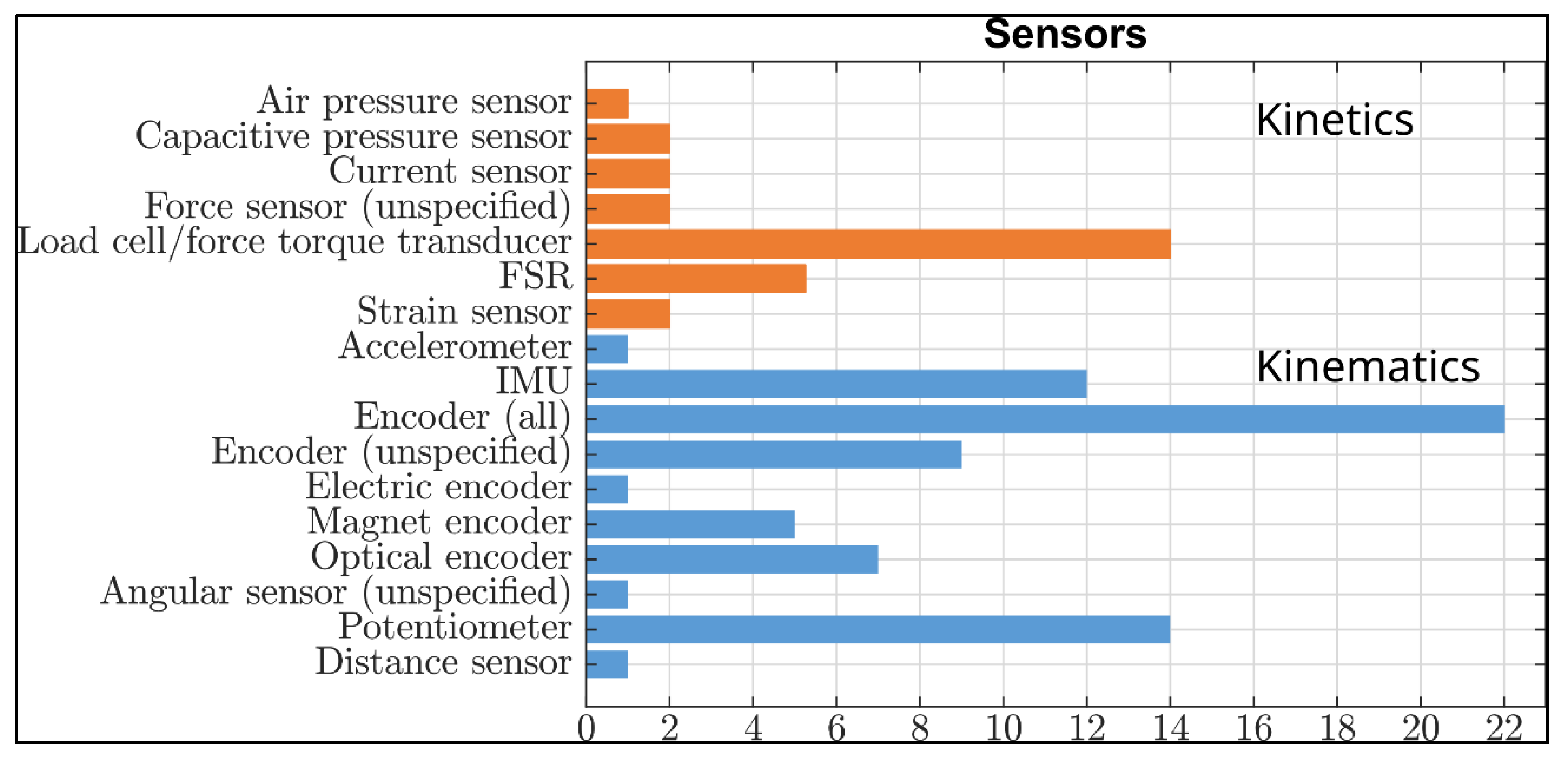
4.2. Parameters of Motor Performance, That Can Be or Have Been Measured by Lower Limb Exoskeletons
4.3. Approaches to Assess Motor Performance through Lower Limb Exoskeletons
4.4. Motor Performance Parameters
4.4.1. Joint Angles
4.4.2. Proprioception
4.4.3. Gait Phase, Spatio–Temporal Gait Parameters and Walking Ability
4.4.4. Muscle strength and Joint Torques
4.4.5. Spasticity/Stiffness/Impedance
4.5. Limitations
4.6. Recommendations for Future Developments and Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kell, R.T.; Bell, G.; Quinney, A. Musculoskeletal fitness, health outcomes and quality of life. Sport. Med. 2001, 31, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation 2003, 107, 346–354. [Google Scholar] [CrossRef] [PubMed]
- van Abellan Kan, G. Epidemiology and consequences of sarcopenia. J. Nutr. Health Aging 2009, 13, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Stathokostas, L.; McDonald, M.W.; Little, R.M.D.; Paterson, D.H. Flexibility of older adults aged 55–86 years and the influence of physical activity. J. Aging Res. 2013, 2013, 743843. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- McGibbon, C.A. Toward a better understanding of gait changes with age and disablement: Neuromuscular adaptation. Exerc. Sport Sci. Rev. 2003, 31, 102–108. [Google Scholar] [CrossRef]
- Granacher, U.; Bridenbaugh, S.A.; Muehlbauer, T.; Wehrle, A.; Kressig, R.W. Age-related effects on postural control under multi-task conditions. Gerontology 2011, 57, 247–255. [Google Scholar] [CrossRef]
- Brault, M.W. Americans with Disabilities: 2010: Household Economic Studies. Washington. 2012. Available online: https://www2.census.gov/library/publications/2012/demo/p70-131.pdf (accessed on 12 January 2022).
