Novel Au Nanoparticle-Modified ZnO Nanorod Arrays for Enhanced Photoluminescence-Based Optical Sensing of Oxygen
Abstract
1. Introduction
2. Materials and Methods
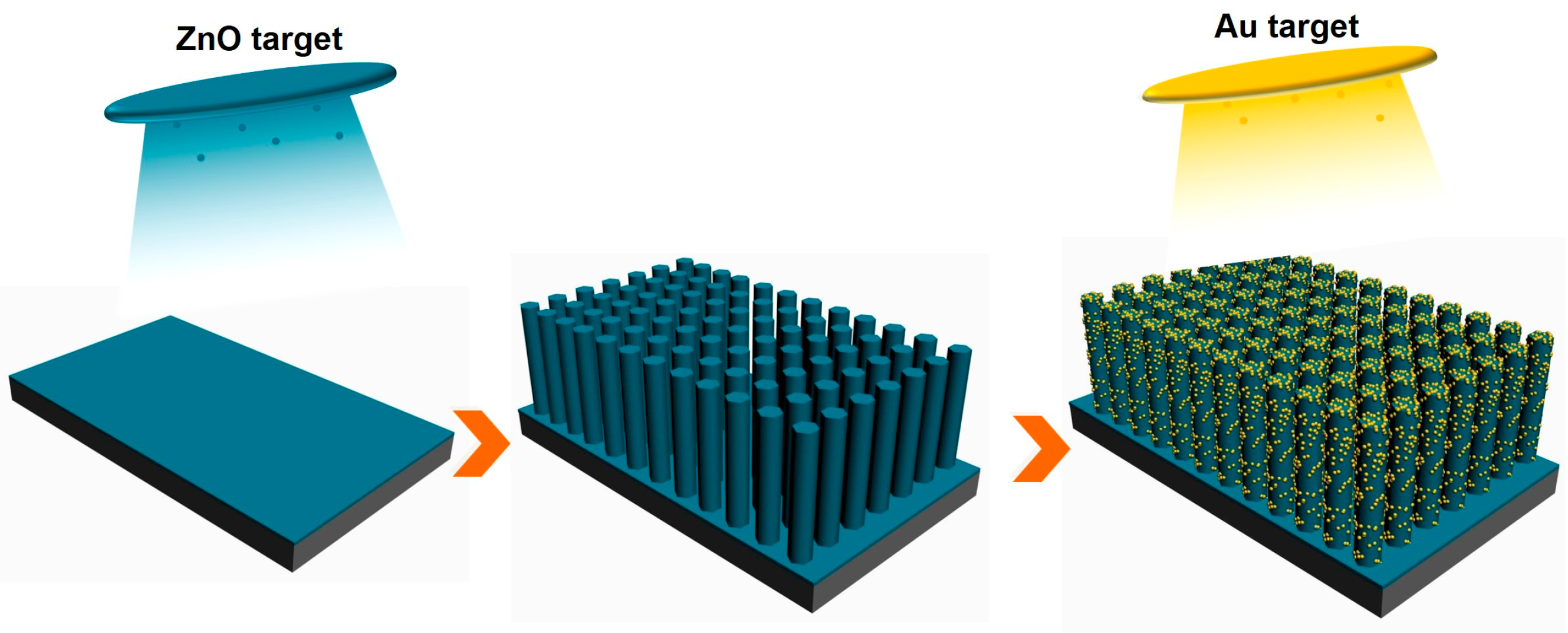
2.1. Preparation of Au/ZnO NR Array Substrate
2.2. Material Characterization and Optical Gas-Sensing Measurements
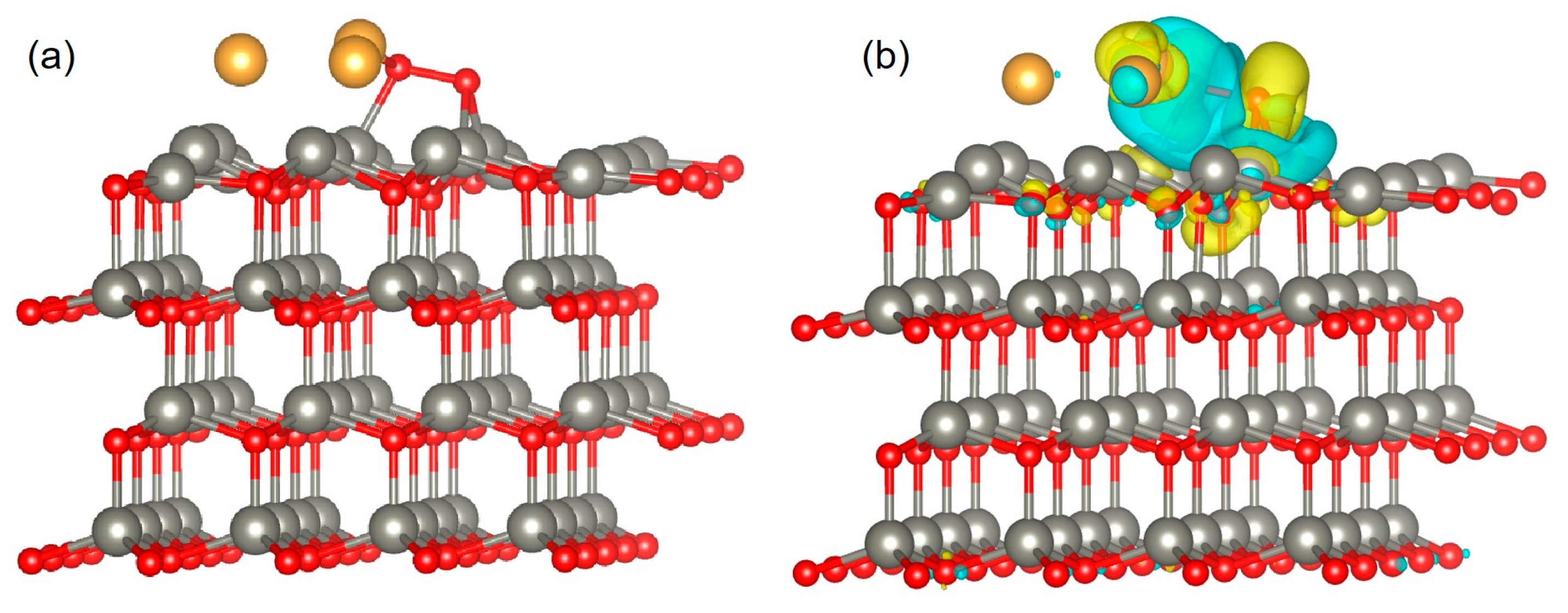
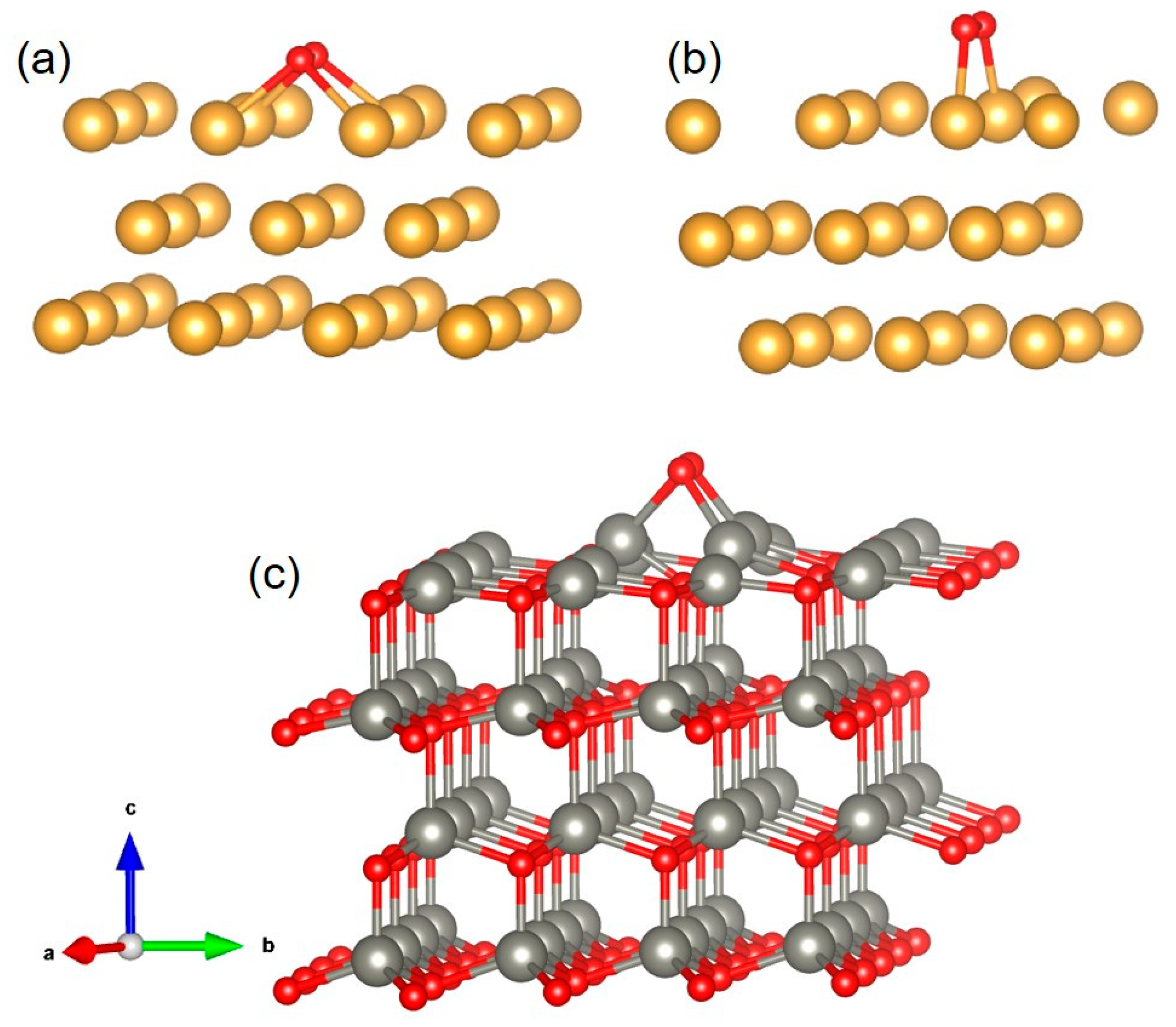
2.3. Material Models and DFT Simulation
3. Results and Discussion
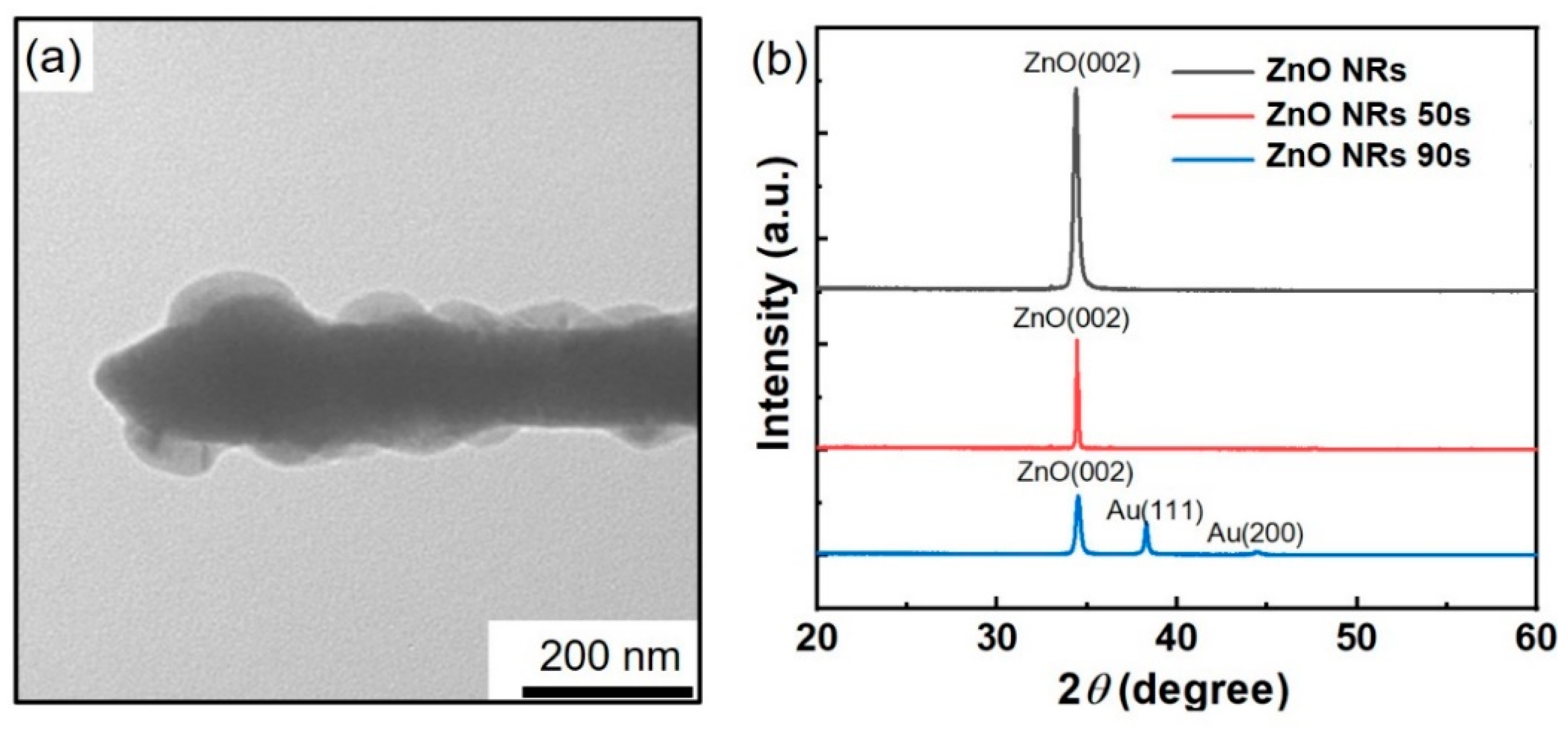
3.1. Morphology and Structure of Au/ZnO NR Samples
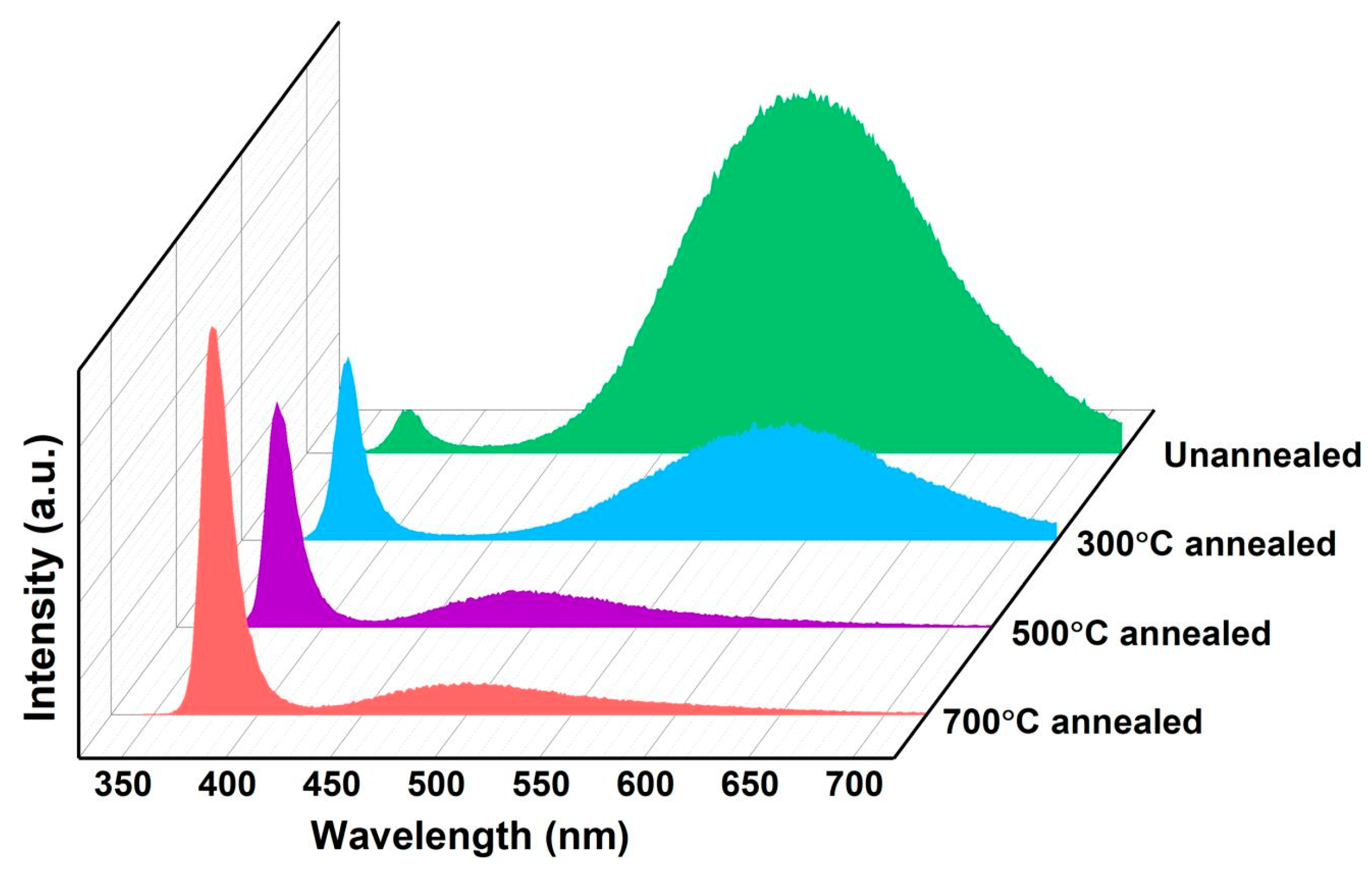
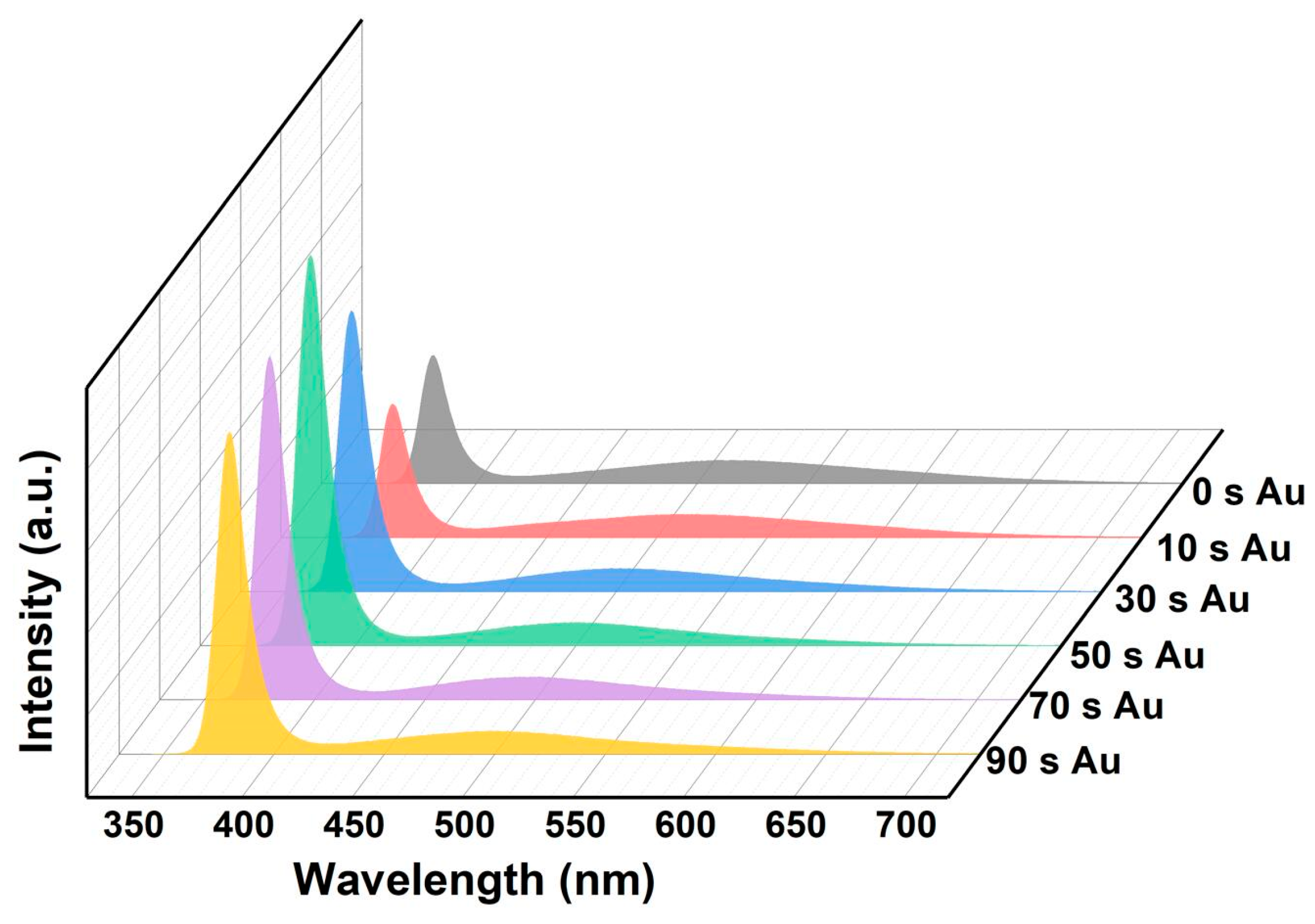
3.2. Optical Properties of Au/ZnO NR Samples
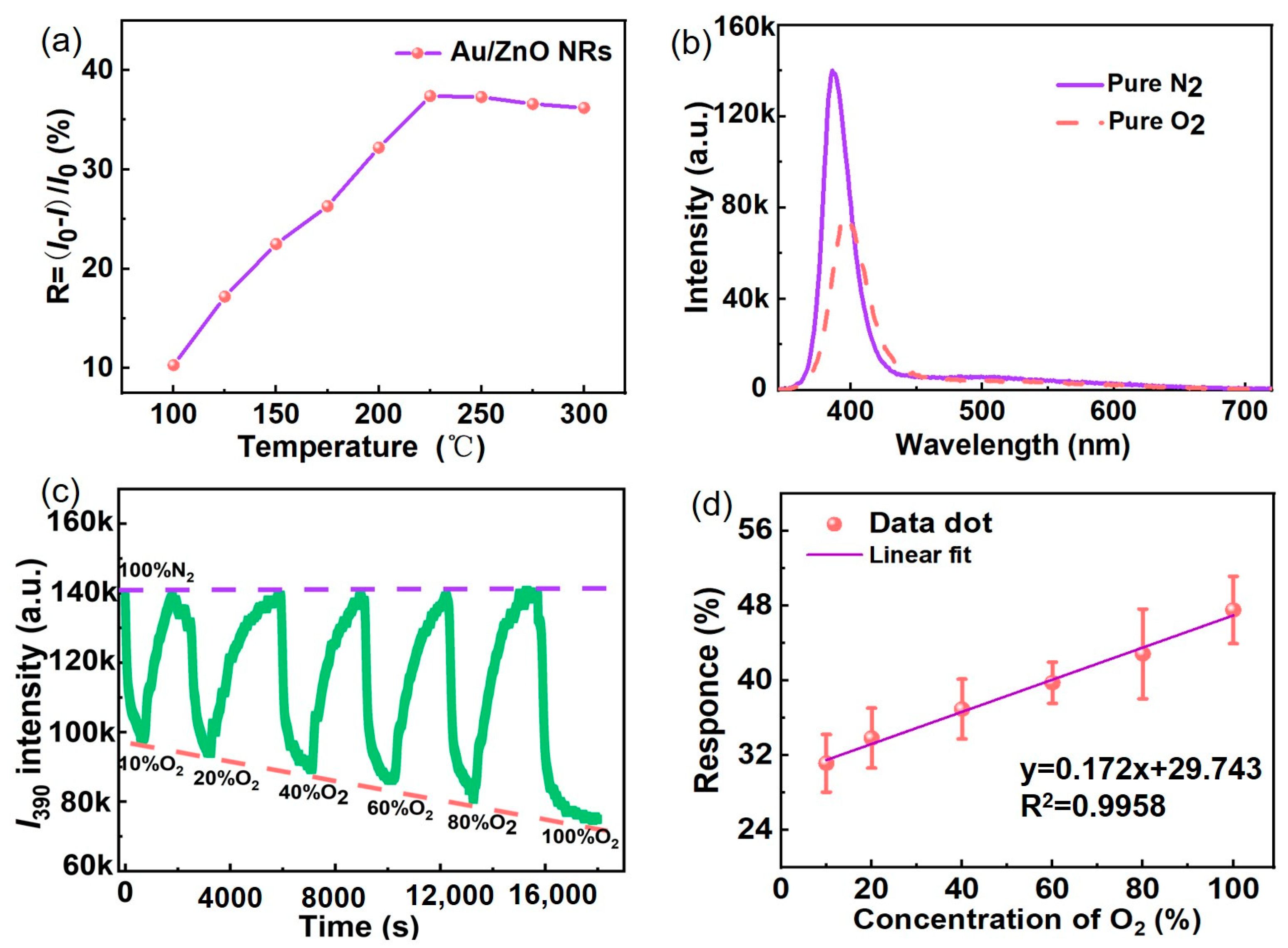
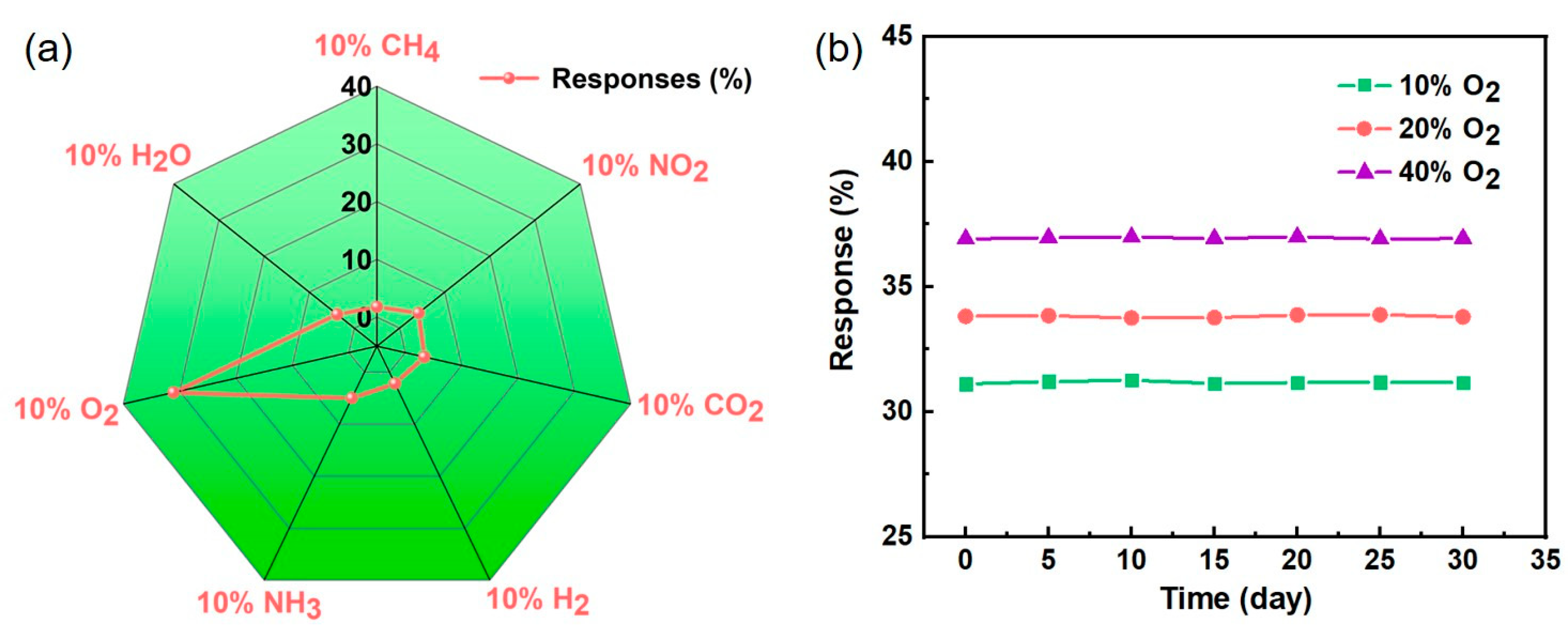
3.3. Gas-Sensing Properties of Au/ZnO NR Sample
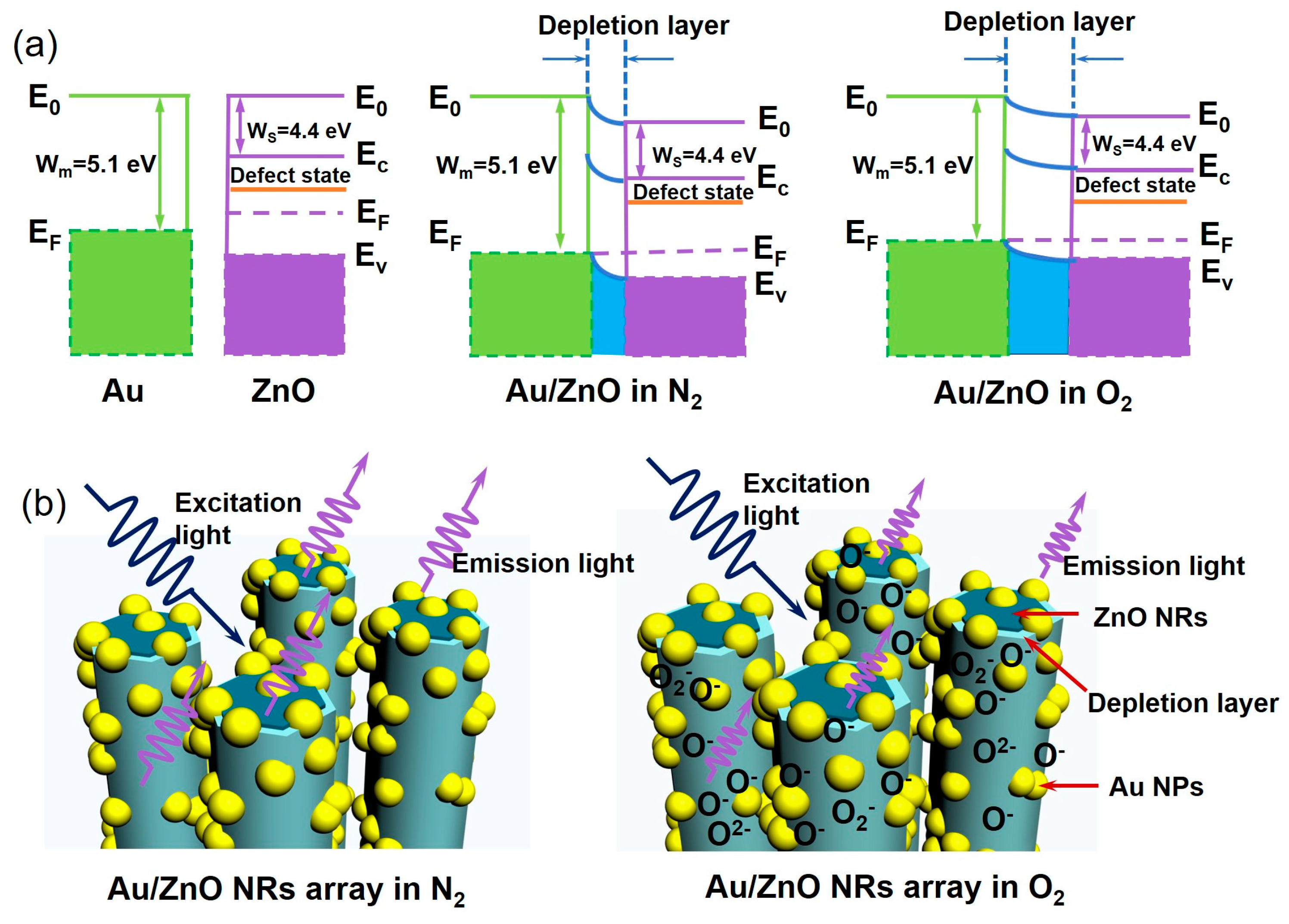
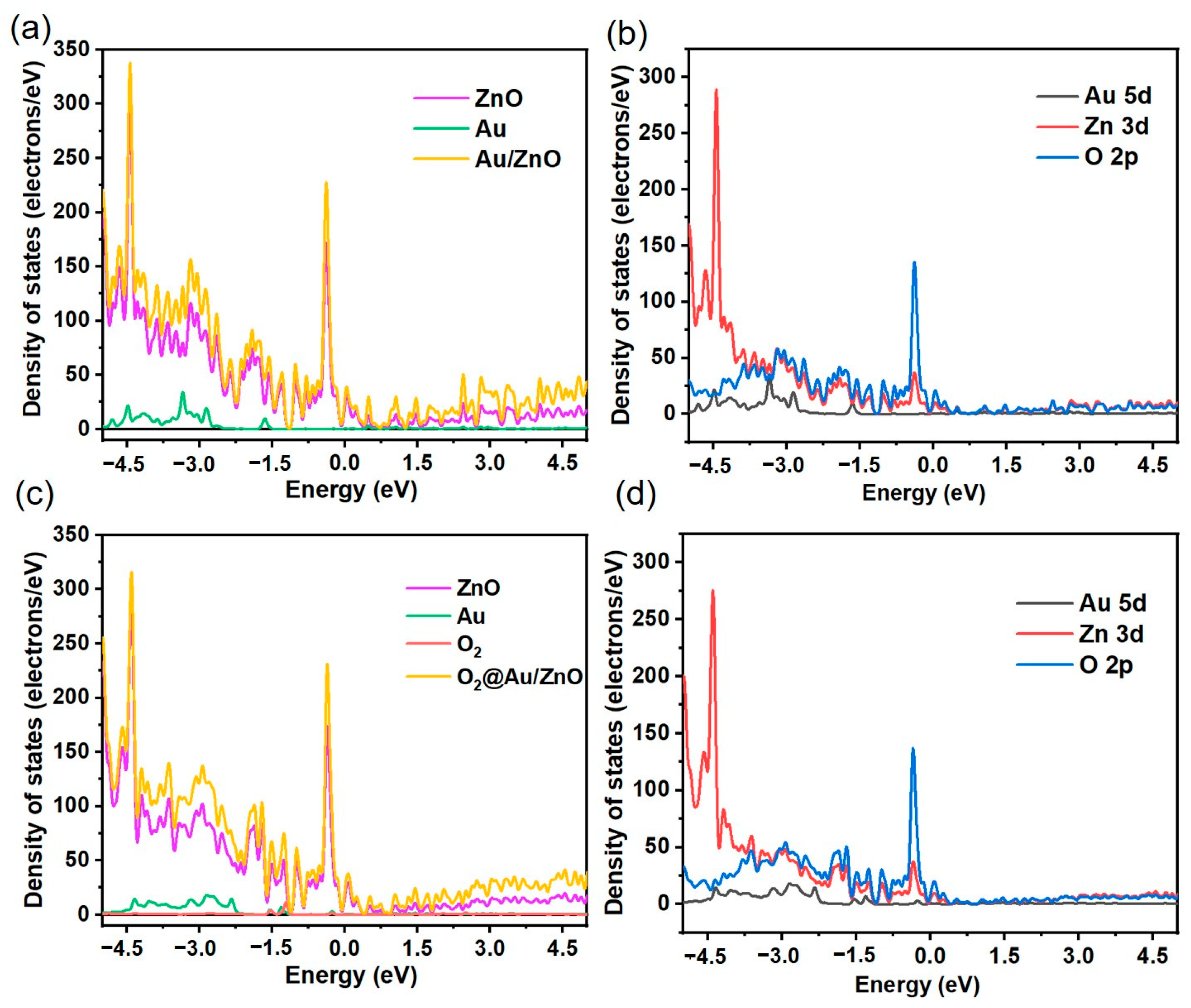
3.4. Gas-Sensing Mechanism of Au/ZnO Sensors
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Berean, K.J.; Ha, N.; Chrimes, A.F.; Xu, K.; Grando, D.; Zhen, J.; Pillai, N.; Campbell, J.L.; Brkljača, R.; et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat. Electron. 2018, 1, 79–87. [Google Scholar] [CrossRef]
- Liu, H.C.; Pan, G.C.; Yang, Z.Q.; Wen, Y.T.; Zhang, X.Y.; Zhang, S.T.; Li, W.J.; Yang, B. Dual-emission of fluorescence and room-temperature phosphorescence for ratiometric and colorimetric oxygen sensing and detection based on dispersion of pure organic thianthrene dimer in polymer host. Adv. Optical Mater. 2022, 10, 2102814. [Google Scholar] [CrossRef]
- Dong, X.Y.; Si, Y.B.; Yang, J.S.; Zhang, C.; Han, Z.; Luo, P.; Wang, Z.Y.; Zang, S.Q.; Mak, T.C.W. Ligand engineering to achieve enhanced ratiometric oxygen sensing in a silver cluster-based metal-organic framework. Nat. Commun. 2020, 11, 3678. [Google Scholar] [CrossRef]
- Roussakis, E.; Li, Z.X.; Nichols, A.J.; Evans, C.L. Oxygen-sensing methods in biomedicine from the macroscale to the microscale. Angew. Chem. Int. Ed. 2015, 54, 8340–8362. [Google Scholar] [CrossRef]
