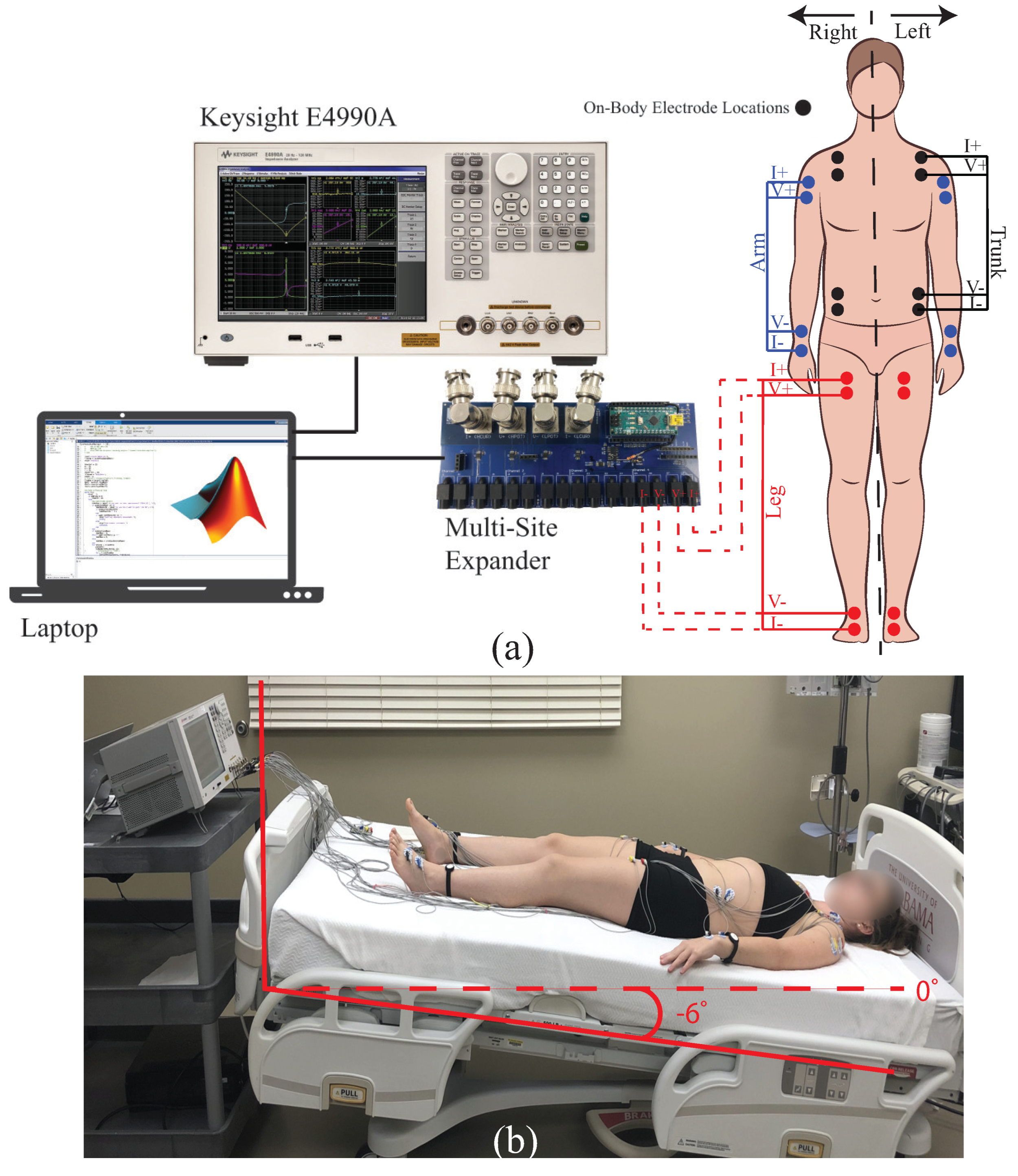
1. Introduction
During space travel, astronauts are subjected to extreme environmental conditions in comparison to conditions on the Earth’s surface. These extreme conditions include high radiation exposure and microgravity. Both of these conditions cause a range of adaptations in the human body [
1]. These adaptations affect the musculoskeletal system (muscle atrophy and loss of bone mineral density), renal system (kidney stone formation), and the vision system (vision impairment). One effect of microgravity on the human body is the redistribution of fluid due to the removal of the hydrostatic gravitational gradient [
1,
2]. Without this gradient, fluid redistributes from the legs toward the chest and head [
1,
3]. This is referred to as a cephalad fluid shift.