- Wheelchair Foundation. Worldwide Need—Wheelchair Foundation. Available online: https://www.wheelchairfoundation.org/fth/analysis-of-wheelchair-need/ (accessed on 24 January 2022).
- World Health Organization, The World Bank. World Report on Disability 2011F; WHO: Geneva, Switzerland, 2011; ISBN 9789241564182. [Google Scholar]
- Sawicki, G.S.; Beck, O.N.; Kang, I.; Young, A.J. The exoskeleton expansion: Improving walking and running economy. J. Neuroeng. Rehabil. 2020, 17, 25. [Google Scholar] [CrossRef]
- Owens, J.G.; Rauzi, M.R.; Kittelson, A.; Graber, J.; Bade, M.J.; Johnson, J.; Nabhan, D. How New Technology Is Improving Physical Therapy. Curr. Rev. Musculoskelet. Med. 2020, 13, 200–211. [Google Scholar] [CrossRef]
- Bogue, R. Robotic exoskeletons: A review of recent progress. Ind. Robot. Int. J. Robot. Res. Appl. 2015, 42, 5–10. [Google Scholar] [CrossRef]
- Lee, H.; Kim, W.; Han, J.; Han, C. The technical trend of the exoskeleton robot system for human power assistance. Int. J. Precis. Eng. Manuf. 2012, 13, 1491–1497. [Google Scholar] [CrossRef]
- Baud, R.; Manzoori, A.R.; Ijspeert, A.; Bouri, M. Review of control strategies for lower-limb exoskeletons to assist gait. J. Neuroeng. Rehabil 2021, 18, 119. [Google Scholar] [CrossRef]
- Neťuková, S.; Bejtic, M.; Malá, C.; Horáková, L.; Kutílek, P.; Kauler, J.; Krupička, R. Lower Limb Exoskeleton Sensors: State-of-the-Art. Sensors 2022, 22, 9091. [Google Scholar] [CrossRef]
- Tiboni, M.; Borboni, A.; Vérité, F.; Bregoli, C.; Amici, C. Sensors and Actuation Technologies in Exoskeletons: A Review. Sensors 2022, 22, 884. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, Y.; Zheng, J.; Dong, D.; Chen, X.; Bai, L. From sensing to control of lower limb exoskeleton: A systematic review. Annu. Rev. Control 2022, 53, 83–96. [Google Scholar] [CrossRef]
- Duncan, E.A.S.; Murray, J. The barriers and facilitators to routine outcome measurement by allied health professionals in practice: A systematic review. BMC Health Serv. Res. 2012, 12, 96. [Google Scholar] [CrossRef]
- Li, X.; Dunn, J.; Salins, D.; Zhou, G.; Zhou, W.; Schüssler-Fiorenza Rose, S.M.; Perelman, D.; Colbert, E.; Runge, R.; Rego, S.; et al. Digital Health: Tracking Physiomes and Activity Using Wearable Biosensors Reveals Useful Health-Related Information. PLoS Biol. 2017, 15, e2001402. [Google Scholar] [CrossRef]
- Mittelmark, M.B.; Bauer, G.F. The Meanings of Salutogenesis. In The Handbook of Salutogenesis; Springer: Cham, Switzerland, 2017; pp. 7–13. [Google Scholar] [CrossRef]
- Nuredini, G.; Saunders, A.; Rajkumar, C.; Okorie, M. Current status of white coat hypertension: Where are we? Ther. Adv. Cardiovasc. Dis. 2020, 14, 1753944720931637. [Google Scholar] [CrossRef]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the medical revolution. Per. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef]
- de Angelis, M.; Lavorgna, L.; Carotenuto, A.; Petruzzo, M.; Lanzillo, R.; Brescia Morra, V.; Moccia, M. Digital Technology in Clinical Trials for Multiple Sclerosis: Systematic Review. J. Clin. Med. 2021, 10, 2328. [Google Scholar] [CrossRef]
- Taborri, J.; Pasinetti, S.; Cardinali, L.; Perroni, F.; Rossi, S. Preventing and Monitoring Work-Related Diseases in Firefighters: A Literature Review on Sensor-Based Systems and Future Perspectives in Robotic Devices. Int. J. Environ. Res. Public Health 2021, 18, 9723. [Google Scholar] [CrossRef] [PubMed]
- Lambercy, O.; Maggioni, S.; Lünenburger, L.; Gassert, R.; Bolliger, M. Robotic and Wearable Sensor Technologies for Measurements/Clinical Assessments. In Neurorehabilitation Technology, 2nd ed.; Reinkensmeyer, D.J., Dietz, V., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 183–207. ISBN 978-3-319-28601-3. [Google Scholar]
- Balasubramanian, S.; Colombo, R.; Sterpi, I.; Sanguineti, V.; Burdet, E. Robotic assessment of upper limb motor function after stroke. Am. J. Phys. Med. Rehabil. 2012, 91, S255–S269. [Google Scholar] [CrossRef] [PubMed]
- Losey, D.P.; McDonald, C.G.; Battaglia, E.; O’Malley, M.K. A Review of Intent Detection, Arbitration, and Communication Aspects of Shared Control for Physical Human–Robot Interaction. Appl. Mech. Rev. 2018, 70, 10804. [Google Scholar] [CrossRef]
- Maggioni, S.; Melendez-Calderon, A.; van Asseldonk, E.; Klamroth-Marganska, V.; Lünenburger, L.; Riener, R.; van der Kooij, H. Robot-aided assessment of lower extremity functions: A review. J. Neuroeng. Rehabil. 2016, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Shirota, C.; van Asseldonk, E.; Matjačić, Z.; Vallery, H.; Barralon, P.; Maggioni, S.; Buurke, J.H.; Veneman, J.F. Robot-supported assessment of balance in standing and walking. J. Neuroeng. Rehabil. 2017, 14, 80. [Google Scholar] [CrossRef]
- Toigo, M.; Flück, M.; Riener, R.; Klamroth-Marganska, V. Robot-assisted assessment of muscle strength. J. Neuroeng. Rehabil. 2017, 14, 103. [Google Scholar] [CrossRef]
- Dobri, S.C.; Ready, H.M.; Davies, T.C. Tools and Techniques Used with Robotic Devices to Quantify Upper-Limb Function in Typically Developing Children: A Systematic Review. Rehabil. Process Outcome 2020, 9, 1179572720979013. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef]
- National Heart, Lung and Blood Institute. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 20 March 2022).