- Chakraborty, A.A.; Laukka, T.; Myllykoski, M.; Ringel, A.E.; Booker, M.A.; Tolstorukov, M.Y.; Meng, Y.J.; Meier, S.R.; Jennings, R.B.; Creech, A.L.; et al. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science 2019, 363, 1217–1222. [Google Scholar] [CrossRef]
- Liang, Y.N.; Wu, Z.X.; Wei, Y.M.; Ding, Q.L.; Zilberman, M.; Tao, K.; Xie, X.; Wu, J. Self-healing; self-adhesive and stable organohydrogel-based stretchable oxygen sensor with high performance at room temperature. Nano-Micro Lett. 2022, 14, 52–71. [Google Scholar] [CrossRef]
- Papkovsky, D.B.; Dmitriev, R.I. Biological detection by optical oxygen sensing. Chem. Soc. Rev. 2013, 42, 8700–8732. [Google Scholar] [CrossRef]
- Arunraja, L.; Thirumoorthy, P.; Karthik, A.; Rajendran, V.; Edwinpaul, L. EDTA-decorated nanostructured ZnO/CdS thin films for oxygen gas sensing applications. J. Electron. Mater. 2016, 45, 4100–4107. [Google Scholar] [CrossRef]
- Zheng, X.C.; Wang, X.; Mao, H.; Wu, W.; Liu, B.R.; Jiang, X.Q. Hypoxia-specific ultrasensitive detection of tumours and cancer cells in vivo. Nat. Commun. 2015, 6, 5834–5846. [Google Scholar] [CrossRef]
- Yadav, K.; Gahlaut, S.K.; Mehta, B.R.; Singh, J.P. Photoluminescence based H2 and O2 gas sensing by ZnO nanowires. Appl. Phys. Lett. 2016, 108, 071602. [Google Scholar] [CrossRef]
- Kraker, E.; Haase, A.; Lamprecht, B.; Jakopic, G. Integrated organic electronic based optochemical sensors using polarization filters. Appl. Phys. Lett. 2008, 92, 033302. [Google Scholar] [CrossRef]
- Xu, R.Y.; Wang, Y.F.; Duan, X.P.; Lu, K.D.; Micheroni, D.; Hu, A.; Lin, W.B. Nanoscale metal–organic frameworks for ratiometric oxygen sensing in live cells. J. Am. Chem. Soc. 2016, 138, 2158–2161. [Google Scholar] [CrossRef]
- Kalyuzhner, Z.; Agdarov, S.; Bennett, A.; Beiderman, Y.; Zalevsky, Z. Remote photonic sensing of blood oxygen saturation via tracking of anomalies in micro-saccades patterns. Opt. Express 2021, 29, 3386–3394. [Google Scholar] [CrossRef]
- Lim, C.J.; Park, J.W. Wearable transcutaneous oxygen sensor for health monitoring. Sensor. Actuat. A Phys. 2019, 298, 111607. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials; spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666. [Google Scholar] [CrossRef]
