Blending Approach Preparation of PVA-g-PMA Films with Embedded “Green” Synthesized Silver Nanoparticles for Acetone Optical Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Copolymer
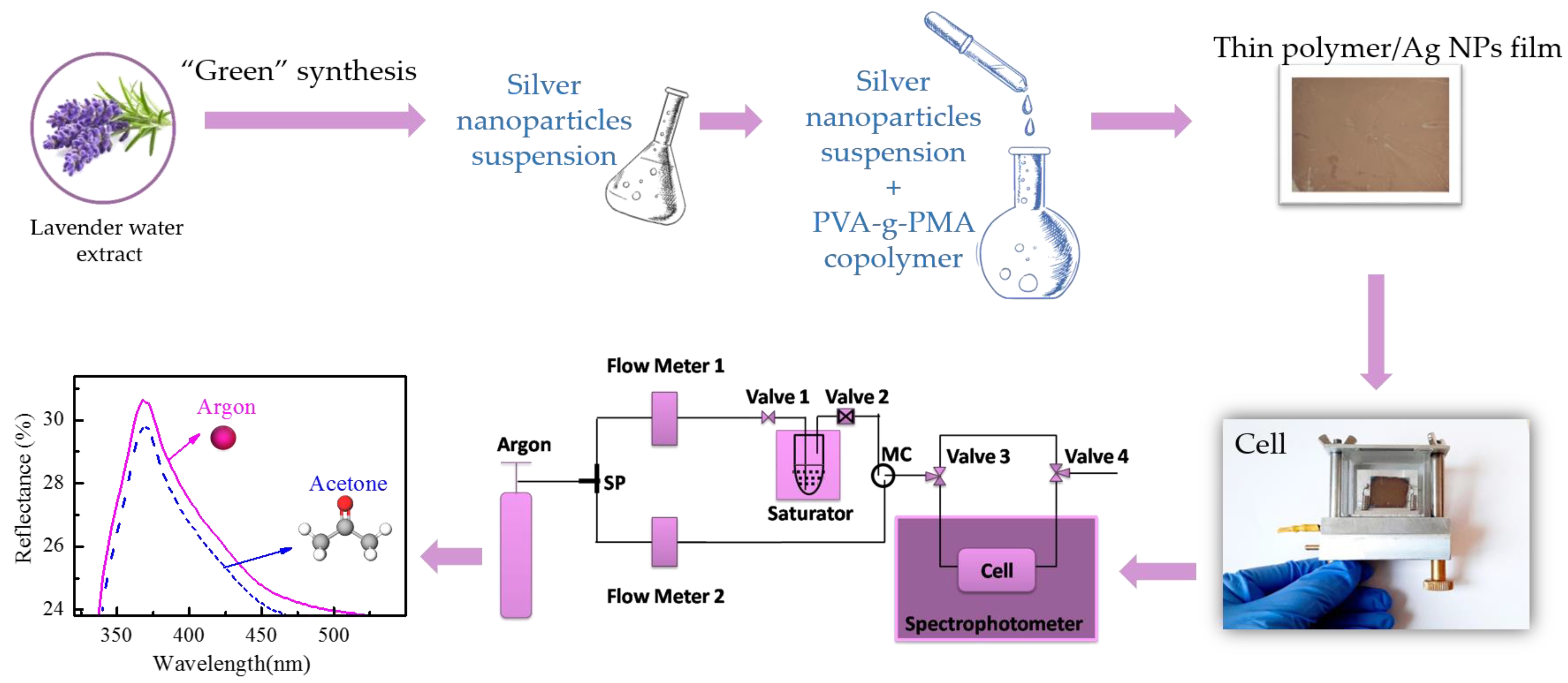
2.2. Silver Nanoparticles Synthesis Method
2.2.1. Preparation of Extracts from Lavender Waste
2.2.2. “Green” Synthesis of AgNPs
2.3. Determination of AgNPs Size, Morphology, Structure, and Concentration
2.4. Composites Thin Film Preparation and Characterization
2.5. Sensing Experiments
3. Results and Discussion
3.1. Characterization of Ag Nanoparticles
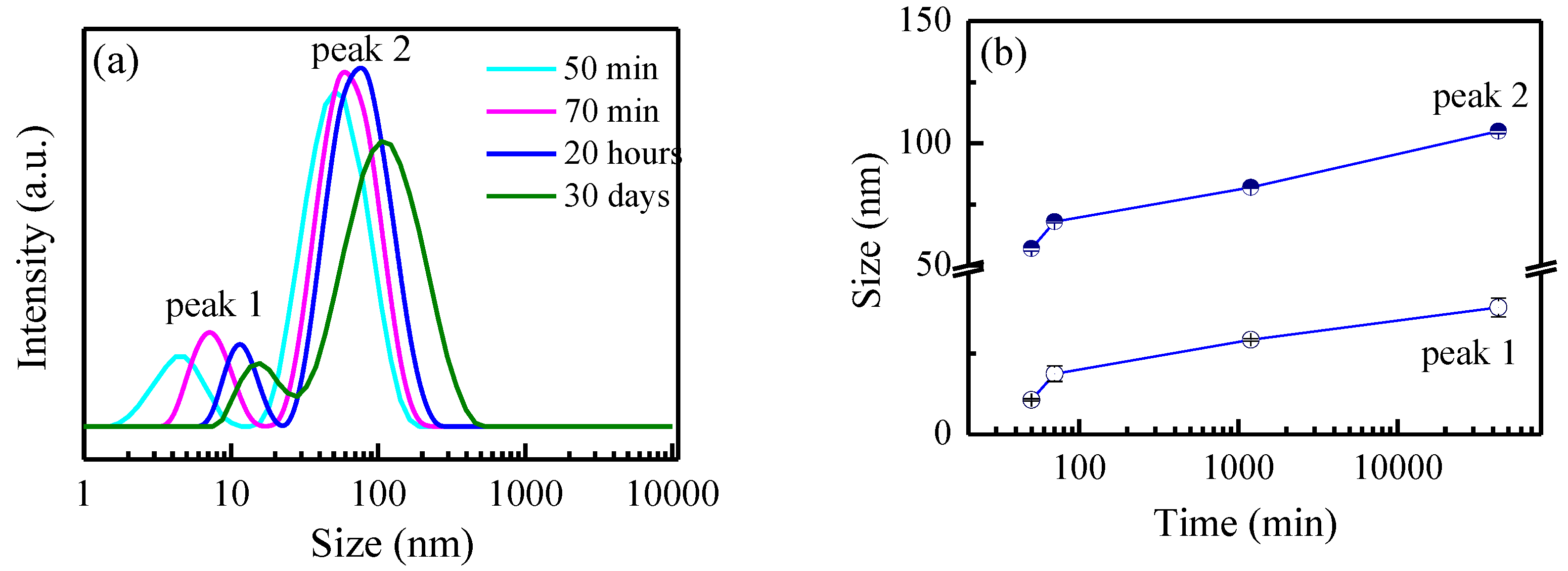
3.1.1. Size Investigation Using DLS
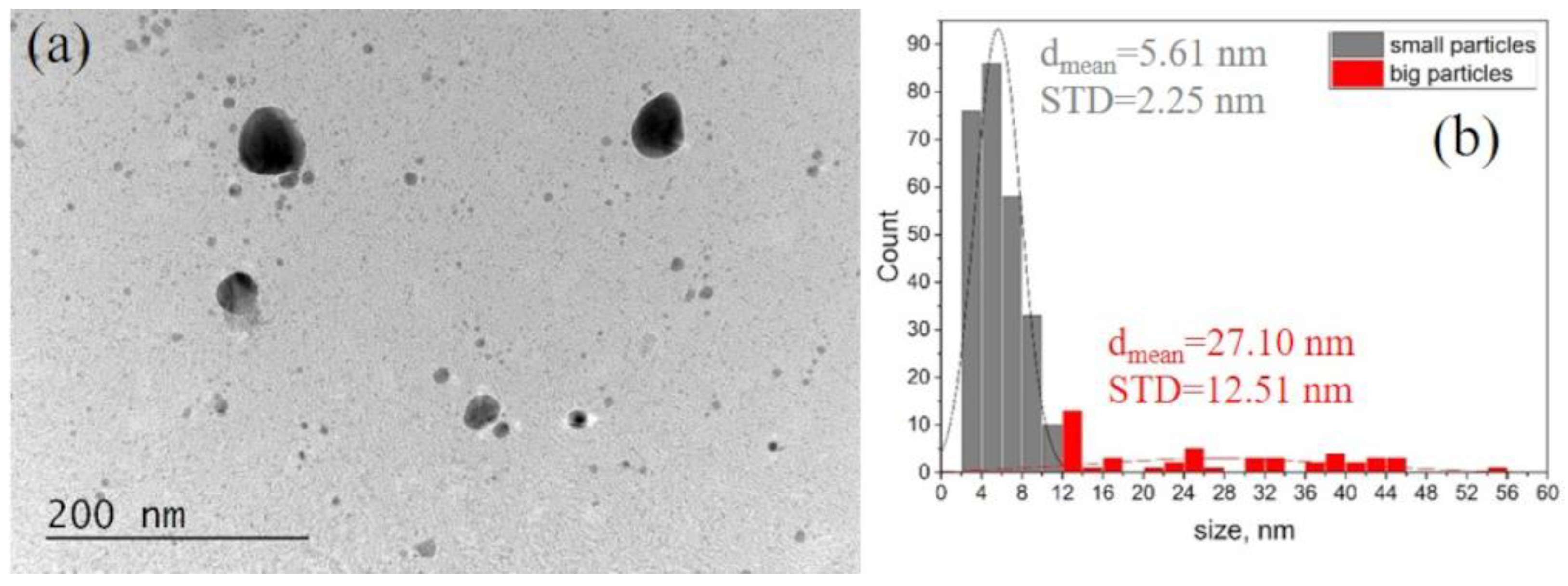
3.1.2. TEM Investigation
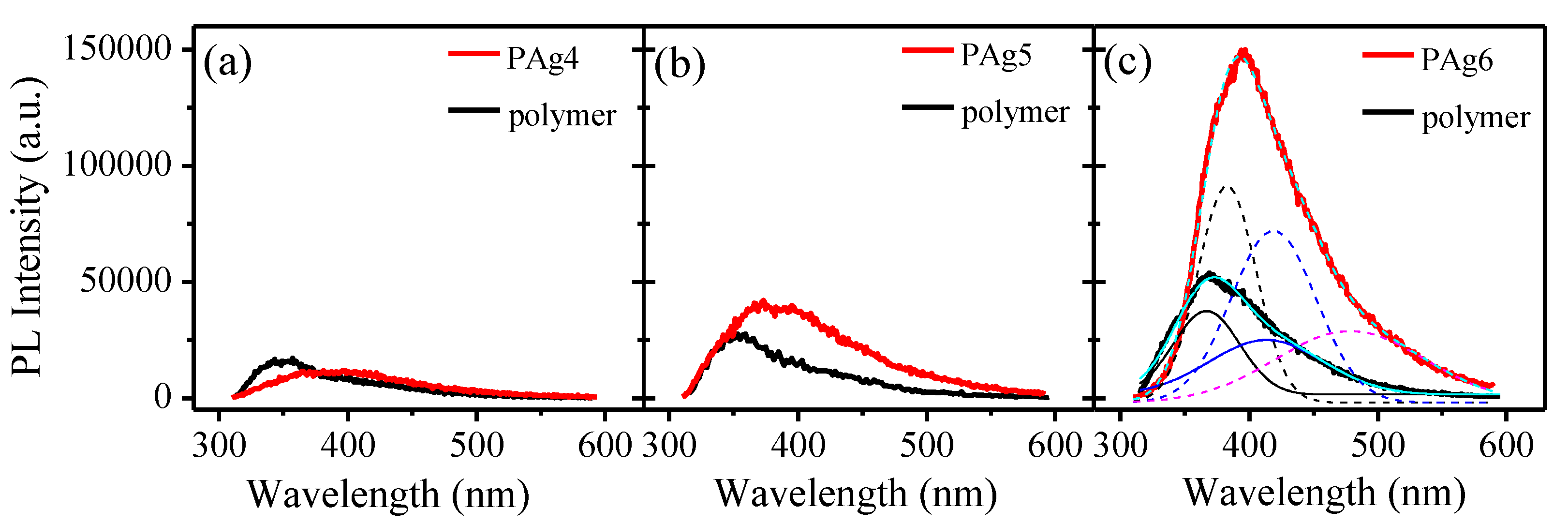
3.2. Thin Films Characterization
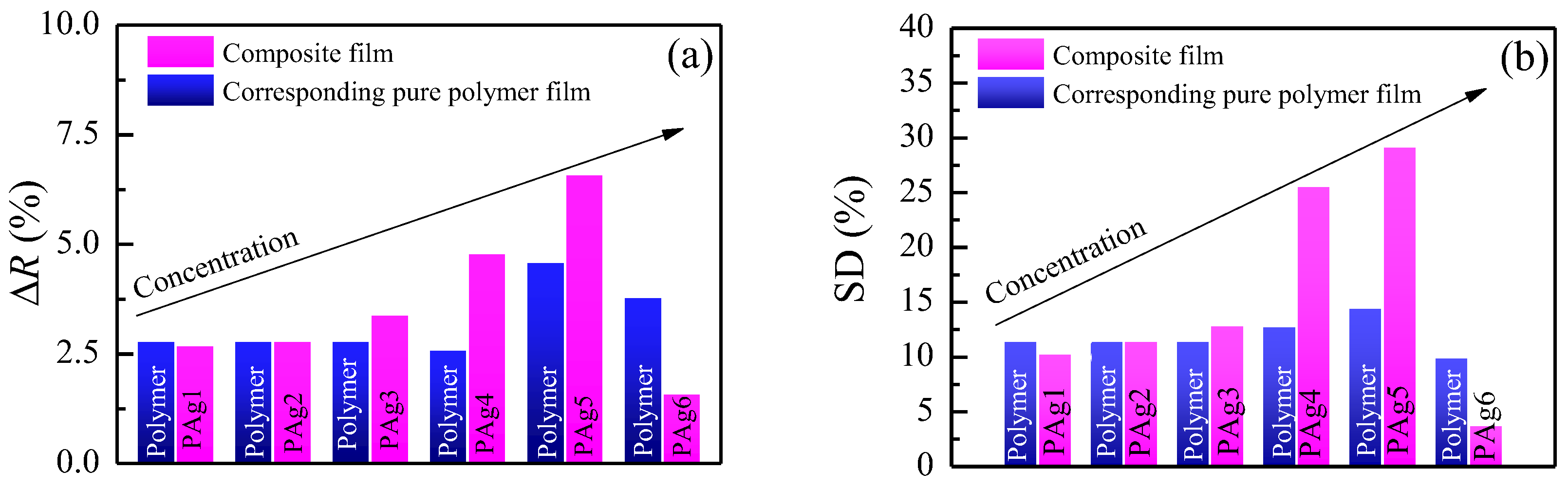
3.3. Sensing Properties of the Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cattaneo, S.; Althahban, S.; Freakley, S.J.; Sankar, M.; Davies, T.; He, Q.; Dimitratos, N.; Kielyab, C.J.; Hutchings, G.J. Synthesis of highly uniform and composition-controlled gold–palladium supported nanoparticles in continuous flow. Nanoscale 2019, 11, 8247–8259. [Google Scholar] [CrossRef] [PubMed]
- Yeshchenko, O.A.; Malynych, S.Z.; Polomarev, S.O.; Galabura, Y.; Chumanov, G.; Luzinov, I. Towards sensor applications of a polymer/Ag nanoparticle nanocomposite film. RSC Adv. 2019, 9, 8498–8506. [Google Scholar] [CrossRef] [PubMed]