From a query of 300 astronauts, Mader et al. reported that approximately
of astronauts on short-duration missions and
of astronauts on long-duration missions experienced a degradation in vision (both distant and near) [
4]. Their hypothesis is that optic nerve and ocular changes may be the result of the cephalad fluid shifts induced by microgravity exposure. Nelson et al. reported in their review of microgravity-induced fluid shifts and ophthalmic changes that permanent loss of visual acuity is one of the highest perceived medical risks of a long spaceflight [
5]. With microgravity-induced fluid shifts expected to be the source of severe medical risks combined with the expectation of space missions increasing in duration, it is critical to have more knowledge of how fluid shifts impact various body systems, how to monitor fluid shifts, and how to develop counter-measures to reduce their impact on astronauts during and after exposure to microgravity.
One measurement methodology that has been investigated for monitoring and quantifying fluid shifts is the electrical impedance of biological tissues [
6,
7,
8,
9,
10]. The passive electrical impedance of tissues (often referred to as tissue bioimpedance) is related to the tissue fluid, type, structure, and geometry. These measurements are collected by exciting the tissue with a low amplitude electrical current (
I, below the perception threshold of an individual) and measuring the voltage response (
V) of the tissue from which the impedance is calculated (
). These measurements are non-invasive and non-ionizing, which make them an attractive monitoring solution. Changes in physiology features are expected to alter the tissue impedance and potentially serve as a biomarker of that underlying mechanism (e.g., fluid shift, tissue damage, tissue swelling). For fluid shift-related applications, the change in the amount (decrease or increase) of fluid within a region of the body is expected to alter the resistance of that body segment (increase or decrease, respectively). Therefore, monitoring the electrical impedance of multiple body segments, such as the arms, legs, and trunks has the potential to reflect fluid distribution (and redistribution).
The use of bioimpedance to measure fluid redistribution (also referred to as impedance plethysmography) for space applications have been reported as early as 1987 with Montgomery providing an overview of 4 specific studies investigating segmental fluid shifts induced by horizontal or −6° head-down bed rest [
6]. Montgomery concluded that calf volume changes due to horizontal or −6° head-down bed rest were immediate, progressive, and uniform across the study participants, not indicative of other segmental changes with time or across subjects. Further, the impedance-derived volumes were highly corrected with anthropometric data [
6]. Additional studies utilized bioimpedance monitoring to compare fluid shifts during horizontal bed rest between men and women (reporting that cephalad shifts between men and women may be different) [
11] and to assess hydration and body composition of cosmonauts onboard the International Space Station [
10]. Additionally, studies of segmental fluid redistribution from transitions in body positions (e.g., sit to stand, sit to supine, etc.) have utilized tissue impedance to measure these alterations [
8,
12,
13,
14]. From this body of knowledge, an emerging trend is that segmental leg resistance changes with body positions as a result of the change in the hydrostatic gravitation gradient. More specifically, the observed trend is that laying in a supine or head-down tilt position increases the segmental leg resistance and is attributed to the movement of fluid out of this body segment. Support for this trend is provided by (1) Fenech and Jaffrin, who reported average increases of
in extra-cellular leg resistance of 15 study participants after 30 min in a supine position [
8], (2) Zhu et al., who reported average increases of
in extra-cellular leg resistance of 6 study participants after 30 min in a supine position [
13], and (3) Berg, who reported an approximately
increase in calf resistance of 7 participants after 120 min in a supine position [
12]. Now, while there are variations across these studies, in terms of electrode configuration and measurement frequency the trend of increasing leg resistance in the supine body position is consistent. The assumption that these increases result from the cephalad fluid shifts supports the application of bioimpedance sensing for monitoring segmental fluid shifts and modeling total body fluid distribution for persons in a microgravity environment.