- Lin, R.B.; Liu, S.Y.; Ye, J.W.; Li, X.Y.; Zhang, J.P. Photoluminescent metal–organic frameworks for gas sensing. Adv. Sci. 2016, 3, 1500434. [Google Scholar] [CrossRef]
- Lew, T.T.S.; Koman, V.B.; Silmore, K.S.; Seo, J.S.; Gordiichuk, P.; Kwak, S.Y.; Park, M.; Ang, M.C.Y.; Khong, D.T.; Lee, M.A.; et al. Real-time detection of wound-induced H2O2 signalling waves in plants with optical nanosensors. Nat. Plants 2020, 6, 404–415. [Google Scholar] [CrossRef]
- Tang, W.; Sun, Y.; Wang, S.C.; Du, B.S.; Yin, Y.Q.; Liu, X.; Yang, B.; Cao, W.W.; Yu, M. Pr3+-Doped (K0.5Na0.5)NbO3 as a high response optical oxygen sensing agent. J. Mater. Chem. C 2016, 4, 11508. [Google Scholar] [CrossRef]
- Du, B.S.; Zheng, Y.Z.; Ye, J.F.; Wang, D.K.; Mao, C.T.; Sun, N.K. Photoluminescence-based sensing of ethanol gas with ultrafine WO3 nanorods. Opt. Lett. 2022, 47, 1145–1148. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B.X. Titanium dioxide nanomaterials for sensor applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Alfè, M.; Gargiulo, V.; Amati, M.; Maraloiu, V.A.; Maddalena, P.; Lettieri, S. Mesoporous TiO2 from metal-organic frameworks for photoluminescence-based optical sensing of oxygen. Catalysts 2021, 11, 795. [Google Scholar] [CrossRef]
- Jiang, T.T.; Du, B.S.; Zhang, H.; Yu, D.F.; Sun, L.; Zhao, G.Y.; Yang, C.H.; Sun, Y.; Yu, M.; Ashfold, M.N.R. High-performance photoluminescence-based oxygen sensing with Pr-modified ZnO nanofibers. Appl. Surf. Sci. 2019, 483, 922–928. [Google Scholar] [CrossRef]
- Liu, X.; Du, B.S.; Sun, Y.; Yu, M.; Yin, Y.Q.; Tang, W.; Chen, C.; Sun, L.; Yang, B.; Cao, W.W.; et al. Sensitive room temperature photoluminescence-based sensing of H2S with novel CuO-ZnO nanorods. ACS Appl. Mater. Interfaces 2016, 8, 16379–16385. [Google Scholar] [CrossRef]
- Lettieri, S.; Setaro, A.; Baratto, C.; Comini, E.; Faglia, G.; Sberveglieri, G.; Maddalena, P. On the mechanism of photoluminescence quenching in tin dioxide nanowires by NO2 adsorption. New J. Phys. 2008, 10, 043013. [Google Scholar] [CrossRef]
