Dioxepine-Peri-Annulated PMIs—Synthesis and Spectral and Sensing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of 1,8-Diamino-3,6,10,13,16,19-hexaazabicyclo-(6,6,6)eicosane (DiAmSar)
2.1.2. Synthesis of Dibutyl 9,10-Dibromo-1,6,7,12-tetrachloroperylene-3,4-dicarboxylate (B2)
2.1.3. Synthesis of Dibutyl 3,4,15,16-Tetrachloronaphtho[2,3-b]peryleno[3,4-ef][1,4]dioxepine-1,18-dicarboxylate (B3)
2.1.4. Synthesis of Dibutyl 3,4,15,16-Tetrachloronaphtho[2,3-b]peryleno[3,4-ef][1,4]dioxepine-1,18-dicarboxylate (B4)
2.1.5. Synthesis of 5,6,17,18-Tetrachloro-2-(2,6-diisopropylphenyl)-1H-naphtho[2″,3″:2′,3′][1,4]dioxepino[5′,6′,7′:9,10]peryleno[3,4-cd]pyridine-1,3(2H)-dione (1a)
2.1.6. Synthesis of 5,6,17,18-Tetrachloro-2-(2-(dimethylamino)ethyl)-1H-naphtho[2″,3″:2′,3′][1,4]dioxepino-[5′,6′,7′:9,10]peryleno[3,4-cd]pyridine-1,3(2H)-dione (1b)
2.1.7. Synthesis of 2-(8-Amino-3,6,10,13,16,19-hexaazabicyclo[6.6.6]icosan-1-yl)-5,6,17,18-tetrachloro-1H-naphtho[2″,3″:2′,3′][1,4]dioxepino[5′,6′,7′:9,10]peryleno[3,4-cd]pyridine-1,3(2H)-dione (1c)
2.2. Computational Details
3. Results and Discussion
3.1. Synthesis of Compounds
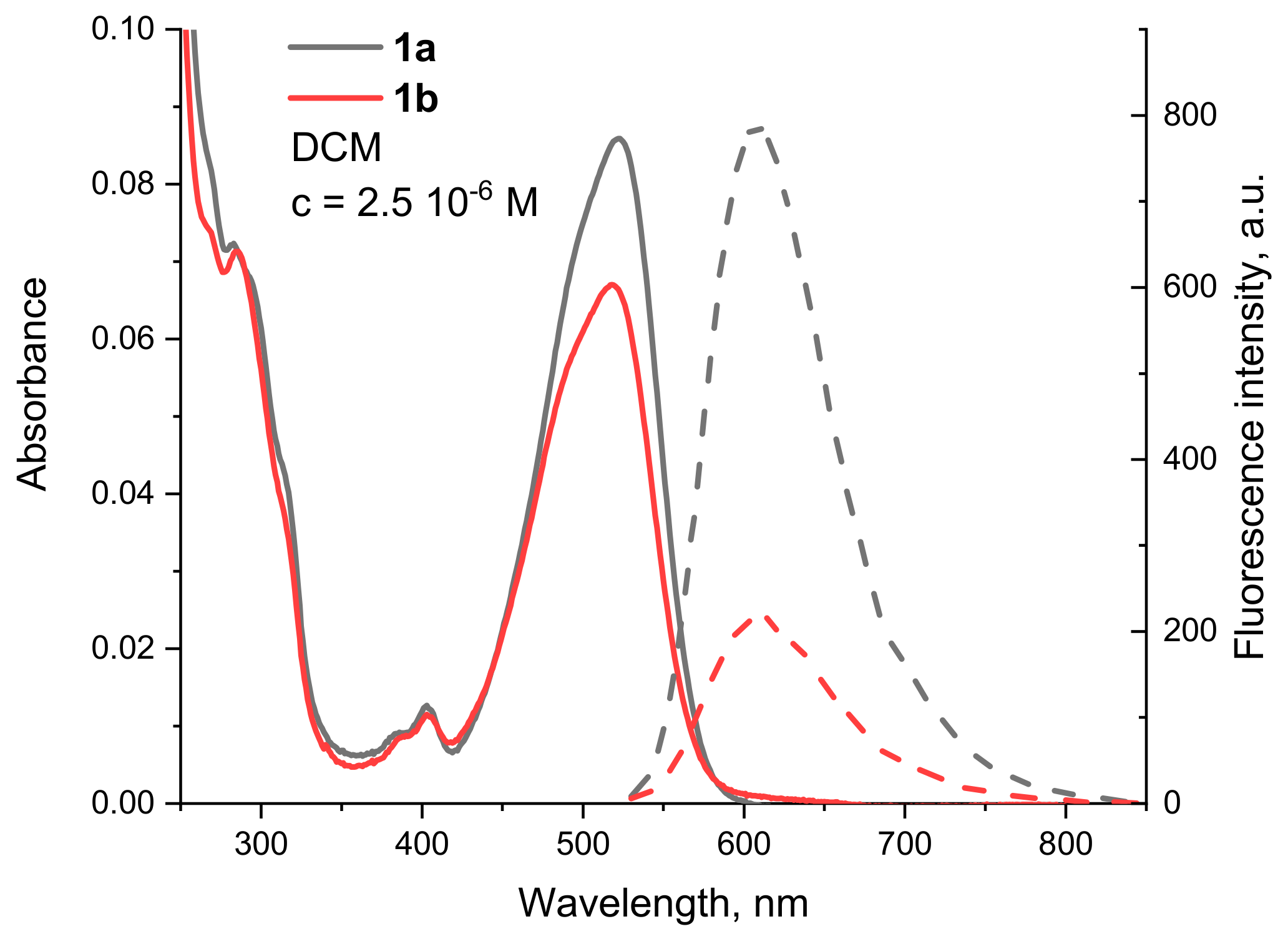
3.2. Spectral Properties
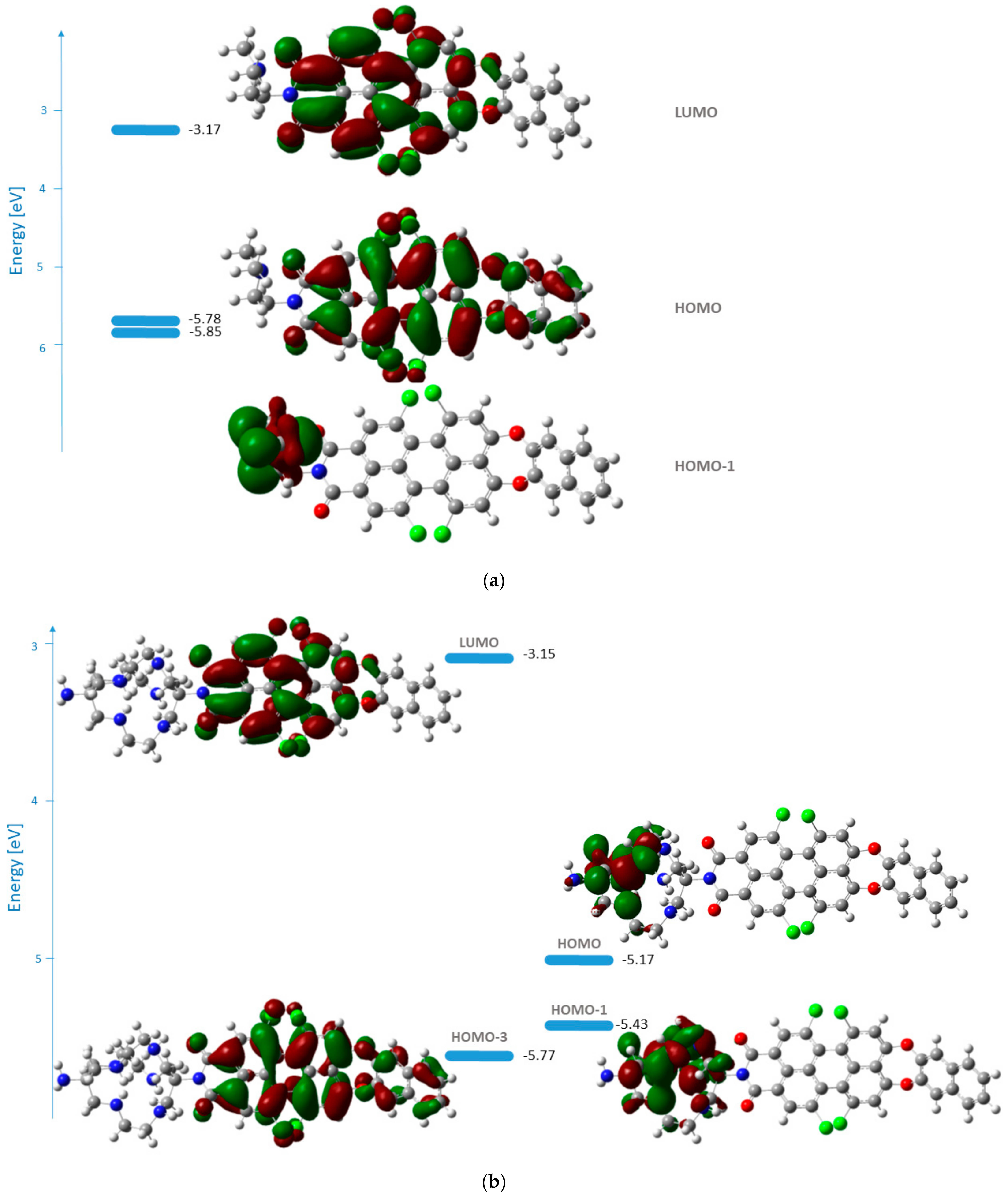
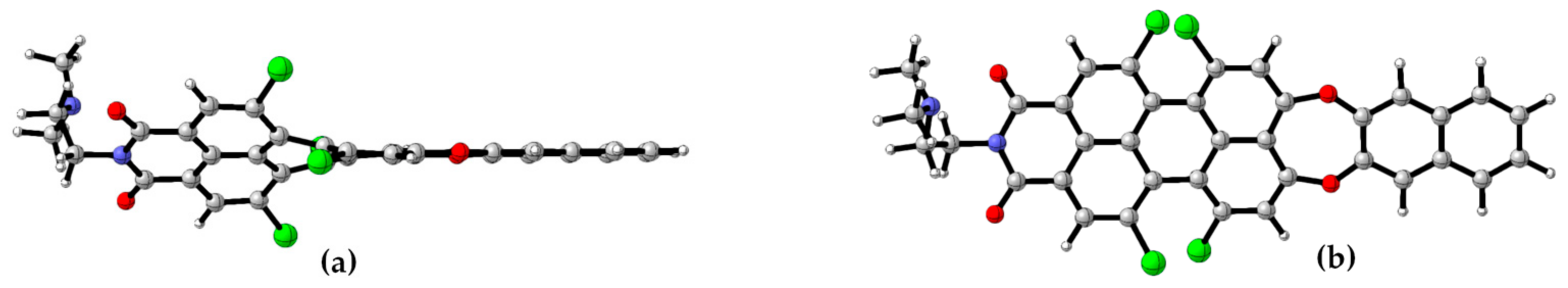
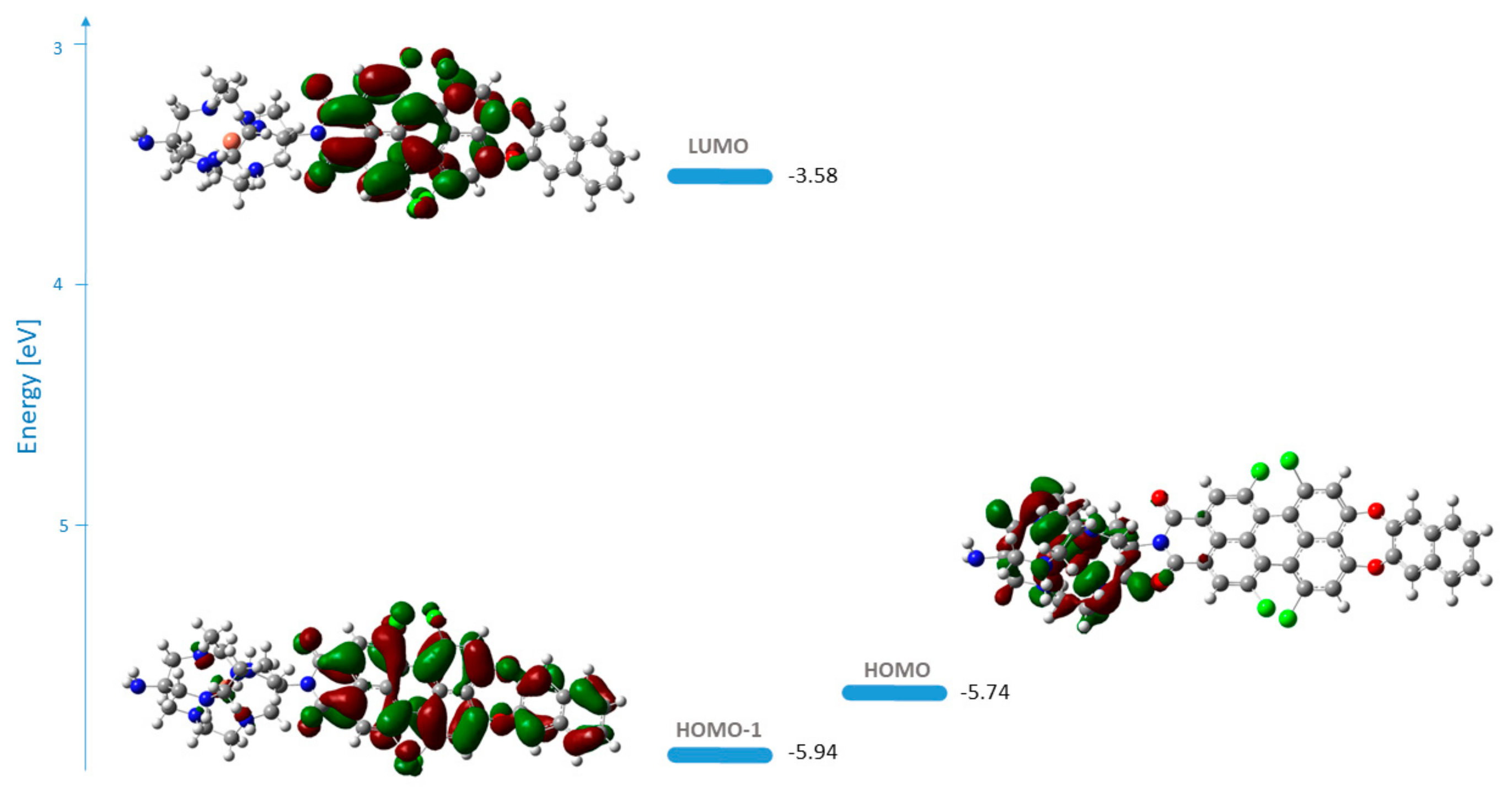
3.3. PET Design Evaluation
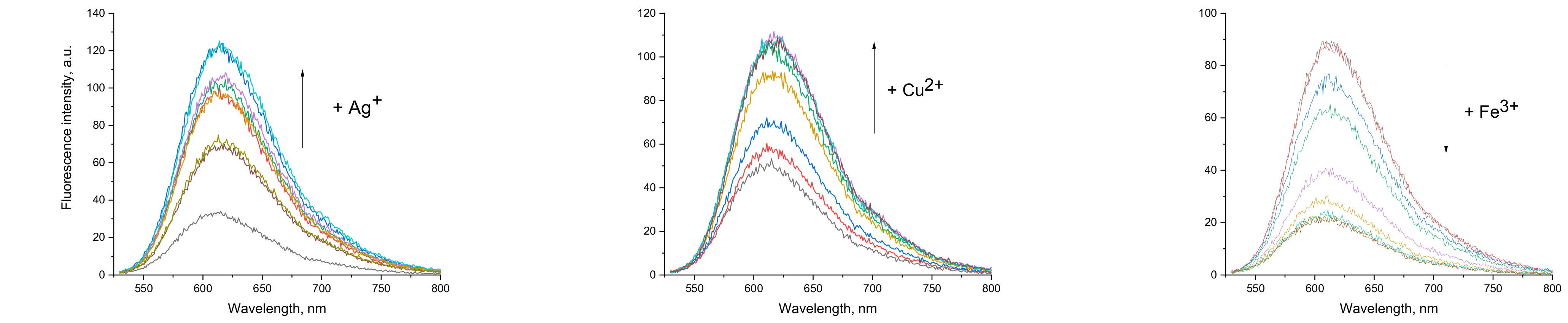
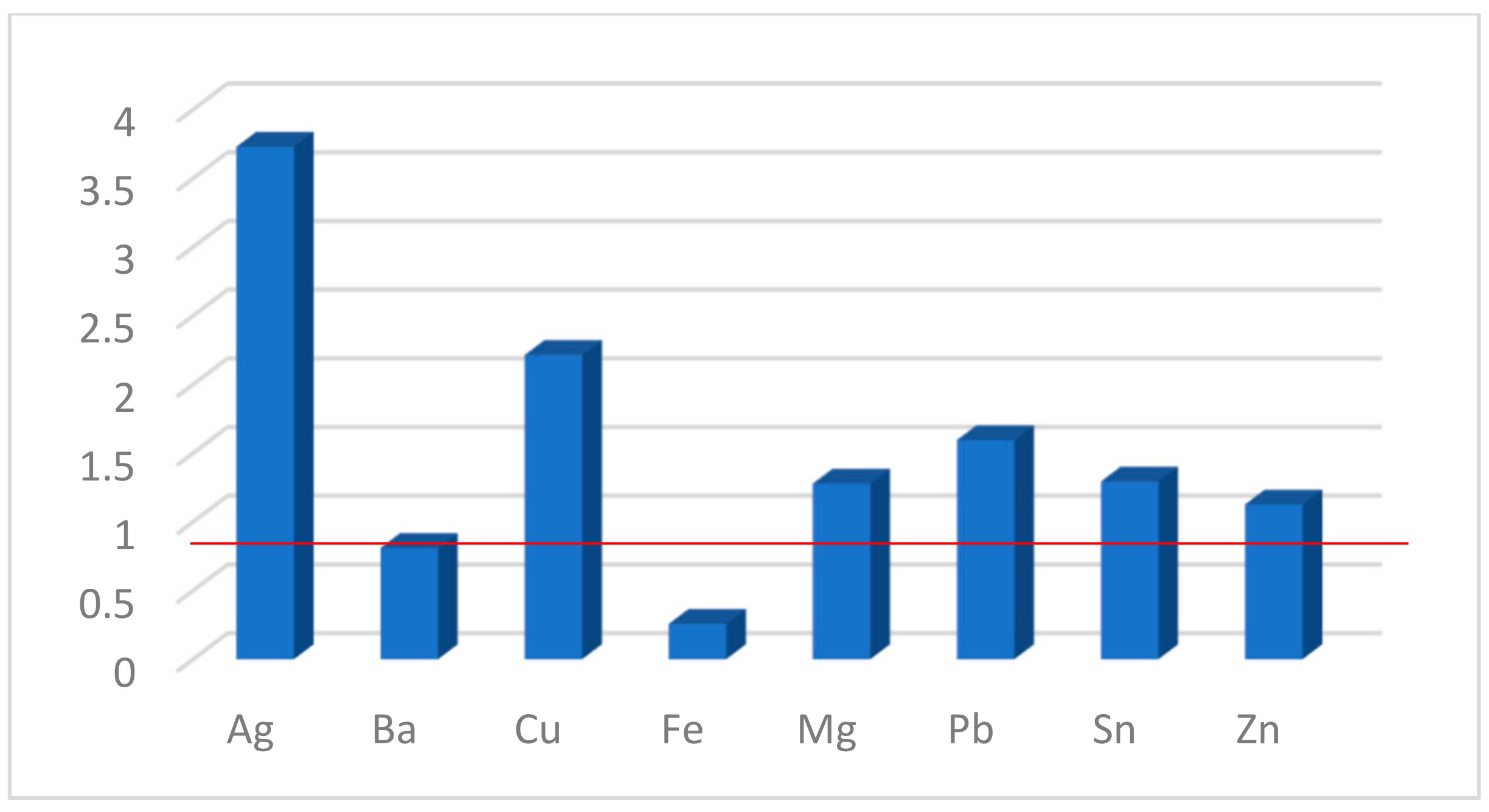
3.4. Influence of Metal Cations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Moustakas, M. The role of metal ions in biology, biochemistry and medicine. Materials. 2021, 14, 549. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egorova, K.S.; Ananikov, V.P. Toxicity of metal compounds: Knowledge and myths. Organometallics 2017, 36, 4071–4090. [Google Scholar] [CrossRef] [Green Version]