One limiting factor toward the adoption of bioimpedance sensing in microgravity environments is that measurements have traditionally required laboratory [
11] instruments or portable equipment [
15]. These instruments prevent unobtrusive and continuous monitoring. Recently, however, the increasing availability of low-cost commercial sensors with bioimpedance capabilities [
16] is facilitating the development of wearable systems [
17,
18] to collect tissue bioimpedance continuously. Wearable, non-invasive, and unobtrusive bioimpedance sensors could accurately quantify the real-time, in-space dynamics of fluid shifts. These sensors could collect data from persons in microgravity conditions to improve fluid models of the human body and even be used to evaluate countermeasures to cephalad fluid shifts. However, further information is needed to determine the necessary requirements for wearable systems focused on this application. Further information to support the development of wearables for this application includes identifying the necessary body segments to monitor, the necessary resolution and measurement range, and the optimum electrode locations. While the arm, leg, and trunk segments have been monitored in previous studies [
8,
11,
13,
14], only a single side of the body has been measured. An implied assumption is that fluid shifts are symmetrical due to the bilateral symmetry of the body. That is, the right and left segments have the same fluid shifts in response to changes in body position or gravitational gradients. However, this assumption has not been validated, which provided the motivation for this study. Specifically, to evaluate the question: are there differences between the resistance alterations of the right and left body segments in response to short-term (4 h) of 6° head-down tilt (HDT) body positioning? The following sections outline methods for collecting segmental tissue resistance from study participants, the methods for analysis, analysis results, and the discussion of these results in the context of previous fluid shift studies and the implications for wearable systems.
3. Results
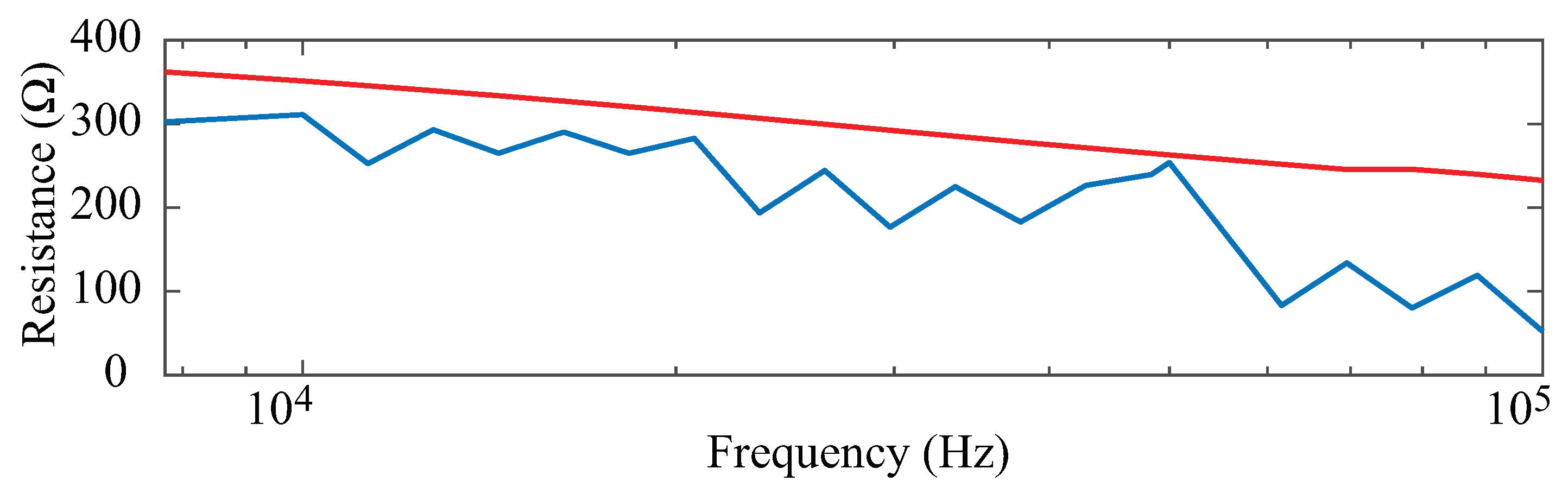