- Pakula, C.; Zaporojtchenko, V.; Strunskus, T.; Zargarani, D.; Herges, R.; Faupel, F. Reversible light-controlled conductance switching of azobenzene-based metal/polymer nanocomposites. Nanotechnology 2010, 21, 465201. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ghadiri, R.; Weigel, T.; Aumann, A.; Gurevich, E.L.; Esen, C.; Medenbach, O.; Cheng, W.; Chichkov, B.; Ostendorf, A. Comparison of in situ and ex situ Methods for Synthesis of Two-Photon Polymerization Polymer Nanocomposites. Polymers 2014, 6, 2037–2050. [Google Scholar] [CrossRef]
- Waters, W.A. Some Comments on the Development of Free Radical Chemistry. Notes Rec. R. Soc. Lond. 1984, 39, 105–124. [Google Scholar] [CrossRef]
- Alonso, A.; Macanás, J.; Davies, G.; Gun’ko, Y.K.; Muñoz, M.; Muraviev, D.N. Environmentally-Safe Polymer-Metal Nanocomposites with Most Favorable Distribution of Catalytically Active and Biocide Nanoparticles. In Advances in Nanocomposite Technology; Hashim, A.A., Ed.; IntechOpen: London, UK, 2011; pp. 175–200. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Wichaita, W.; Thérien-Aubin, H. Influence of the Architecture of Soft Polymer-Functionalized Polymer Nanoparticles on Their Dynamics in Suspension. Polymers 2020, 12, 1844. [Google Scholar] [CrossRef]
- Bolla, P.A.; Huggias, S.; Serradell, M.A.; Ruggera, J.F.; Casella, M.L. Synthesis and Catalytic Application of Silver Nanoparticles Supported on Lactobacillus kefiri S-Layer Proteins. Nanomaterials 2020, 10, 2322. [Google Scholar] [CrossRef]
- Zhang, J.; Ahmadi, M.; Fargas, G.; Perinka, N.; Reguera, J.; Lanceros-Méndez, S.; Llanes, L.; Jiménez-Piqué, E. Silver Nanoparticles for Conductive Inks: From Synthesis and Ink Formulation to Their Use in Printing Technologies. Metals 2022, 12, 234. [Google Scholar] [CrossRef]