- Lvova, L. Chemical sensors for heavy metals/toxin detection. Chemosensors 2020, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, J.; Xu, S.; Li, C.; Dong, B. Recent progress in fluorescent probes for metal Ion detection. Front. Chem. 2022, 10, 1–15. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Shetty, N.S.; Mahesha, P.; Gaonkar, S.L. A Comprehensive Review on Thiophene Based Chemosensors; Springer: New York, NY, USA, 2022; Volume 32, ISBN 0123456789. [Google Scholar]
- Park, S.H.; Kwon, N.; Lee, J.H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [Green Version]
- You, L.; Zha, D.; Anslyn, E.V. Recent advances in supramolecular analytical chemistry using optical sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef]
- Park, J.-K.; Shin, J.; Jang, S.; Seol, M.-L.; Kang, J.; Choi, S.; Eom, H.; Kwon, O.; Park, S.; Noh, D.-Y.; et al. Rational design of fluorescent/colorimetric chemosensors for detecting transition metal ions by varying functional groups. Inorganics 2022, 10, 189. [Google Scholar] [CrossRef]
- Daly, B.; Ling, J.; De Silva, A.P. Current developments in fluorescent PET (photoinduced electron transfer) sensors and switches. Chem. Soc. Rev. 2015, 44, 4203–4211. [Google Scholar] [CrossRef] [Green Version]
- De Silva, A.P.; Moody, T.S.; Wright, G.D. Fluorescent PET (Photoinduced Electron Transfer) sensors as potent analytical tools. Analyst 2009, 134, 2385–2393. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Dimitrova, M.D.; Asiri, A.M.; Alamry, K.A.; Bojinov, V.B. Synthesis, sensor activity and logic behaviour of a novel bichromophoric system based on rhodamine 6G and 1,8-naphthalimide. Dye. Pigment. 2015, 115, 172–180. [Google Scholar] [CrossRef]