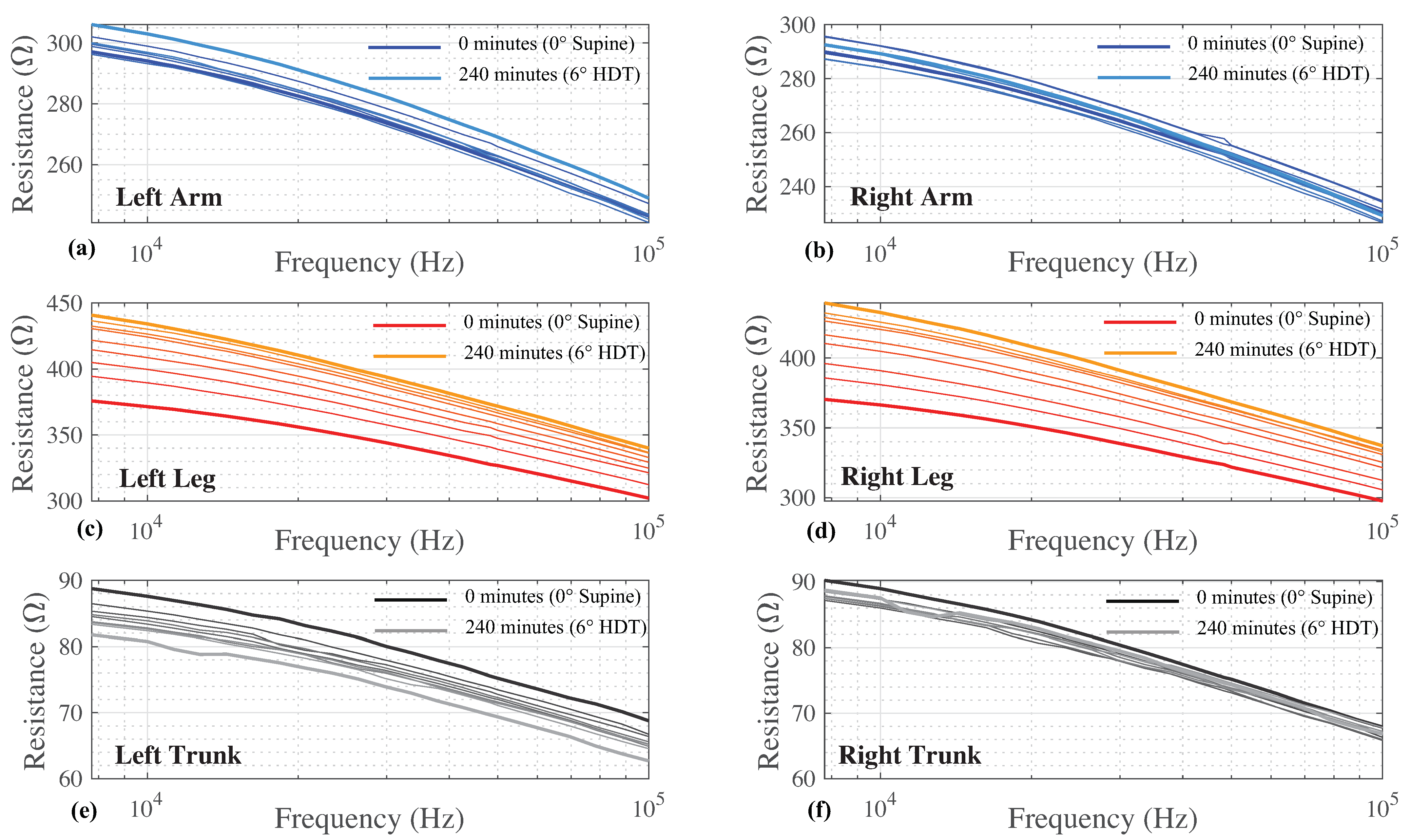
The multi-frequency (7 to 100 kHz) resistance for all time points and body segments from a single participant is provided in
Figure 3 as a sample of participant data included for analysis. The 8 sets of resistance data for each body segment (left and right arm/leg/trunk) are shown in
Figure 3a–f. Different color shades represent measurements collected at different time points with the darkest shade representing data at 0 min and the lightest shade at 240 min. Each segmental resistance shows a decreasing resistance value with increasing frequency, which is typical of biological tissues. For this particular participant, there is a general trend of increasing segmental resistance (at all measured frequencies) between the measurements at 0 min and 240 min for the arms and legs, with the opposite trend (decreasing resistance) for the trunk.
For further analysis resistances at two discrete frequencies, 10 kHz and 100 kHz, were utilized. “Low” and “high” frequency resistances are often attributed to extra-cellular and total tissue fluids, respectively, supporting their independent analysis here. The median 10 kHz and 100 kHz resistances of all participants segmented (left/right leg, arm, and trunk) are provided in
Table 2 and
Table 3, respectively.
3.1. Statistical Analysis
To evaluate if there were statistically significant differences in the 10 kHz and 100 kHz resistances of each segment resulting from the length of time in the HDT position, a Friedman test (SPSS Statistics) was applied to the repeated measures data. For this testing, the dependent variable was segmental resistance and the independent variable was time. The testing of the 10 kHz and 100 kHz right and left leg segments rejected the null hypothesis supporting that there were statistically significant differences of median resistance at the different time points during the HDT protocol (10 kHz left leg: , ; 10 kHz right leg: , , 100 kHz left leg: , , 100 kHz right leg: , ). The 10 kHz and 100 kHz right trunks were also statistically different at the different time points (10 kHz right trunk: , ; 100 kHz right trunk: , ).