- Caillosse, E.; Zaier, M.; Mezghani, M.; Hajjar-Garreau, S.; Vidal, L.; Lougnot, D.; Balan, L. Photo-Induced Self-Assembly of Silver Nanoparticles for Rapid Generation of First and Second Surface Mirrors. ACS Appl. Nano Mater. 2020, 3, 6531–6540. [Google Scholar] [CrossRef]
- Yu, C.-X.; Xiong, F.; Liu, L.-L. Electrochemical Biosensors with Silver Nanoparticles as Signal Labels. Int. J. Electrochem. 2020, 15, 3869–3890. [Google Scholar] [CrossRef]
- Dodevska, T.; Vasileva, I.; Denev, P.; Karashanova, D.; Georgieva, B.; Kovacheva, D.; Yantcheva, N.; Slavov, A. Rosa damascena waste mediated synthesis of silver nanoparticles: Characteristics and application for an electrochemical sensing of hydrogen peroxide and vanillin. Mater. Chem. Phys. 2019, 231, 335–343. [Google Scholar] [CrossRef]
- Prosposito, P.; Mochi, F.; Ciotta, E.; Casalboni, M.; De Matteis, F.; Venditti, I.; Fontana, L.; Testa, G.; Fratoddi, I. Hydrophilic silver nanoparticles with tunable optical properties: Application for the detection of heavy metals in water. Beilstein J. Nanotechnol. 2016, 7, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Sanjeevappa, H.K.; Nilogal, P.; Rayaraddy, R.; Martis, L.J.; Osman, S.M.; Badiadka, N.; Yallappa, S. Biosynthesized unmodified silver nanoparticles: A colorimetric optical sensor for detection of Hg2+ ions in aqueous solution. Results Chem. 2022, 4, 100–507. [Google Scholar] [CrossRef]
- Venditti, I. Metal Nanoparticles–Polymers Hybrid Materials I. Polymers 2022, 14, 3117. [Google Scholar] [CrossRef] [PubMed]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Feng Chong, K.; Ali, G.A.M. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef]