- Yordanova-Tomova, S.; Cheshmedzhieva, D.; Stoyanov, S.; Dudev, T.; Grabchev, I. Synthesis, photophysical characterization, and sensor activity of new 1,8-naphthalimide derivatives. Sensors 2020, 20, 3892. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. pH sensor potential and antimicrobial activity of a new PPA dendrimer modified with benzanthrone fluorophores in solution and on viscose fabric. J. Photochem. Photobiol. A Chem. 2019, 375, 24–29. [Google Scholar] [CrossRef]
- Abebe, F.; Perkins, P.; Shaw, R.; Tadesse, S. A rhodamine-based fluorescent sensor for selective detection of Cu2+ in aqueous media: Synthesis and spectroscopic properties. J. Mol. Struct. 2020, 1205, 127594. [Google Scholar] [CrossRef]
- Staneva, D.; Manov, H.; Yordanova, S.; Vasileva-Tonkova, E.; Stoyanov, S.; Grabchev, I. Synthesis, spectral properties and antimicrobial activity of a new cationic water-soluble pH-dependent poly(propylene imine) dendrimer modified with 1,8-naphthalimides. Luminescence 2020, 35, 947–954. [Google Scholar] [CrossRef]
- Staneva, D.; Yordanova, S.; Vasileva-Tonkova, E.; Stoyanov, S.; Grabchev, I. Synthesis of a new fluorescent poly(propylene imine) dendrimer modified with 4-nitrobenzofurazan. Sensor and antimicrobial activity. J. Photochem. Photobiol. A Chem. 2020, 395, 112506. [Google Scholar] [CrossRef]