- Sailor, M.J.; Wu, E.C. Photoluminescence-based sensing with porous silicon films, microparticles, and nanoparticles. Adv. Funct. Mater. 2009, 19, 3195–3280. [Google Scholar] [CrossRef]
- Nalwa, K.S.; Cai, Y.K.; Thoeming, A.L.; Shinar, J.; Shinar, R.; Chaudhary, S. Polythiophene-fullerene based photodetectors: Tuning of spectral response and application in photoluminescence based (Bio)chemical sensors. Adv. Mater. 2010, 22, 4157–4161. [Google Scholar] [CrossRef]
- Alenezi, M.R.; Henley, S.J.; Emerson, N.G.; Silva, S.R.P. From 1D and 2D ZnO nanostructures to 3D hierarchical structures with enhanced gas sensing properties. Nanoscale 2014, 6, 235–247. [Google Scholar] [CrossRef]
- Agarwal, S.; Rai, P.; Gatell, E.N.; Llobet, E.; Güell, F.; Kumar, M.; Awasthi, K. Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sens. Actuators B Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef]
- Xu, Y.S.; Zheng, L.L.; Yang, C.; Zheng, W.; Liu, X.H.; Zhang, J. Oxygen vacancies enabled porous SnO2 thin films for highly sensitive detection of triethylamine at room temperature. ACS Appl. Mater. Interfaces 2020, 12, 20707–20713. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.K.; Si, G.K.; Li, Y.; Zhang, S.W.; Deng, X.L.; Xu, X.J. Oxygen vacancy defects engineering on Ce-doped α-Fe2O3 gas sensor for reducing gases. Sens. Actuators B Chem. 2020, 302, 127165. [Google Scholar] [CrossRef]
- Qu, X.; Yang, R.; Tong, F.; Zhao, Y.; Wang, M.H. Hierarchical ZnO microstructures decorated with Au nanoparticles for enhanced gas sensing and photocatalytic properties. Powder Technol. 2018, 330, 259–265. [Google Scholar] [CrossRef]
- Liu, R.C.; Xie, D.C.; Adedokun, G.; Xue, F.; Xu, L.; Wu, F. A low power bridge-type gas sensor with enhanced sensitivity to oxygen by sandwiched ZnO/Au/ZnO film sputtered in O2 atmosphere. IEEE Sens. J. 2021, 21, 18578–18587. [Google Scholar] [CrossRef]
- Du, B.S.; Tang, C.C.; Zhao, D.; Zhang, H.; Yu, D.F.; Yu, M.; Balram, K.C.; Gersen, H.; Yang, B.; Cao, W.W.; et al. Diameter-optimized high-order waveguide nanorods for fluorescence enhancement applied in ultrasensitive bioassays. Nanoscale 2019, 11, 14322–14329. [Google Scholar] [CrossRef]