Pairwise comparisons were performed (SPSS Statistics) with a Bonferroni correction for multiple (8) comparisons between the measurements at 0 min and all other time points. The 10 kHz leg resistance (for both left and right segments) was statistically significantly different (
) at 120, 150, 180, 210, and 240 min compared to the baseline value. These specific time points are denoted by the
symbol in
Table 2. The median 10 kHz values between 0 min and 240 min of the left/right legs had an increase of 34.5 Ω and 38.3 Ω, respectively. The 100 kHz leg resistances (for both left and right segments) were also statistically significantly different (
) at 90, 120, 150, 180, 210, and 240 min compared to the baseline value. These specific time points are denoted by the
symbol in
Table 3. The median 100 kHz values between 0 min and 240 min of the left/right legs had an increase of 21.1 Ω and 21.8 Ω, respectively. From the pairwise comparisons for both the segmental arms and trunk resistances there were no statistically significant differences between baseline and later time points.
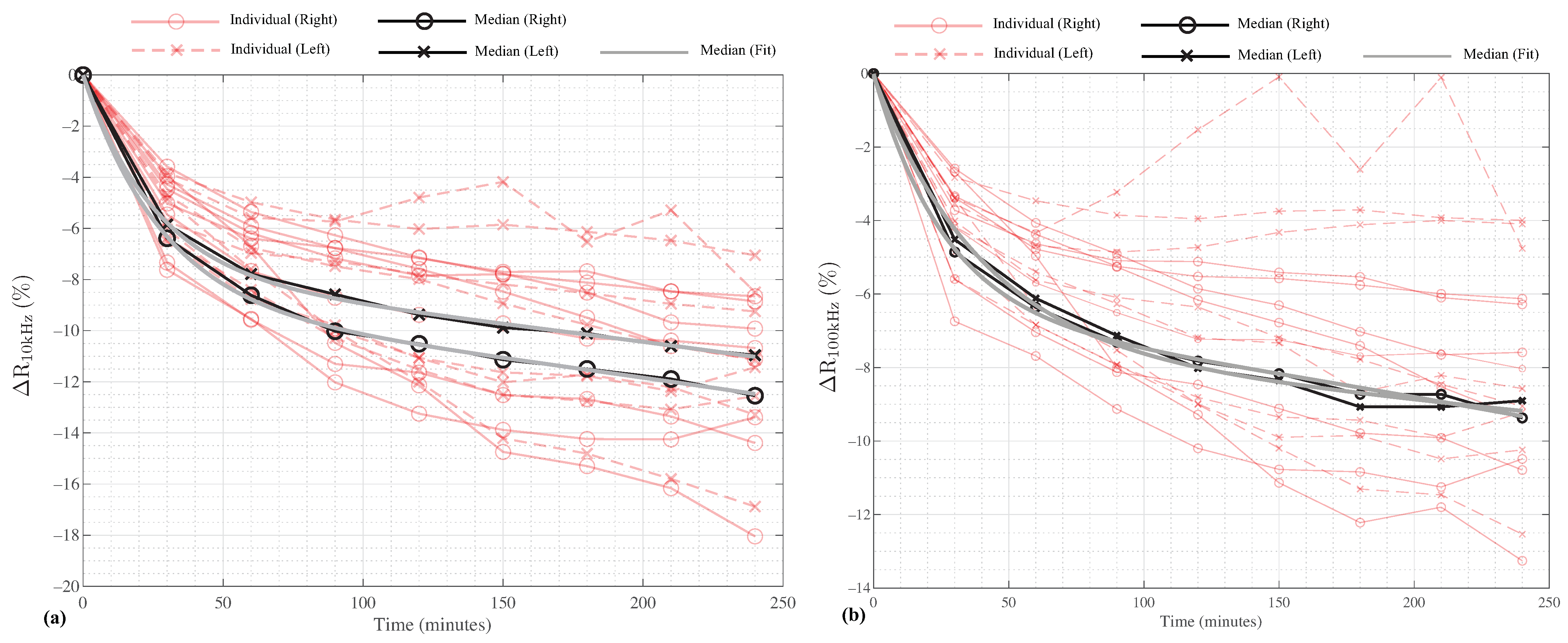
3.2. Relative Differences
The relative differences between the 10 kHz and 100 kHz segmental resistances from
min to
min (in comparison to the baseline at
) were calculated using:
where
is the relative difference,
is the resistance at
min, and
is the resistance at time point
x, where
min. The calculated relative differences for the participants are given in
Figure 4a,b for the 10 kHz and 100 kHz resistances, respectively, as red lines. For reference, the median values (previously reported in
Table 2 and
Table 3) are also shown as solid black lines for both the left and right segments.