- Chen, S.; Xue, Z.; Gao, N.; Yang, X.; Zang, L. Perylene diimide-based fluorescent and colorimetric sensors for environmental detection. Sensors. 2020, 20, 917. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Khan, A.; Chatterjee, O.; Bhunia, S.; Koner, A.L. Perylene monoimide as a versatile fluoroprobe: The past, present, and future. Org. Mater. 2021, 3, 417–454. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Said, A.I.; Toshkova, R.A.; Tzoneva, R.D.; Bojinov, V.B. A novel water-soluble perylenetetracarboxylic diimide as a fluorescent pH probe: Chemosensing, biocompatibility and cell imaging. Dye. Pigment. 2019, 160, 28–36. [Google Scholar] [CrossRef]
- Herrmann, A.; Müllen, K. From industrial colorants to single photon sources and biolabels: The fascination and function of rylene dyes. Chem. Lett. 2006, 35, 978–985. [Google Scholar] [CrossRef]
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 4, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Facchetti, A.; Barlow, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R.; Marder, S.R. Rylene and related diimides for organic electronics. Adv. Mater. 2011, 23, 268–284. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wonneberger, H. Perylene imides for organic photovoltaics: Yesterday, today, and tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Jung, C.; Wallis, P.; Schnitzler, T.; Li, C.; Müllen, K.; Bräuchle, C. Photophysics of new photostable rylene derivatives: Applications in single-molecule studies and membrane labelling. Chem. Phys. Chem. 2011, 12, 1588–1595. [Google Scholar] [CrossRef]
- Kozma, E.; Mróz, W.; Villafiorita-Monteleone, F.; Galeotti, F.; Andicsová-Eckstein, A.; Catellani, M.; Botta, C. Perylene diimide derivatives as red and deep red-emitters for fully solution processable OLEDs. RSC Adv. 2016, 6, 61175–61179. [Google Scholar] [CrossRef]