- Alex, K.V.; Pavai, P.T.; Rugmini, R.; Prasad, M.S.; Kamakshi, K.; Sekhar, K.C. Green Synthesized Ag Nanoparticles for Bio-Sensing and Photocatalytic Applications. ACS Omega 2020, 5, 13123–13129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Cumbal, L. Biosynthesis of silver nanoparticles using lavender leaf and their applications for catalytic, sensing, and antioxidant activities. Nanotechnol. Rev. 2016, 5, 521–528. [Google Scholar] [CrossRef]
- Carmona, E.R.; Benito, N.; Plaza, T.; Recio-Sánchez, G. Green synthesis of silver nanoparticles by using leaf extracts from the endemic Buddleja globosa hope. Green Chem. Lett. Rev. 2017, 10, 250–256. [Google Scholar] [CrossRef]
- Melkamu, W.W.; Bitew, L.T. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) J.F. Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon 2021, 7, 2405–8440. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Bujňáková, Z.B.; Balážová, L.; Tkáčiková, L. Green Synthesis of Silver Nanoparticles with Antibacterial Activity Using Various Medicinal Plant Extracts: Morphology and Antibacterial Efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, H.; Magnusson, R. Development of tuned refractive-index nanocomposites to fabricate nanoimprinted optical devices. Opt. Mater. Express 2018, 8, 175–183. [Google Scholar] [CrossRef]
- Kulkarni, A.G.; Britto, S.D.; Jogaiah, S. 18—Economic considerations and limitations of green synthesis vs. chemical synthesis of nanomaterials. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Jogaiah, S., Singh, H.B., Fraceto, L.F., de Lima, R., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2021; pp. 459–468. ISBN 978-0-12-820092-6. [Google Scholar]