- Lunenburger, L.; Colombo, G.; Riener, R.; Dietz, V. Clinical Assessments Performed During Robotic Rehabilitation by the Gait Training Robot Lokomat. In Proceedings of the 2005 IEEE 9th International Conference on Rehabilitation Robotics, Chicago, IL, USA, 28 June–1 July 2005; IEEE Operations Center: Piscataway, NJ, USA, 2005; pp. 345–348, ISBN 0-7803-9003-2. [Google Scholar]
- Chaparro-Rico, B.D.M.; Cafolla, D.; Tortola, P.; Galardi, G. Assessing Stiffness, Joint Torque and ROM for Paretic and Non-Paretic Lower Limbs during the Subacute Phase of Stroke Using Lokomat Tools. Appl. Sci. 2020, 10, 6168. [Google Scholar] [CrossRef]
- Hu, G.; Jiang, J.; Lee, K. Parametric and Noise Effects on Magnetic Sensing System for Monitoring Human-Joint Motion of Lower Extremity in Sagittal Plane. IEEE Sens. J. 2023, 23, 4729–4739. [Google Scholar] [CrossRef]
- Bryan, G.M.; Franks, P.W.; Song, S.; Voloshina, A.S.; Reyes, R.; O’Donovan, M.P.; Gregorczyk, K.N.; Collins, S.H. Optimized hip-knee-ankle exoskeleton assistance at a range of walking speeds. J. Neuroeng. Rehabil. 2021, 18, 1–12. [Google Scholar] [CrossRef]
- Agrawal, S.K.; Banala, S.K.; Fattah, A.; Sangwan, V.; Krishnamoorthy, V.; Scholz, J.P.; Hsu, W.-L. Assessment of motion of a swing leg and gait rehabilitation with a gravity balancing exoskeleton. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 410–420. [Google Scholar] [CrossRef]
- Banala, S.K.; Agrawal, S.K.; Fattah, A.; Krishnamoorthy, V.; Hsu, W.-L.; Scholz, J.; Rudolph, K. Gravity-Balancing Leg Orthosis and Its Performance Evaluation. IEEE Trans. Rob. 2006, 22, 1228–1239. [Google Scholar] [CrossRef]
- Veneman, J.F.; Kruidhof, R.; Hekman, E.E.G.; Ekkelenkamp, R.; van Asseldonk, E.H.F.; van der Kooij, H. Design and evaluation of the LOPES exoskeleton robot for interactive gait rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 379–386. [Google Scholar] [CrossRef]
- Fan, Y.J.; Yin, Y.H. Active and Progressive Exoskeleton Rehabilitation Using Multisource Information Fusion from EMG and Force-Position EPP. IEEE Trans. Biomed. Eng. 2013, 60, 3314–3321. [Google Scholar] [CrossRef]
- Koginov, G.; Sternberg, K.; Wolf, P.; Schmidt, K.; Duarte, J.E.; Riener, R. An algorithm to reduce human-robot interface compliance errors in posture estimation in wearable robots. Wearable Technol. 2022, 3, e30. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Zhou, M.; Xu, W. An adaptive framework of real-time continuous gait phase variable estimation for lower-limb wearable robots. Robot. Amd. Auton. Syst. 2021, 143, 103842. [Google Scholar] [CrossRef]
- Molinaro, D.D.; Kang, I.; Camargo, J.; Young, A.J. Biological Hip Torque Estimation using a Robotic Hip Exoskeleton. In Proceedings of the 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), New York, NY, USA, 29 November–1 December 2020; IEEE: Piscataway, NJ, USA, 2020. [Google Scholar]
- d’Elia, N.; Vanetti, F.; Cempini, M.; Pasquini, G.; Parri, A.; Rabuffetti, M.; Ferrarin, M.; Molino Lova, R.; Vitiello, N. Physical human-robot interaction of an active pelvis orthosis: Toward ergonomic assessment of wearable robots. J. Neuroeng. Rehabil. 2017, 14, 29. [Google Scholar] [CrossRef]
- Buesing, C.; Fisch, G.; O’Donnell, M.; Shahidi, I.; Thomas, L.; Mummidisetty, C.K.; Williams, K.J.; Takahashi, H.; Rymer, W.Z.; Jayaraman, A. Effects of a wearable exoskeleton stride management assist system (SMA®) on spatiotemporal gait characteristics in individuals after stroke: A randomized controlled trial. J. Neuroeng. Rehabil. 2015, 12, 69. [Google Scholar] [CrossRef]
- Pinheiro, C.; Figueiredo, J.; Magalhaes, N.; Santos, C.P. Wearable Biofeedback Improves Human-Robot Compliance during Ankle-Foot Exoskeleton-Assisted Gait Training: A Pre-Post Controlled Study in Healthy Participants. Sensors 2020, 20, 5876. [Google Scholar] [CrossRef] [PubMed]
- Bolus, N.B.; Teague, C.N.; Inan, O.T.; Kogler, G.F. Instrumented Ankle–Foot Orthosis: Toward a Clinical Assessment Tool for Patient-Specific Optimization of Orthotic Ankle Stiffness. IEEE–ASME Trans. Mechatron. 2017, 22, 2492–2501. [Google Scholar] [CrossRef]
- Satici, A.C.; Erdogan, A.; Patoglu, V. Design of a reconfigurable ankle rehabilitation robot and its use for the estimation of the ankle impedance. In Proceedings of the 2009 IEEE International Conference on Rehabilitation Robotics, ICORR 2009, Kyoto, Japan, 23–26 June 2009; pp. 257–264. [Google Scholar] [CrossRef]
- Park, Y.-L.; Chen, B.; Young, D.; Stirling, L.; Wood, R.J.; Goldfield, E.; Nagpal, R. Bio-inspired active soft orthotic device for ankle foot pathologies. In Proceedings of the 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems, San Francisco, CA, USA, 25–30 September 2011; IEEE: Piscataway, NJ, USA; pp. 4488–4495, ISBN 978-1-61284-456-5. [Google Scholar]