- Paterson, B.M.; Buncic, G.; McInnes, L.E.; Roselt, P.; Cullinane, C.; Binns, D.S.; Jeffery, C.M.; Price, R.I.; Hicks, R.J.; Donnelly, P.S. Bifunctional 64Cu-labelled macrobicyclic cage amine isothiocyanates for immuno-positron emission tomography. Dalt. Trans. 2015, 44, 4901–4909. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Conti, P. Development of multi-functional chelators based on sarcophagine cages. Molecules 2014, 19, 4246–4255. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.S.; Ma, M.T.; Sunassee, K.; Shaw, K.P.; Williams, J.D.; Paul, R.L.; Donnelly, P.S.; Blower, P.J. Comparison of 64cu-complexing bifunctional chelators for radioimmunoconjugation: Labeling efficiency, specific activity, and in vitro/in vivo stability. Bioconjug. Chem. 2012, 23, 1029–1039. [Google Scholar] [CrossRef] [Green Version]
- Brand, C.; Abdel-Atti, D.; Zhang, Y.; Carlin, S.; Clardy, S.M.; Keliher, E.J.; Weber, W.A.; Lewis, J.S.; Reiner, T. In vivo imaging of GLP-1R with a targeted bimodal PET/fluorescence imaging agent. Bioconjug. Chem. 2014, 25, 1323–1330. [Google Scholar] [CrossRef]
- Hooshyar, Z.; Rezanejade Bardajee, G.; Kakavand, N.; Khanjari, M.; Dianatnejad, N. Investigations on the interactions of DiAmsar with serum albumins: Insights from spectroscopic and molecular docking techniques. Luminescence 2015, 30, 538–548. [Google Scholar] [CrossRef]
- Bottomley, G.A.; Clark, I.J.; Creaser, I.I.; Engelhardt, L.M.; Geue, R.J.; Hagen, K.S.; Harrowfield, J.M.; Lawrance, G.A.; Lay, P.A.; Sargeson, A.M.; et al. The synthesis and structure of encapsulating ligands: Properties of bicyclic hexamines. Aust. J. Chem. 1994, 47, 143–179. [Google Scholar] [CrossRef]