- Ahmad, H.; Venugopal, K.; Rajagopal, K.; De Britto, S.; Nandini, B.; Pushpalatha, H.G.; Konappa, N.; Udayashankar, A.C.; Geetha, N.; Jogaiah, S. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Eucalyptus globules and Their Fungicidal Ability Against Pathogenic Fungi of Apple Orchards. Biomolecules 2020, 10, 425. [Google Scholar] [CrossRef]
- Sergeev, A.A.; Mironenko, A.Y.; Nazirov, A.E.; Leonov, A.A.; Voznesenskii, S.S. Nanocomposite polymer structures for optical sensors of hydrogen sulfide. Technol. Phys. 2017, 62, 1277–1280. [Google Scholar] [CrossRef]
- Bozhilova, S.; Lazarova, K.; Ivanova, S.; Karashanova, D.; Babeva, T.; Christova, D. Colloidal Aqueous Dispersions of Methyl (meth)Acrylate-Grafted Polyvinyl Alcohol Designed for Thin Film Applications. Coatings 2022, 12, 1882. [Google Scholar] [CrossRef]
- Lazarova, Y.L.; Dodevska, T.M.; Slavov, A.M.; Karashanova, D.B.; Georgieva, B.C. Biosynthesized silver nanoparticles: Electrochemical application. Bulg. Chem. Com. 2019, 51, 173–177. [Google Scholar]
- ImageJ. Available online: https://imagej.net/ij/index.html (accessed on 30 January 2023).
- Lazarova, K.; Vasileva, M.; Marinov, G.; Babeva, T. Optical characterization of sol–gel derived Nb2O5 thin films. Opt. Laser Technol. 2014, 58, 114–118. [Google Scholar] [CrossRef]
- Lazarova, K.; Awala, H.; Thomas, S.; Vasileva, M.; Mintova, S.; Babeva, T. Vapor Responsive One-Dimensional Photonic Crystals from Zeolite Nanoparticles and Metal Oxide Films for Optical Sensing. Sensors 2014, 14, 12207–12218. [Google Scholar] [CrossRef]
- Maxwell-Garnett, J.C. Colours in metal glasses and in metallic films. Philos. Trans. R. Soc. Lond. 1904, 203A, 385–420. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370. [Google Scholar] [CrossRef]