The median resistances show a decreasing rate of change with increasing time in the HDT position. This implies that there are potentially two-decay modes (e.g., different mechanisms governing the rates of change). To model this behavior, the median datasets were fit to the two-term exponential function given by:
where
are the exponential coefficients,
are the exponential time constants, and
t is the time (in min). Using the curve-fitting interactive application in MATLAB, the coefficients that best fit (
2) to the median segmental leg resistance are:
where the notation LL and RR denote the left leg and right leg, respectively. The functions given by (3) through (6) show very good agreement with the median data, with
(proportion of the total sum of squares explained by the model) values of
,
,
, and
, respectively. For reference, these functions are also plotted in
Figure 4 as solid gray lines. The MATLAB curve-fitting application uses a non-linear least squares approach with the trust-region algorithm. For the fittings in this effort, the default application configuration was utilized. In addition to the good agreement from the
values, the
confidence intervals for each parameter are given in
Table 4. Notice that most of the intervals in
Table 4 do not include the value zero, confirming that the null hypothesis (e.g., the coefficient is zero) can be rejected for these coefficients. The one exception is the
coefficient for the LL-100 kHz model, which supports that these data can likely be modeled with a single-term exponential function.
3.3. Symmetry Differences
A Wilcoxon signed-rank test was conducted to evaluate the study question: Are there differences between the left and right segment resistance alterations induced by 240 min in the 6° HDT position? Only leg resistances were tested because they were the only segments with statistically significant differences during the study protocol. The symmetry differences (
) for this test were calculated using:
where
and
denote the resistance measurements at 240 min and 0 min, respectively, and
or
superscripts denote the left or right leg. From the 8 study participants the median
values were 2.29 Ω and 2.88 Ω for the 10 kHz and 100 kHz resistances, respectively. From the Wilcoxon signed-rank test, there was not a significant median difference between the 10 kHz (
,
) and 100 kHz (
,
)
values. That is, there were no significant differences between the resistance increases of the left and right leg segments.
4. Discussion
From the results presented in
Table 2 and
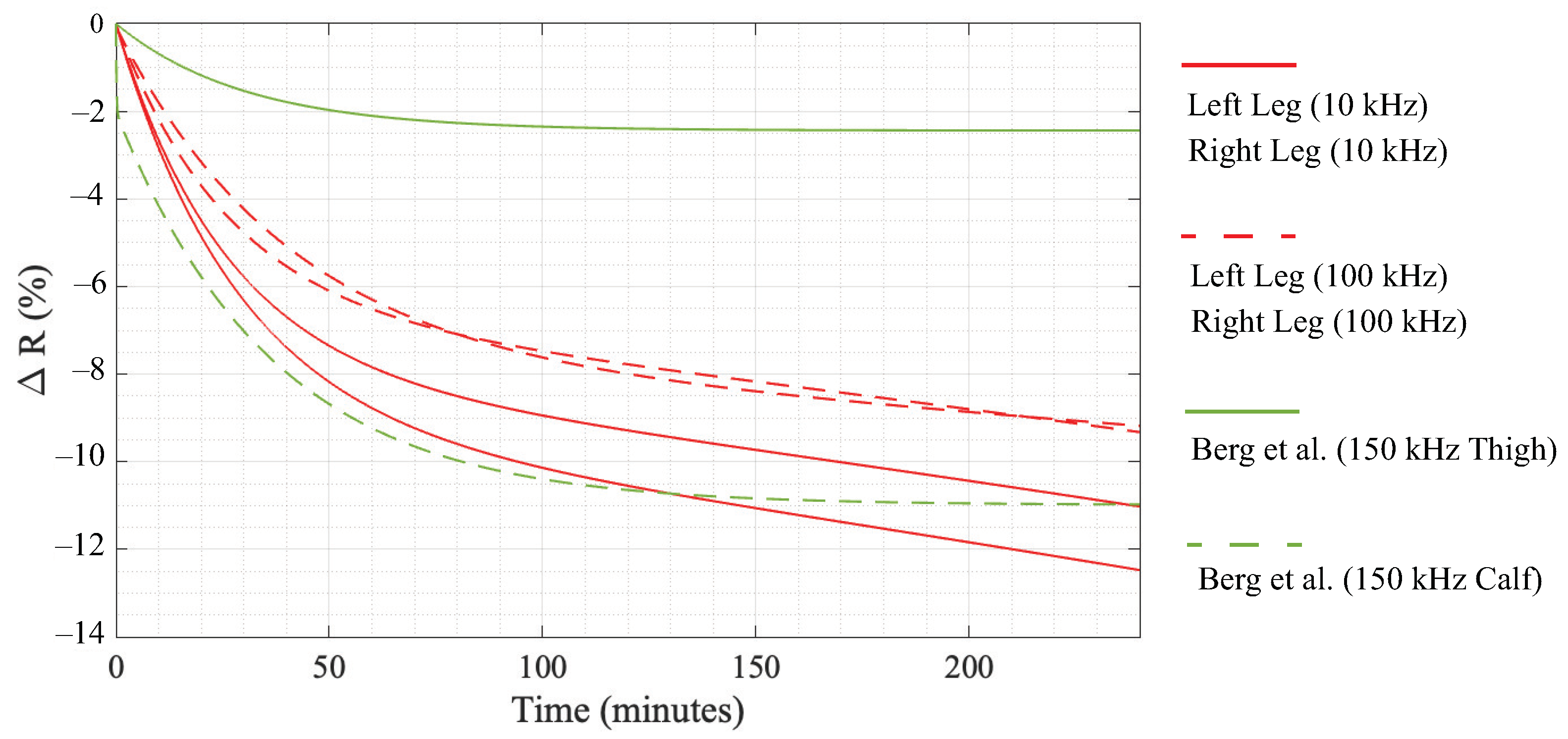
Table 3, the participant’s time in the 6° HDT position resulted in statistically significant increases in the median 10 kHz and 100 kHz resistance. These differences are attributed to the movement of fluid away from the legs and into the trunk of each participant and support that resistance measurements are sensitive to changes in body position (and the resulting fluid redistribution). Comparing the relative differences, the 10 kHz resistance had a larger rate of change until approximately 80 min. This is observed visually in