- Aíin, G.; Barroso, F.A.; Moreno, J.C.; Pons, J.L. Assessment of the suitability of the Motorized ankle-foot orthosis as a diagnostic and rehabilitation tool for gait. In Proceedings of the NEUROTECHNIX 2013—Proceedings of the International Congress on Neurotechnology, Electronics and Informatics, Vilamoura, Algarve, Portugal, 18–20 September 2013; pp. 161–166. [Google Scholar]
- Durandau, G.; Rampeltshammer, W.F.; Kooij, H.; Sartori, M. Neuromechanical Model-Based Adaptive Control of Bilateral Ankle Exoskeletons: Biological Joint Torque and Electromyogram Reduction Across Walking Conditions. IEEE Trans. Rob. 2022, 38, 1380–1394. [Google Scholar] [CrossRef]
- Dambreville, C.; Pairot de Fontenay, B.; Blanchette, A.K.; Roy, J.-S.; Mercier, C.; Bouyer, L. Ankle proprioception during gait in individuals with incomplete spinal cord injury. Physiol. Rep. 2019, 7, e14328. [Google Scholar] [CrossRef]
- Chisholm, A.E.; Domingo, A.; Jeyasurya, J.; Lam, T. Quantification of Lower Extremity Kinesthesia Deficits Using a Robotic Exoskeleton in People with a Spinal Cord Injury. Neurorehabil. Neural. Repair. 2016, 30, 199–208. [Google Scholar] [CrossRef]
- Domingo, A.; Marriott, E.; de Grave, R.B.; Lam, T. Quantifying lower limb joint position sense using a robotic exoskeleton: A pilot study. IEEE Int. Conf. Rehabil. Robot. 2011, 2011, 5975455. [Google Scholar] [CrossRef]
- Domingo, A.; Lam, T. Reliability and validity of using the Lokomat to assess lower limb joint position sense in people with incomplete spinal cord injury. J. Neuroeng. Rehabil. 2014, 11, 167. [Google Scholar] [CrossRef]
- Maggioni, S.; Lunenburger, L.; Riener, R.; Melendez-Calderon, A. Robot-aided assessment of walking function based on an adaptive algorithm. In Proceedings of the 2015 IEEE International Conference on Rehabilitation Robotics (ICORR 2015), Singapore, 11–14 August 2015; Yu, H., Ed.; IEEE: Piscataway, NJ, USA, 2015; pp. 804–809, ISBN 978-1-4799-1808-9. [Google Scholar]
- Lonini, L.; Shawen, N.; Scanlan, K.; Rymer, W.Z.; Kording, K.P.; Jayaraman, A. Accelerometry-enabled measurement of walking performance with a robotic exoskeleton: A pilot study. J. Neuroeng. Rehabil. 2016, 13, 35. [Google Scholar] [CrossRef]
- Gambon, T.M.; Schmiedeler, J.P.; Wensing, P.M. Effects of User Intent Changes on Onboard Sensor Measurements During Exoskeleton-Assisted Walking. IEEE Access 2020, 8, 224071–224082. [Google Scholar] [CrossRef]
- Li, C.; He, Y.; Chen, T.; Chen, X.; Tian, S. Real-Time Gait Event Detection for a Lower Extremity Exoskeleton Robot by Infrared Distance Sensors. IEEE Sens. J. 2021, 21, 27116–27123. [Google Scholar] [CrossRef]
- Xia, Y.; Li, J.; Yang, D.; Wei, W. Gait Phase Classification of Lower Limb Exoskeleton Based on a Compound Network Model. Symmetry 2023, 15, 163. [Google Scholar] [CrossRef]
- Kang, I.; Kunapuli, P.; Hsu, H.; Young, A.J. Electromyography (EMG) Signal Contributions in Speed and Slope Estimation Using Robotic Exoskeletons. In Proceedings of the IEEE International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 548–553. [Google Scholar] [CrossRef]
- Kang, I.; Kunapuli, P.; Young, A.J. Real-Time Neural Network-Based Gait Phase Estimation Using a Robotic Hip Exoskeleton. IEEE Trans. Med. Robot. Bionics 2020, 2, 28–37. [Google Scholar] [CrossRef]
- Kang, I.; Molinaro, D.D.; Duggal, S.; Chen, Y.; Kunapuli, P.; Young, A.J. Real-Time Gait Phase Estimation for Robotic Hip Exoskeleton Control During Multimodal Locomotion. IEEE Robot. Autom. 2021, 6, 3491–3497. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Hu, J.; Zheng, J.; Wang, X.; Deng, J.; Wan, Z.; Wang, H.; Wang, Y. Gait Pattern Identification and Phase Estimation in Continuous Multilocomotion Mode Based on Inertial Measurement Units. IEEE Sens. J. 2022, 22, 16952–16962. [Google Scholar] [CrossRef]
- Crea, S.; Manca, S.; Parri, A.; Zheng, E.; Mai, J.; Lova, R.M.; Vitiello, N.; Wang, Q. Controlling a Robotic Hip Exoskeleton with Noncontact Capacitive Sensors. IEEE/ASME Trans. Mechatron. 2019, 24, 2227–2235. [Google Scholar] [CrossRef]
- Cao, W.; Ma, Y.; Chen, C.; Zhang, J.; Wu, X. Hardware Circuits Design and Performance Evaluation of a Soft Lower Limb Exoskeleton. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 384–394. [Google Scholar] [CrossRef]
- Yu, S.; Yang, J.; Huang, T.-H.; Zhu, J.; Visco, C.J.; Hameed, F.; Stein, J.; Zhou, X.; Su, H. Artificial Neural Network-Based Activities Classification, Gait Phase Estimation, and Prediction. Ann. Biomed. Eng. 2023. [Google Scholar] [CrossRef]