- Tan, S.T.; Tan, C.H.; Chong, W.Y.; Chi, C.Y.; Umar, A.A.; Ginting, R.T.; Lee, H.B.; Lim, K.S.M.Y.; Salleh, M.M. Microwave-assisted hydrolysis preparation of highly crystalline ZnO nanorod array for room temperature photoluminescence-based CO gas sensor. Sens. Actuators B 2016, 227, 304–312. [Google Scholar]
- Liu, X.; Sun, Y.; Yu, M.; Yin, Y.; Yang, B.; Cao, W.W.; Ashfold, M.N.R. Incident fluence dependent morphologies; photoluminescence and optical oxygen sensing properties of ZnO nanorods grown by pulsed laser deposition. J. Mater. Chem. C 2015, 3, 2557–2562. [Google Scholar] [CrossRef]
- Valerini, D.; Cretì, A.; Caricato, A.P.; Lomascolo, M.; Rella, R.; Martino, M. Optical gas sensing through nanostructured ZnO films with different morphologies. Sens. Actuators B 2010, 145, 167–173. [Google Scholar] [CrossRef]
- Madel, M.; Jakob, J.; Huber, F.; Neuschl, B.; Bauer, S.; Xie, Y.; Tischer, I.; Thonke, K. Optical gas sensing by micro-photoluminescence on multiple and single ZnO nanowires. Phys. Status Solidi 2015, 212, 1810–1816. [Google Scholar] [CrossRef]
- Cretì, A.; Valerini, D.; Taurino, A.; Quaranta, F.; Lomascolo, M.; Rella, R. Photoluminescence quenching processes by NO2 adsorption in ZnO nanostructured films. J. Appl. Phys. 2012, 111, 073520. [Google Scholar] [CrossRef]
- Aad, R.; Simic, V.; Le, C.L.; Rocha, L.; Sallet, V.; Sartel, C.; Lusson, A.; Couteaua, C.; Lerondel, G. ZnO nanowires as effective luminescent sensing materials for nitroaromatic derivatives. Nanoscale 2013, 5, 9176–9180. [Google Scholar] [CrossRef]
- Lei, M.L.; Gao, M.Q.; Yang, X.Y.; Zou, Y.D.; Alghamdi, A.; Ren, Y.; Deng, Y.H. Size-controlled Au nanoparticles incorporating mesoporous ZnO for sensitive oxygen sensing. ACS Appl. Mater. Interfaces 2021, 13, 51933–51944. [Google Scholar] [CrossRef]
- Xue, D.P.; Zhang, Z.Y.; Wang, Y. Enhanced methane sensing performance of SnO2 nanoflowers based sensors decorated with Au nanoparticles. Mater. Chem. Phys. 2019, 237, 121864. [Google Scholar] [CrossRef]