Figure 5 where the 10 and 100 kHz data are given as solid and dashed lines, respectively. This may suggest that the extra-cellular fluid (associated with low-frequency resistance) responds faster to the change in body position. From approximately 80 min to 240 min, both 10 and 100 kHz have similar rates of change. Overall, the relative differences for the 10 kHz segmental leg resistances (in the range from
to
) were greater than 100 kHz relative differences (approximately
after 240 min. It is important to note though that a limitation of this study is the small number of participants (8), which limits the power of this study. Therefore, further research with a greater number of participants is recommended to confirm that the trends reported in this effort are observed.
Overall, the trend of the segmental leg resistance increasing over time aligns with previous reports by Montgomery [
6,
11] and Berg et al. [
12] who similarly measured segmental leg resistances in adults during bed rest. Berg et al. reported relative differences of thigh and calf 150 kHz resistance given by
and
. The relative differences modeled by these equations are plotted in
Figure 5 as solid and dashed green lines, respectively [
12]. The median relative differences of our study lie between Bergs reports of calf and thigh resistance (but are similar in magnitude and rate of change for
min). Specifically, the calf resistance reported by Berg et al. had a relative increase of approximately
after 120 in a supine position, which is similar to the approximately
increase and 9–11% increase of the 100 and 10 kHz resistance at 120 min shown in
Figure 5 for the participants in this study. The differences in magnitude between studies are attributed to differences in the measurement setups. Berg et al. measured the leg as two segments (with greater changes in the calf than thigh) in comparison to one segment in this work. Therefore, our results capture changes in both calf/thigh servings as the “average” in comparison to those of Berg et al. Berg et al. only measured participants for 120 min and also only had participants in a supine position. This lower period of measurement and different body positions are expected to be the source of their models deviating from the trends reported here. Yadollahi et al. also reported statistically significant decreases in segmental leg volume (estimated from segmental leg resistance) after 90 min in the supine body position [
14]. Yadollahi et al. reported that the changes were well modeled by exponential functions (but complete details of those functions and the raw resistance were not provided limiting direct comparison here). Overall, these combined studies support that the relative changes in segmental resistance are captured by exponential models with similar rates of change across studies and conditions.