- Galen, S.S.; Clarke, C.J.; Mclean, A.N.; Allan, D.B.; Conway, B.A. Isometric hip and knee torque measurements as an outcome measure in robot assisted gait training. NeuroRehabilitation 2014, 34, 287–295. [Google Scholar] [CrossRef]
- Tan, A.Q.; Dhaher, Y.Y. Evaluation of lower limb cross planar kinetic connectivity signatures post-stroke. J. Biomech. 2014, 47, 949–956. [Google Scholar] [CrossRef]
- Cherni, Y.; Girardin-Vignola, G.; Ballaz, L.; Begon, M. Reliability of maximum isometric hip and knee torque measurements in children with cerebral palsy using a paediatric exoskeleton—Lokomat. Neurophysiol. Clin. 2018, 49, 335–342. [Google Scholar] [CrossRef]
- Bolliger, M.; Banz, R.; Dietz, V.; Lünenburger, L. Standardized voluntary force measurement in a lower extremity rehabilitation robot. J. Neuroeng. Rehabil. 2008, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Cruz, T.H.; Dhaher, Y.Y. Evidence of abnormal lower-limb torque coupling after stroke: An isometric study. Stroke 2008, 39, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Rea, R.; Beck, C.; Rovekamp, R.; Diftler, M.; Neuhaus, P. X1: A robotic exoskeleton for in-space countermeasures and dynamometry. In Proceedings of the AIAA SPACE 2013 Conference and Exposition, San Diego, CA, USA, 10–12 September 2013. [Google Scholar]
- Naghavi, N.; Akbarzadeh, A.; Tahamipour-Z, S.M.; Kardan, I. Assist-As-Needed control of a hip exoskeleton based on a novel strength index. Robot. Amd. Auton. Syst. 2020, 134, 103667. [Google Scholar] [CrossRef]
- Riener, R.; Brunschweiler, A.; Lünenburger, L.; Colombo, G. Robot-supported spasticity evaluation. In Proceedings of the 9th Annual Conference of the International FES Society, Bournemouth, UK, 6–9 September 2004. [Google Scholar]
- Cherni, Y.; Ballaz, L.; Girardin-Vignola, G.; Begon, M. Intra- and inter-tester reliability of spasticity assessment in standing position in children and adolescents with cerebral palsy using a paediatric exoskeleton. Disabil. Rehabil. 2021, 43, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Koopman, B.; van Asseldonk, E.F.; van der Kooij, H. In vivo measurement of human knee and hip dynamics using MIMO system identification. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2010, 2010, 3426–3429. [Google Scholar] [CrossRef]
- Mendoza-Crespo, R.; Soto, R.; Pons, J.L. Velocity dependant spasticity detection for active exoskeleton based therapies. Biosyst. Biorobotics 2017, 15, 1491–1495. [Google Scholar] [CrossRef]
- Nazon, Y.; Doshi, R.; Rouse, E. Validation of Methods for Estimation of Knee Joint Mechanical Impedance During Locomotion Using a Torque-Controllable Knee Exoskeleton. J. Biomech. Eng. 2021, 144, 41005. [Google Scholar] [CrossRef]
- Roy, A.; Krebs, H.I.; Williams, D.J.; Bever, C.T.; Forrester, L.W.; Macko, R.M.; Hogan, N. Robot-Aided Neurorehabilitation: A Novel Robot for Ankle Rehabilitation. IEEE Trans. Robot. 2009, 25, 569–582. [Google Scholar] [CrossRef]
- Roy, A.; Krebs, H.I.; Bever, C.T.; Forrester, L.W.; Macko, R.F.; Hogan, N. Measurement of passive ankle stiffness in subjects with chronic hemiparesis using a novel ankle robot. J. Neurophysiol. 2011, 105, 2132–2149. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: A scoping review. Syst. Rev. 2017, 6, 263. [Google Scholar] [CrossRef]
- Riener, R. Technology of the Robotic Gait Orthosis Lokomat. In Neurorehabilitation Technology, 1st ed.; Dietz, V., Nef, T., Rymer, W.Z., Eds.; Springer Limited: London, UK, 2012; pp. 221–232. ISBN 978-1-4471-2276-0. [Google Scholar]
- Taylor, M.M.; Creelman, C.D. PEST: Efficient Estimates on Probability Functions. J. Acoust. Soc. Am. 1967, 41, 782–787. [Google Scholar] [CrossRef]
- Marino, R.J.; Barros, T.; Biering-Sorensen, F.; Burns, S.P.; Donovan, W.H.; Graves, D.E.; Haak, M.; Hudson, L.M.; Priebe, M.M. International standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 2003, 26 (Suppl. 1), S50–S56. [Google Scholar] [CrossRef]
- Bezold, J.; Krell-Roesch, J.; Eckert, T.; Jekauc, D.; Woll, A. Sensor-based fall risk assessment in older adults with or without cognitive impairment: A systematic review. Eur. Rev. Aging Phys. Act. 2021, 18, 15. [Google Scholar] [CrossRef]
- Ibrahim, H.K.; Gabli, M.; Peyrodie, L. Anticipation of falls from a structure for paraplegics by intelligent methods. In Proceedings of the 2022 IEEE 3rd International Conference on Electronics, Control, Optimization and Computer Science (ICECOCS), Fez, Morocco, North Africa, 1–2 December 2022; IEEE: Piscataway, NJ, USA; pp. 1–4, ISBN 978-1-6654-5723-1. [Google Scholar]