- Liu, H.Y.; Yang, W.; Wang, M.X.; Xiao, L.; Liu, S.T. Fabrication of lotus-like Au@TiO2 nanocomposites with enhanced gas-sensing properties. Sens. Actuators B Chem. 2016, 236, 490–498. [Google Scholar] [CrossRef]
- Yan, H.H.; Song, P.; Zhang, S.; Zhang, J.; Yang, Z.X.; Wang, Q. Au nanoparticles modified MoO3 nanosheets with their enhanced properties for gas sensing. Sens. Actuators B Chem. 2016, 236, 201–207. [Google Scholar] [CrossRef]
- Lee, J.S.; Katoch, A.; Kim, J.H.; Kim, S.S. Effect of Au nanoparticle size on the gas-sensing performance of p-CuO nanowires. Sens. Actuators B Chem. 2016, 222, 307–314. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yan, Y.Z.; Yang, L.X.; Xing, C.; Zeng, Y.; Zhao, Y.; Jiang, Y.J. Ultraviolet luminescence enhancement of planar wide bandgap semiconductor film by a hybrid microsphere cavity/dual metallic nanoparticles sandwich structure. Opt. Express 2019, 27, 15399–15412. [Google Scholar] [CrossRef]
- Li, X.H.; Zhang, Y.; Ren, X.J. Effects of localized surface plasmons on the photoluminescence properties of Au-coated ZnO films. Opt. Express 2009, 17, 8735–8740. [Google Scholar]
- Chen, Y.Z.; Wang, Z.H.; Fu, H.F.; Han, D.M.; Gu, F.B. Insights into the effect of Au particle size on triethylamine sensing properties based on a Au–ZnO nanoflower sensor. J. Mater. Chem. C 2022, 10, 3318–3328. [Google Scholar] [CrossRef]
- Xia, J.; Diao, K.D.; Zheng, Z.; Cui, X.D. Porous Au/ZnO nanoparticles synthesised through a metal organic framework (MOF) route for enhanced acetone gas-sensing. RSC Adv. 2017, 7, 38444. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Li, Y.X.; Feng, B.X.; Wu, Y.; Zhu, Y.H.; Wei, J. Self-templated synthesis of mesoporous Au-ZnO nanospheres for seafood freshness detection. Sens. Actuators B Chem. 2022, 360, 131662. [Google Scholar] [CrossRef]
- Cao, P.J.; Huang, Q.G.; Navale, S.T.; Fang, M.; Liu, X.K.; Zeng, Y.X.; Liu, W.J.; Stadler, F.J.; Lu, Y.M. Integration of mesoporous ZnO and Au@ZnO nanospheres into sensing device for the ultrasensitive CH3COCH3 detection down to ppb levels. Appl. Surf. Sci. 2020, 518, 146223. [Google Scholar] [CrossRef]