- Cai, H.; Fissekis, J.; Conti, P.S. Synthesis of a novel bifunctional chelator AmBaSar based on sarcophagine for peptide conjugation and 64Cu radiolabelling. Dalt. Trans. 2009, 27, 5395–5400. [Google Scholar] [CrossRef]
- Zagranyarski, Y.; Chen, L.; Zhao, Y.; Wonneberger, H.; Li, C.; Müllen, K. Facile transformation of perylene tetracarboxylic acid dianhydride into strong donor-acceptor chromophores. Org. Lett. 2012, 14, 5444–5447. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision 16.A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Labanowski, J.K.; Andzelm, J.W. (Eds.) Density Functional Methods in Chemistry; Springer: New York, NY, USA, 1991. [Google Scholar]
- Gross, E.K.U.; Dobson, J.F.; Petersilka, M. Density functional theory of time-dependent phenomena. In Density Functional Theory II; Springer: Berlin/Heidelberg, Germany, 1996; pp. 81–172. [Google Scholar]
- Cheshmedzhieva, D.; Ivanova, P.; Stoyanov, S.; Tasheva, D.; Dimitrova, M.; Ivanov, I.; Ilieva, S. Experimental and theoretical study on the absorption and fluorescence properties of substituted aryl hydrazones of 1,8-naphthalimide. Phys. Chem. Chem. Phys. 2011, 13, 18530–18538. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Zagranyarski, Y.; Chen, L.; Jänsch, D.; Gessner, T.; Li, C.; Müllen, K. Toward perylene dyes by the hundsdiecker reaction. Org. Lett. 2014, 16, 2814–2817. [Google Scholar] [CrossRef]
- Manov, H.; Staneva, D.; Vasileva-Tonkova, E.; Kukeva, R.; Stoyanov, S.; Grabchev, I.; Alexandrova, R.; Stoyanova, R. A new Cu(II) complex of PAMAM dendrimer modified with 1,8-naphthalimide: Antibacterial and anticancer activity. Biointerface Res. Appl. Chem. 2021, 12, 5534–5547. [Google Scholar] [CrossRef]