- Saini, I.; Rozra, J.; Chandak, N.; Aggarwal, S.; Sharma, P.K.; Sharma, A. Tailoring of electrical, optical and structural properties of PVA by addition of Ag nanoparticles. Mater. Chem. Phys. 2013, 139, 802–810. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Dmitruk, I.M.; Alexeenko, A.A.; Losytskyy, M.Y.; Kotko, A.V.; Pinchuk, A.O. Size-dependent surface-plasmon-enhanced photoluminescence from silver nanoparticles embedded in silica. Phys. Rev. B 2009, 79, 235–438. [Google Scholar] [CrossRef]
- Berberova-Buhova, N.; Nedelchev, L.; Mateev, G.; Stoykova, E.; Strijkova, V.; Nazarova, D. Influence of the size of Au nanoparticles on the photoinduced birefringence and diffraction efficiency of polarization holographic gratings in thin films of azopolymer nanocomposites. Opt. Mater. 2021, 121, 11560. [Google Scholar] [CrossRef]
- Nedelchev, L.; Nazarova, D.; Dragostinova, V.; Karashanova, D. Increase of photoinduced birefringence in a new type of anisotropic nanocomposite: Azopolymer doped with ZnO nanoparticles. Opt. Lett. 2012, 37, 2676–2678. [Google Scholar] [CrossRef] [PubMed]
- Nedelchev, L.; Mateev, G.; Strijkova, V.; Salgueiriño, V.; Schmool, D.S.; Berberova-Buhova, N.; Stoykova, E.; Nazarova, D. Tunable Polarization and Surface Relief Holographic Gratings in Azopolymer Nanocomposites with Incorporated Goethite (α-FeOOH) Nanorods. Photonics 2021, 8, 306. [Google Scholar] [CrossRef]

| Film Code | Cm (wt%) | fAg (%) |
|---|---|---|
| PAg1 | 0.07 | 0.008 |
| PAg2 | 0.14 | 0.016 |
| PAg3 | 0.28 | 0.032 |
| PAg4 | 0.57 | 0.065 |
| PAg5 | 1.14 | 0.129 |
| PAg6 | 2.30 | 0.260 |
| Sample | nc | np | kc | kp | dc (nm) | dp (nm) | Δncalc |
|---|---|---|---|---|---|---|---|