- Li, X.W.; Zhou, X.; Guo, H.; Wang, C.; Liu, J.Y.; Sun, P.; Liu, F.M.; Lu, G. Design of Au@ZnO yolk–shell nanospheres with enhanced gas sensing properties. ACS Appl. Mater. Interfaces 2014, 6, 18661–18667. [Google Scholar] [CrossRef]
- Cao, P.J.; Cai, Y.Z.; Pawar, D.; Han, S.; Xu, W.Y.; Fang, M.; Liu, X.K.; Zeng, Y.X.; Liu, W.J.; Lu, Y.M.; et al. Au@ZnO/rGO nanocomposite-based ultra-low detection limit highly sensitive and selective NO2 gas sensor. J. Mater. Chem. C 2022, 10, 4295–4305. [Google Scholar] [CrossRef]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO plasmonic nanostructures for enhanced UV photodetector and room temperature NO sensing devices. Sci. Rep. 2014, 4, 6483–6492. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, S.L.; Liu, J.; Cai, Y.Y.; Liang, C.H. Laser irradiation-induced Au-ZnO nanospheres with enhanced sensitivity and stability in ethanol sensing. Phys. Chem. Chem. Phys. 2016, 18, 22503–22508. [Google Scholar] [CrossRef]
- Yu, S.G.; Zhang, H.Y.; Chen, C.; Lin, C.C. Investigation of humidity sensor based on Au modified ZnO nanosheets via hydrothermal method and first principle. Sens. Actuators B Chem. 2019, 287, 526–534. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, J.; Zhang, K.W.; Yang, X.D.; Zhang, M.Z. The modification effect of Fe2O3 nanoparticles on ZnO nanorods improves the adsorption and detection capabilities of TEA. Inorg. Chem. Front. 2022, 9, 259–266. [Google Scholar] [CrossRef]
- Song, X.P.; Li, L.; Chen, X.; Xu, Q.; Song, B.; Pan, Z.Y.; Liu, Y.N.; Juan, F.Y.; Xu, F.; Cao, B.Q. Enhanced triethylamine sensing performance of α-Fe2O3 nanoparticle/ZnO nanorod heterostructures. Sens. Actuators B Chem. 2019, 298, 126917. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Pan, W.J.; Zhou, L.J.; Yu, S.J. Room-temperature benzene sensing with Au-doped ZnO nanorods/exfoliated WSe2 nanosheets and density functional theory simulations. ACS Appl. Mater. Interfaces 2021, 13, 33392–33403. [Google Scholar] [CrossRef]


| Sensitive Materials | Method | Detection Range | Detection Limit | Stability | Reference |