- Yordanova, S.; Grabchev, I.; Stoyanov, S.; Petkov, I. New detectors for metal cations and protons based on PAMAM dendrimers modified with 1,8-naphthalimide units. J. Photochem. Photobiol. A Chem. 2014, 283, 1–7. [Google Scholar] [CrossRef]
- Costabel, D.; Skabeev, A.; Nabiyan, A.; Luo, Y.; Max, J.B.; Rajagopal, A.; Kowalczyk, D.; Dietzek, B.; Wächtler, M.; Görls, H.; et al. 1,7,9,10-tetrasubstituted PMIs accessible through decarboxylative bromination: Synthesis, characterization, photophysical studies, and hydrogen evolution catalysis. Chem. A Eur. J. 2021, 27, 4081–4088. [Google Scholar] [CrossRef]
- Sahoo, D.; Sharma, V.; Roy, R.; Varghese, N.; Mohanta, K.; Koner, A.L. Synthesis of highly-soluble push-pull perylenemonoimide derivatives by regioselective peri-functionalization for switchable memory applications. Chem. Commun. 2019, 55, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Legault, C.Y. CYLview, 1.0b; Université de Sherbrooke: Sherbrooke, QC, Canada, 2009; Available online: https://www.cylview.org (accessed on 26 February 2023).
- Chen, Z.; Baumeister, U.; Tschierske, C.; Würthner, F. Effect of core twisting on self-assembly and optical properties of perylene bisimide dyes in solution and columnar liquid crystalline phases. Chem. A Eur. J. 2007, 13, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Briggs, E.A.; Besley, N.A. Density functional theory based analysis of photoinduced electron transfer in a triazacryptand based K + sensor. J. Phys. Chem. A 2015, 119, 2902–2907. [Google Scholar] [CrossRef] [PubMed] [Green Version]