- Li, H.; Yu, H.; Chen, Y.; Tang, X.; Wang, D.; Meng, Q.; Du, Q. Design of a Minimally Actuated Lower Limb Exoskeleton with Mechanical Joint Coupling. J. Bionic. Eng. 2022, 19, 370–389. [Google Scholar] [CrossRef]
- Hnat, S.K.; Audu, M.L.; Triolo, R.J.; Quinn, R.D. Estimating Center of Mass Kinematics During Perturbed Human Standing Using Accelerometers. J. Appl. Biomech. 2021, 37, 415–424. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Hadjiosif, A.M.; Xu, J.; Wong, A.L.; Haith, A.M. Motor Learning. Compr. Physiol. 2019, 9, 613–663. [Google Scholar] [CrossRef]
- Bartenbach, V. Constraints Caused by Lower Extremity Exoskeletons. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 2017. [Google Scholar]
- Poggensee, K.L.; Collins, S.H. How adaptation, training, and customization contribute to benefits from exoskeleton assistance. Sci. Robot. 2021, 6, eabf1078. [Google Scholar] [CrossRef]
- Zhou, L.; Fischer, E.; Tunca, C.; Brahms, C.M.; Ersoy, C.; Granacher, U.; Arnrich, B. How We Found Our IMU: Guidelines to IMU Selection and a Comparison of Seven IMUs for Pervasive Healthcare Applications. Sensors 2020, 20, 4090. [Google Scholar] [CrossRef]
- Payton, C.J. Biomechanical Evaluation of Movement in Sport and Exercise: The British Association of Sport and Exercise Sciences Guide, 2nd ed.; Taylor and Francis: Florence, Italy, 2016; ISBN 978-0-415-63264-5. [Google Scholar]
- Chen, X.; Qu, X. Age-Related Differences in the Relationships Between Lower-Limb Joint Proprioception and Postural Balance. Hum. Factors 2019, 61, 702–711. [Google Scholar] [CrossRef]
- Otalora, S.; Ballen-Moreno, F.; Arciniegas-Mayag, L.; Munera, M.; Cifuentes, C.A. The AGoRA V2 Unilateral Lower-Limb Exoskeleton: Mechatronic Integration and Biomechanical Assessment. IEEE Robot. Autom. 2022, 7, 7928–7933. [Google Scholar] [CrossRef]
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. Quantitative gait markers and incident fall risk in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, S.; Betschart, M.; Bethoux, F. Gait analysis for poststroke rehabilitation: The relevance of biomechanical analysis and the impact of gait speed. Phys. Med. Rehabil. Clin. N. Am. 2013, 24, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.J. Influence of signal filtering and sample rate on isometric torque—Time parameters using a traditional isokinetic dynamometer. J. Biomech. 2019, 83, 235–242. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, L.L.; de Koff, M.A.C.; Ribbers, G.M.; Selles, R.W. The diagnostic levels of evidence of instrumented devices for measuring viscoelastic joint properties and spasticity; a systematic review. J. Neuroeng. Rehabil. 2022, 19, 16. [Google Scholar] [CrossRef]
- Sposito, M.; Poliero, T.; Di Natali, C.; Semprini, M.; Barresi, G.; Laffranchi, M.; Caldwell, D.G.; de Michieli, L.; Ortiz, J. Exoskeletons in Elderly Healthcare. In Internet of Things for Human-Centered Design: Application to Elderly Healthcare, 1st ed.; Scataglini, S., Imbesi, S., Marques, G., Eds.; Springer: Singapore, 2022; pp. 353–374. ISBN 978-981-16-8487-6. [Google Scholar]
- Moeller, T.; Krell-Roesch, J.; Woll, A.; Stein, T. Effects of Upper-Limb Exoskeletons Designed for Use in the Working Environment-A Literature Review. Front. Robot. AI 2022, 9, 858893. [Google Scholar] [CrossRef]
- Gao, M.; Wang, Z.; Pang, Z.; Sun, J.; Li, J.; Li, S.; Zhang, H. Electrically Driven Lower Limb Exoskeleton Rehabilitation Robot Based on Anthropomorphic Design. Machines 2022, 10, 266. [Google Scholar] [CrossRef]
- Ringhof, S.; Patzer, I.; Beil, J.; Asfour, T.; Stein, T. Does a Passive Unilateral Lower Limb Exoskeleton Affect Human Static and Dynamic Balance Control? Front. Sport. Act. Living 2019, 1, 22. [Google Scholar] [CrossRef]
- Lambercy, O.; Lünenburger, L.; Gassert, R.; Bolliger, M. Robots for Measurement/Clinical Assessment. In Neurorehabilitation Technology, 1st ed.; Dietz, V., Nef, T., Rymer, W.Z., Eds.; Springer Limited: London, UK, 2012; pp. 443–456. ISBN 978-1-4471-2276-0. [Google Scholar]
- United Nations. World Population Ageing: 2017 Highlights; United Nations: New York, NY, USA, 2017; ISBN 978921151551. [Google Scholar]

| Author | Exoskeleton | Participants | Validation Tool | Protocol | Results |
|---|---|---|---|---|---|
| Joint Angles | |||||
| Hu et al. [38] | Lower extremity exoskeleton (full limb, stationary) | N = 1 (1♂) health: all healthy | Vicon motion capture system (Oxford Metric, Oxford, UK) | Sit to stand exercise | Human-robot hip angle deviation
|
| Veneman et al. [42] | LOPES Exoskeleton (hip and knee; stationary) | N = 10 age: 26 health: all unimpaired | PTI-VZ4000 mocap system from PhoeniX Technologies (Campbell, CA, USA) | Treadmill walking |