|---|---|---|---|---|---|
| PAM-CS DN | Electrochemical | 1–100% O2 | 1% O2 | Temperature and humidity | [6], 2022 |
| ZnO/CdS-EDTA | Electrical | 10–50 ppm | 10 ppm | Long-term | [8], 2016 |
| ZnO nanowires | Photoluminescence | 9% O2 (10–50 sccm) | 9% O2 | Flow | [10], 2016 |
| Dye | Optochemical | 5–100% O2 | 5% O2 | [11], 2008 | |
| PVDC | Photoluminescence | 2–30% O2 | 2% O2 | Temperature | [14], 2019 |
| Pr3+/(K0.5 Na0.5)Nb3 | Photoluminescence | 2–100% O2 | 2% O2 | [18], 2016 | |
| Pd/TiO2 | Electrical | 100–1000 ppm | 100 ppm | Flow | [20], 2007 |
| Mesoporous TiO2 | Photoluminescence | 2–20% O2 | 2% O2 | [21], 2021 | |
| Pr/ZnO nanofibers | Photoluminescence | 3–20% O2 | 3% O2 | [22], 2019 | |
| Au/ZnO nanorods | Photoluminescence | 1–100% O2 | 1% O2 | Long-term | This work |
| Structure | Etotal | EO2 | Ebase | Eadsorption |
|---|---|---|---|---|
| Au (200) | −103 | −9.85 | −92.209 | −0.941 |
| Au (111) | −107 | −9.85 | −96.191 | −0.959 |
| ZnO (002) | −574 | −9.85 | −559.87 | −4.28 |
| Au/ZnO | −585 | −9.85 | −573.62 | −1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.; Zhang, M.; Ye, J.; Wang, D.; Han, J.; Zhang, T. Novel Au Nanoparticle-Modified ZnO Nanorod Arrays for Enhanced Photoluminescence-Based Optical Sensing of Oxygen. Sensors 2023, 23, 2886. https://doi.org/10.3390/s23062886
Du B, Zhang M, Ye J, Wang D, Han J, Zhang T. Novel Au Nanoparticle-Modified ZnO Nanorod Arrays for Enhanced Photoluminescence-Based Optical Sensing of Oxygen. Sensors. 2023; 23(6):2886. https://doi.org/10.3390/s23062886
Chicago/Turabian StyleDu, Baosheng, Meng Zhang, Jifei Ye, Diankai Wang, Jianhui Han, and Tengfei Zhang. 2023. "Novel Au Nanoparticle-Modified ZnO Nanorod Arrays for Enhanced Photoluminescence-Based Optical Sensing of Oxygen" Sensors 23, no. 6: 2886. https://doi.org/10.3390/s23062886
APA StyleDu, B., Zhang, M., Ye, J., Wang, D., Han, J., & Zhang, T. (2023). Novel Au Nanoparticle-Modified ZnO Nanorod Arrays for Enhanced Photoluminescence-Based Optical Sensing of Oxygen. Sensors, 23(6), 2886. https://doi.org/10.3390/s23062886