| PAg1 | 1.39 | 1.39 | 0.020 | 0.019 | 29 | 28 | <10−4 |
| PAg2 | 1.41 | 1.39 | 0.020 | 0.019 | 28 | 28 | 1 × 10−4 |
| PAg3 | 1.42 | 1.39 | 0.020 | 0.019 | 31 | 28 | 1 × 10−4 |
| PAg4 | 1.39 | 1.39 | 0.020 | 0.019 | 40 | 39 | 2 × 10−4 |
| PAg5 | 1.37 | 1.36 | 0.020 | 0.019 | 48 | 48 | 5 × 10−4 |
| PAg6 | 1.37 | 1.37 | 0.020 | 0.019 | 75 | 71 | 9 × 10−4 |
| errors | ±0.01 | ±0.01 | ±0.005 | ±0.005 | ±1 | ±1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarova, K.; Christova, D.; Karashanova, D.; Georgieva, B.; Marovska, G.; Slavov, A.; Babeva, T. Blending Approach Preparation of PVA-g-PMA Films with Embedded “Green” Synthesized Silver Nanoparticles for Acetone Optical Detection. Sensors 2023, 23, 2941. https://doi.org/10.3390/s23062941
Lazarova K, Christova D, Karashanova D, Georgieva B, Marovska G, Slavov A, Babeva T. Blending Approach Preparation of PVA-g-PMA Films with Embedded “Green” Synthesized Silver Nanoparticles for Acetone Optical Detection. Sensors. 2023; 23(6):2941. https://doi.org/10.3390/s23062941
Chicago/Turabian StyleLazarova, Katerina, Darinka Christova, Daniela Karashanova, Biliana Georgieva, Gergana Marovska, Anton Slavov, and Tsvetanka Babeva. 2023. "Blending Approach Preparation of PVA-g-PMA Films with Embedded “Green” Synthesized Silver Nanoparticles for Acetone Optical Detection" Sensors 23, no. 6: 2941. https://doi.org/10.3390/s23062941
APA StyleLazarova, K., Christova, D., Karashanova, D., Georgieva, B., Marovska, G., Slavov, A., & Babeva, T. (2023). Blending Approach Preparation of PVA-g-PMA Films with Embedded “Green” Synthesized Silver Nanoparticles for Acetone Optical Detection. Sensors, 23(6), 2941. https://doi.org/10.3390/s23062941