|
| Koginov et al. [44] | Myosuit (hip and knee, mobile) | N = 8 (4♂, 4♀) health: all healthy | Vicon motion capture system (Oxford Metric, Oxford, UK) | Standing and Treadmill walking with different support modes (1-3) and different speed (0.8 m/s, 1.3 m/s) | Human-robot hip angle deviation RMSE:
|
| d’Elia et al. [47] | Active pelvis orthosis (APO) (hip; mobile) | N = 5 age: 29.2 ± 6.3 health: all healthy | optoelectronic system (SmartD, BTS, Milan, Italy) | Treadmill walking with three different speeds (slow, normal and fast; depending on leg length) and different modes (transparent, low, moderate and high assistance) | Human-robot hip angle deviation RMSE:
|
| Park et al. [52] | Active Soft Orthotic Device (ankle; mobile) | n.d. | Goniometer | Moving the ankle freely in plantar and dorsiflexion | Mean error:
|
| Proprioception | |||||
| Chisholm et al. [56] | Lokomat (full leg; stationary) | N = 34 (26♂, 8♀) age: 39.5 ± 10.2 (abled bodied) 39.5 ± 9.7 (SCI) health: n = 17 abled bodied n = 17 SCI | Manual assessment | 2 assessments of robotic lower limb joint proprioception separated by one week; manual assessment of proprioception | Test–retest reliability Hip:
|
| Domingo and Lam [58] | Lokomat (full leg; stationary) | N = 46 (28♂, 18♀) age: 37.8 ± 14.1 (abled bodied) 40.5 ± 14.0 (SCI) health: n = 23 abled bodied n = 23 SCI | Manual assessment | First participant was moved to the target position for 5 s and second to the starting position passively; participant must replicate the target position; manual assessment of proprioception | Test–retest reliability Hip:
|
| Dambreville et al. [55] | Electrohydraulic robotized ankle–foot orthosis (ankle; mobile) | N = 25 (13♂, 12♀) age: 22.88 ± 2.63 health: all healthy | Only reliability study | Treadmill walking; exoskeleton induces perturbations during gait; push a button when they felt a perturbation |
|
| Gait Phase, Spatio-temporal Gait Parameters and Walking Ability | |||||
| Lonini et al. [60] | ReWalk (full leg; mobile) | N = 11 (6♂, 5♀) age: 26.9 ± 14 health: n = 6 abled bodied n = 5 SCI | Number of steps (accelerometer) | Two 6MWT (1 min pause between) on a 30 m walkway |
|
| Kang et al. [64] | Powered hip exoskeleton (hip; mobile) | Young n = 4 (3♂, 1♀) age: 23.5 ± 3.3 Elderly n = 2 (2♀) age: 72.5 health: all healthy | Treadmill | Treadmill walking (different speed) | Walking speed (RMSE)
|
| Li et al. [62] | Unilateral rehabilitation exoskeleton robot (full leg; mobile) | N = 10 (8♂, 2♀) age: 25 ± 4 health: all healthy | Vicon motion capture system (Oxford Metric, Oxford, UK) |
| Gait phase estimation (MAE in ms) (self-selected speed; 2 km/h; 4 km/h; 6 km/h)
|
| Xia et al. [63] | Passive lower limb weight-bearing exoskeleton (full leg; mobile) | N = 7 (6♂, 1♀) age: 25–30 health: all healthy | Image acquisition system (manual labelling) |
| Gait phase estimation accuracy (correct classified data points/total data points)) average: 92.989% left foot lift, right foot hang: 69% left foot lift, right foot support: 96% left foot hang, right foot lift: 82% left foot hang, right foot support: 97% left foot support, right foot lift: 94% left foot support, right foot hang: 98% left foot support, right foot support: 81% |
| Zhang et al. [45] | Single-joint robotic hip exoskeleton (hip; mobile) | N = 7 (5♂, 2♀) age: 25.9 ± 3.8 health: all healthy | FSR Sensors in foot insole (offline) |
| Gait phase estimation (RMSE)
|
| Crea et al. [68] | Active pelvis orthosis (APO) (hip; mobile) | N = 7 (4♂, 3♀) age: 28.6 ± 4.9 health: all healthy | Sensor insoles | Treadmill walking with fast and slow speed in two modes (assistive and transparent) | Gait phase estimation (RMSE)
|
| Yu et al. [70] | Portable knee exoskeleton (knee; mobile) | N = 3 age: 25.3 ± 0.94 | Foot switches | Walking on a treadmill and stair walking (ascending + descending) at steady and varying speed | Gait phase estimation (RMSE) Steady speed:
|
| Joint Torques and Strength | |||||
| Cherni et al. [73] | Lokomat (full leg; stationary) | N = 17 (9♂, 8♀) age: 10.0 ± 3.2 health: all CP | Handheld dynamometer | Isometric force measurement fixed joints angles (30° hip flexion, 45° knee flexion); producing and holding maximum strength for 5 s, each muscle group (hip flexors/extensors and knee flexors/extensors) measured separately | Test–retest reliability Inter-tester (single measurement):
|
| Galen et al. [71] | Lokomat (full leg; stationary) | N = 18 (14♂, 4♀) age: 49.3 ± 11 health: all iSCI | Standard neurological classification of spinal cord injury (ASIA) scoring system | Isometric force fixed joints angles; producing and holding maximum strength for 5 s, muscle groups: hip flexors/extensors and knee flexors/extensors |