Of particular interest regarding the segmental changes in this work is that the segmental trunk resistance did not show statistically significant differences at any time point in HDT compared to the pre-tilt measures. An unexpected finding as the fluid redistribution away from the legs while in HDT is expected to flow to the trunk and decrease the segmental resistance. In comparison to previous studies, Montgomery reported increases in segmental volume change after short-term (4 h) bed rest estimated from segmental trunk resistances but these increases were not statistically significant. Fenech and Jaffrin also reported no statistically significant changes in extra-cellular resistance (an alternative proxy for low-frequency resistance) for the trunk after 30 min supine [
8]. However, Yadollahi et al. did report statistically significant differences in segmental trunk volumes of men and women in supine positions after 90 min [
14]. In comparison to this work and the works of Montgomery [
11], and Fenech and Jaffrin [
8], the trunk was measured by Yadollahi et al. as two segments (thorax, abdomen). In this configuration thorax values had a statistically significant increase in fluid (e.g., a decrease in resistance) in both men and women but no increase in abdomen values [
14]. This may suggest that the trunk should be monitored as multiple segments to capture fluid redistribution and why changes were not observed here.
Reviewing the last measured segment (arm), there were no statistically significant changes in the participants of this study. Similar to the trunk this was unexpected as the HDT position was expected to induce a shift of fluid from the arms towards the trunk/neck (resulting in a resistance increase of the arm segment). Fenech and Jaffrin reported a statistically significant increase in extra-cellular resistance (e.g., a proxy for low-frequency resistance) in participants during their 30 min supine protocol, supporting that supine positions did cause a change in arm fluid/resistance. The lack of changes observed in this study may be caused by participants being allowed to move and reposition their arms throughout the HDT protocol (and only required to be still during measurements). The muscle contractions during movement may reduce or counter the expected cephalad fluid shifts.
Focusing on the main study question the comparison of the right and left segmental leg resistances, there were no statistically significant differences in either 10 or 100 kHz measurements based on the side of the body measured after 240 min of 6° HDT. These results support the implied assumption of earlier studies that fluid shifts would be similar for both left and right bodies (suggesting that only a single side of the body can be measured to estimate segmental fluids).
For the future design of wearable bioimpedance systems aimed at monitoring segmental fluid shifts in microgravity environments, the results of this study and previous works [
6,
8,
11,
12] suggest that systems can measure a single side of the body (left or right) and that the legs and trunk should be the segments of focus. This can potentially reduce the amount of hardware and processing required of a wearable system. Further, accurately estimating trunk fluid distributions will likely require monitoring the trunk as multiple segments (e.g., thorax, abdomen). Future efforts will also be necessary to explore the number of trunk "segments" for this application and investigate measuring the neck resistance (which has not been measured in the previous studies) as an additional segment to quantify fluid shifts towards the head. The exponentially decreasing relative difference observed at both 10 kHz and 100 kHz resistance for the segmental leg measurements suggests it may be possible to further simplify the requirements of a wearable bioimpedance system to monitor only a single frequency, potentially only 10 kHz (which corresponded to the larger rate of change observed in this study). Though further research is needed to confirm if a single frequency is appropriate for capturing changes across longer-time scales. It may be that intra-cellular fluid (associated with higher frequencies) may become more significant at longer time scales and require monitoring of both low and high-frequency segmental resistance.
Another important aspect of the adoption of wearable bioimpedance systems for fluid shift monitoring is the effect of movement and muscle contraction on tissue resistance. Muscle contractions were reported by Kitchin and Freeborn to increase bicep tissue resistance up to 6.75 Ω when curling a 15 lb dumbbell [
20]. Therefore, muscle contractions due to activity could lead to incorrect interpretation of fluid shifts in resistance data. Consider that the median left/right 10 kHz segmental arm resistance of this study, given in
Table 2, had a difference of 3.8 Ω and 3.6 Ω, respectively, between the measurements at 0 and 240 min. While there was no statistically significant difference between these values in this study, additional increases of 6 Ω or greater due to leg muscle contractions have the potential to alter the study results and be interpreted as a fluid shift. Therefore, systems aimed at providing continuous, real-time monitoring will require processing techniques to identify artifacts (such as contraction events) and remove or correct them towards accurately estimating fluid distributions across conditions. Another important consideration is that studies of fluid shifts to date (including this study) have required participants to remain motionless. However, this is not realistic of the expected use conditions by astronauts in microgravity environments. In fact, fluid shift monitoring during activities and evaluation of counter-measures (such as lower-body negative pressure [
21]) will require monitoring during movement. Future studies should explore sensing modalities that complement bioimpedance to capture movement (e.g., inertial measurement units) and muscle activity (e.g., surface electromyography). The additional sensing modalities are expected to increase the accuracy of fluid estimates by helping identify when motion-related artifacts are introduced to the resistance data, which can be used to either remove or correct degraded data. These future efforts are expected to help advance solutions to provide comprehensive fluid shift monitoring to support the personalized and continuous monitoring of astronauts in microgravity conditions.