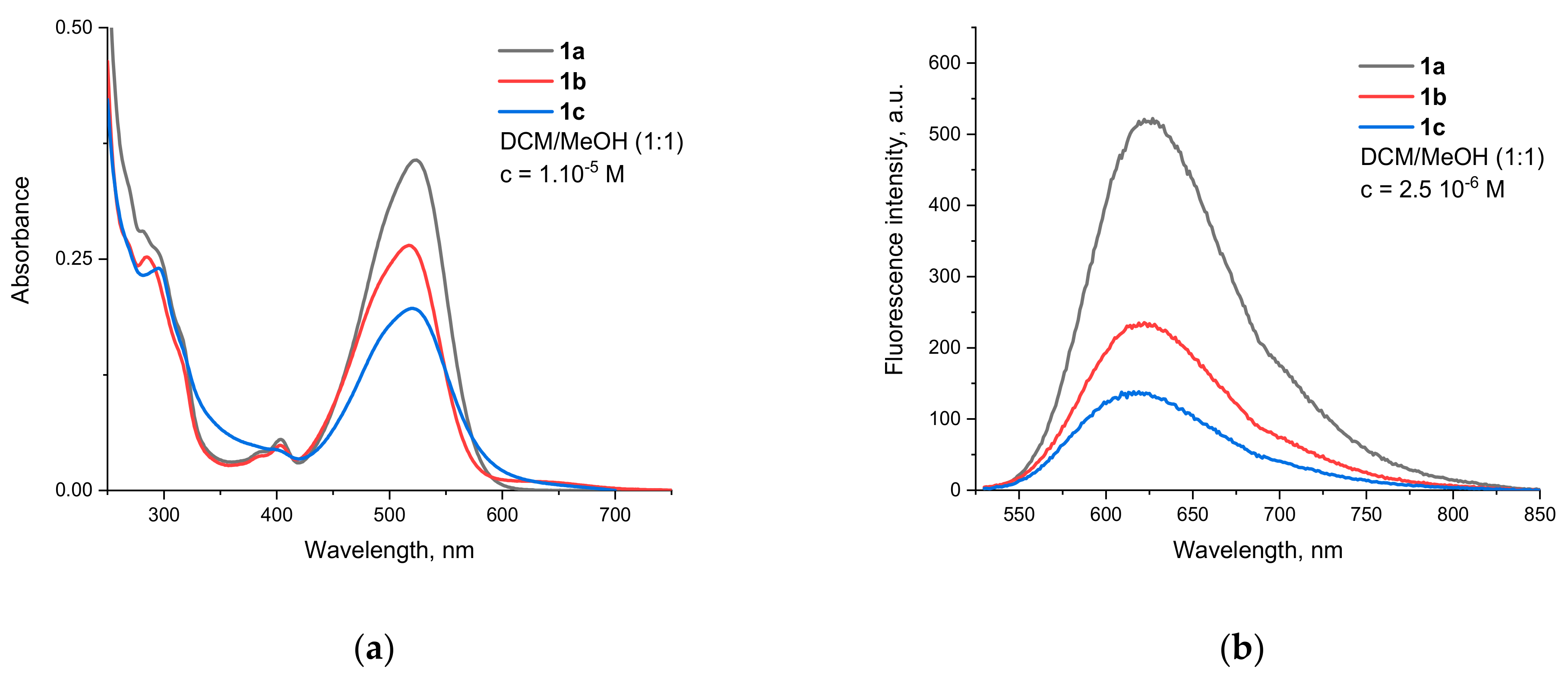


| Compound | ε, l. mol−1 cm−1 | RQY *, % | |||
|---|---|---|---|---|---|
| 1a | 523 | 35,700 | 625 | 3120 | 100 # |
| 1b | 519 | 26,500 | 622 | 3191 | 61 |
| 1c | 520 | 19,700 | 619 | 3076 | 48 |
| B3LYP/6-31G(d,p) | PBE0/6-311+G(2d,p) | Experiment * | ||||
|---|---|---|---|---|---|---|
| λmax [eV] | f | Electronic Transition Contribution [%] | λmax [eV] | f | λmax [eV] | |
| 1a | 2.26 | 0.6632 | HOMO–LUMO 99.4 | 2.30 | 0.6862 | 2.37 |
| 1b | 2.28 | 0.6224 | HOMO–LUMO 99.4 | 2.31 | 0.6429 | 2.39 |
| 1c | 2.19 | 0.6863 | HOMO-4–LUMO 99.2 | 2.32 | 0.6928 | 2.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagranyarski, Y.; Cheshmedzhieva, D.V.; Mutovska, M.; Ahmedova, A.; Stoyanov, S. Dioxepine-Peri-Annulated PMIs—Synthesis and Spectral and Sensing Properties. Sensors 2023, 23, 2902. https://doi.org/10.3390/s23062902
Zagranyarski Y, Cheshmedzhieva DV, Mutovska M, Ahmedova A, Stoyanov S. Dioxepine-Peri-Annulated PMIs—Synthesis and Spectral and Sensing Properties. Sensors. 2023; 23(6):2902. https://doi.org/10.3390/s23062902
Chicago/Turabian StyleZagranyarski, Yulian, Diana Valentinova Cheshmedzhieva, Monika Mutovska, Anife Ahmedova, and Stanimir Stoyanov. 2023. "Dioxepine-Peri-Annulated PMIs—Synthesis and Spectral and Sensing Properties" Sensors 23, no. 6: 2902. https://doi.org/10.3390/s23062902
APA StyleZagranyarski, Y., Cheshmedzhieva, D. V., Mutovska, M., Ahmedova, A., & Stoyanov, S. (2023). Dioxepine-Peri-Annulated PMIs—Synthesis and Spectral and Sensing Properties. Sensors, 23(6), 2902. https://doi.org/10.3390/s23062902