|
| Bolliger et al. [74] | Lokomat (full leg; stationary) | N = 30 (8♂, 32♀) age: 25.7 ± 3.8 (healthy) 53.5 ± 16.5 (neurological disorders) health: n = 16 healthy n = 14 neurological disorders | Only reliability study | Isometric force measurement fixed joints angles (30° hip flexion, 45° knee flexion); producing and holding maximum strength for 5 s, each muscle group (hip flexors/extensors and knee flexors/extensors) measured separately | Healthy Inter-tester reliability
Inter-tester reliability
|
| Rea et al. [76] | X1 exoskeleton (full leg; mobile) | N = 8 | Biodex system; dynamometer | n.d. |
|
| Molinaro et al. [46] | Robotic hip exoskeleton (hip; mobile) | N = 5 age: 23.0 ± 2.1 health: all healthy | Vicon motion capture system (Oxford Metric, Oxford, UK) + Bertec force plates (Bertec, Columbus, OH, USA) + OpenSim | Walking on a treadmill Level ground Ramp ascent Ramp descent | RMSE of estimated hip torque compared to ground truth: Level ground:
|
| Stiffness/Spasticity/Impedance | |||||
| Lunenburger et al. [36] | Lokomat (full leg; stationary) | N = 42 health: all with neurological disorders | Modified Ashworth score | Automated movement of the tested joints; participant‘s legs are 100% unloaded |
|
| Cherni et al. [79] | Lokomat Pediatric version (full leg; stationary) | N = 16 (9♂, 7♀) age: 20 ± 3 health: all CP | Only reliability study | Lokomat L-STIFF Tool; Exoskeleton displace each joint with three different velocities (slow/medium/fast) | Test–retest reliability Intra-tester (same day):
|
| Author/Item | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bolliger et al. [74] | 1 | 1 | 1 | na | 1 | 1 | 1 | na | na | 1 | 1 | na | 8 |
| Cherni et al. [73] | 1 | 1 | 1 | na | 1 | 1 | 1 | na | na | 1 | 1 | na | 8 |
| Cherni et al. [79] | 1 | 1 | 1 | na | 1 | 1 | 1 | na | na | 1 | 1 | na | 8 |
| Chisholm et al. [56] | 1 | 1 | 1 | na | 1 | 1 | 1 | na | na | 1 | 0 | na | 7 |
| Crea et al. [68] | 1 | 1 | 0 | na | 0 | 1 | 1 | na | na | 1 | 0 | na | 5 |
| Dambreville et al. [55] | 1 | 1 | 1 | na | 1 | 1 | 1 | na | na | 1 | 1 | na | 8 |
| d’Elia et al. [47] | 1 | 1 | 1 | na | 0 | 1 | 1 | na | na | 1 | 0 | na | 6 |
| Domingo and Lam [58] | 1 | 1 | 1 | na | 1 | 1 | 1 | na | na | 1 | 0 | na | 7 |
| Galen et al. [71] | 1 | 1 | 1 | na | 1 | 1 | 1 | na | na | 1 | 1 | na | 8 |
| Hu et al. [38] | 1 | 0 | 0 | na | 0 | 1 | 1 | na | na | 0 | 0 | na | 3 |
| Kang et al. [64] | 1 | 1 | 1 | na | 0 | 1 | 1 | na | na | 0 | 0 | na | 5 |
| Koginov et al. [44] | 1 | 1 | 0 | na | 1 | 1 | 1 | na | na | 1 | 1 | na | 7 |
| Li et al. [62] | 1 | 0 | 0 | na | 1 | 1 | 1 | na | na | 1 | 1 | na | 6 |
| Lonini et al. [60] | 1 | 1 | 1 | na | 0 | 1 | 1 | na | na | 1 | 0 | na | 6 |
| Lunenburger et al. [36] | 1 | 0 | 0 | na | 1 | 1 | 1 | na | na | 0 | 0 | na | 4 |
| Molinaro et al. [46] | 1 | 1 | 1 | na | 0 | 1 | 1 | na | na | 1 | 0 | na | 6 |
| Park et al. [52] | 1 | 0 | 0 | na | 0 | 1 | 1 | na | na | 0 | 0 | na | 3 |
| Rea et al. [76] | 0 | 0 | 0 | na | 0 | 0 | 0 | na | na | 0 | 0 | na | 0 |
| Veneman et al. [42] | 1 | 0 | 0 | na | 0 | 1 | 1 | na | na | 0 | 0 | na | 3 |
| Xia et al. [63] | 0 | 0 | 0 | na | 0 | 1 | 1 | na | na | 1 | 0 | na | 3 |
| Yu et al. [70] | 1 | 0 | 0 | na | 0 | 1 | 1 | na | na | 0 | 1 | na | 4 |
| Zhang et al. [45] | 1 | 1 | 0 | na | 0 | 1 | 1 | na | na | 0 | 0 | na | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moeller, T.; Moehler, F.; Krell-Roesch, J.; Dežman, M.; Marquardt, C.; Asfour, T.; Stein, T.; Woll, A. Use of Lower Limb Exoskeletons as an Assessment Tool for Human Motor Performance: A Systematic Review. Sensors 2023, 23, 3032. https://doi.org/10.3390/s23063032
Moeller T, Moehler F, Krell-Roesch J, Dežman M, Marquardt C, Asfour T, Stein T, Woll A. Use of Lower Limb Exoskeletons as an Assessment Tool for Human Motor Performance: A Systematic Review. Sensors. 2023; 23(6):3032. https://doi.org/10.3390/s23063032
Chicago/Turabian StyleMoeller, Tobias, Felix Moehler, Janina Krell-Roesch, Miha Dežman, Charlotte Marquardt, Tamim Asfour, Thorsten Stein, and Alexander Woll. 2023. "Use of Lower Limb Exoskeletons as an Assessment Tool for Human Motor Performance: A Systematic Review" Sensors 23, no. 6: 3032. https://doi.org/10.3390/s23063032
APA StyleMoeller, T., Moehler, F., Krell-Roesch, J., Dežman, M., Marquardt, C., Asfour, T., Stein, T., & Woll, A. (2023). Use of Lower Limb Exoskeletons as an Assessment Tool for Human Motor Performance: A Systematic Review. Sensors, 23(6), 3032. https://doi.org/10.3390/s23063032