Sol-Gel Dipping Devices for H2S Visualization
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. General
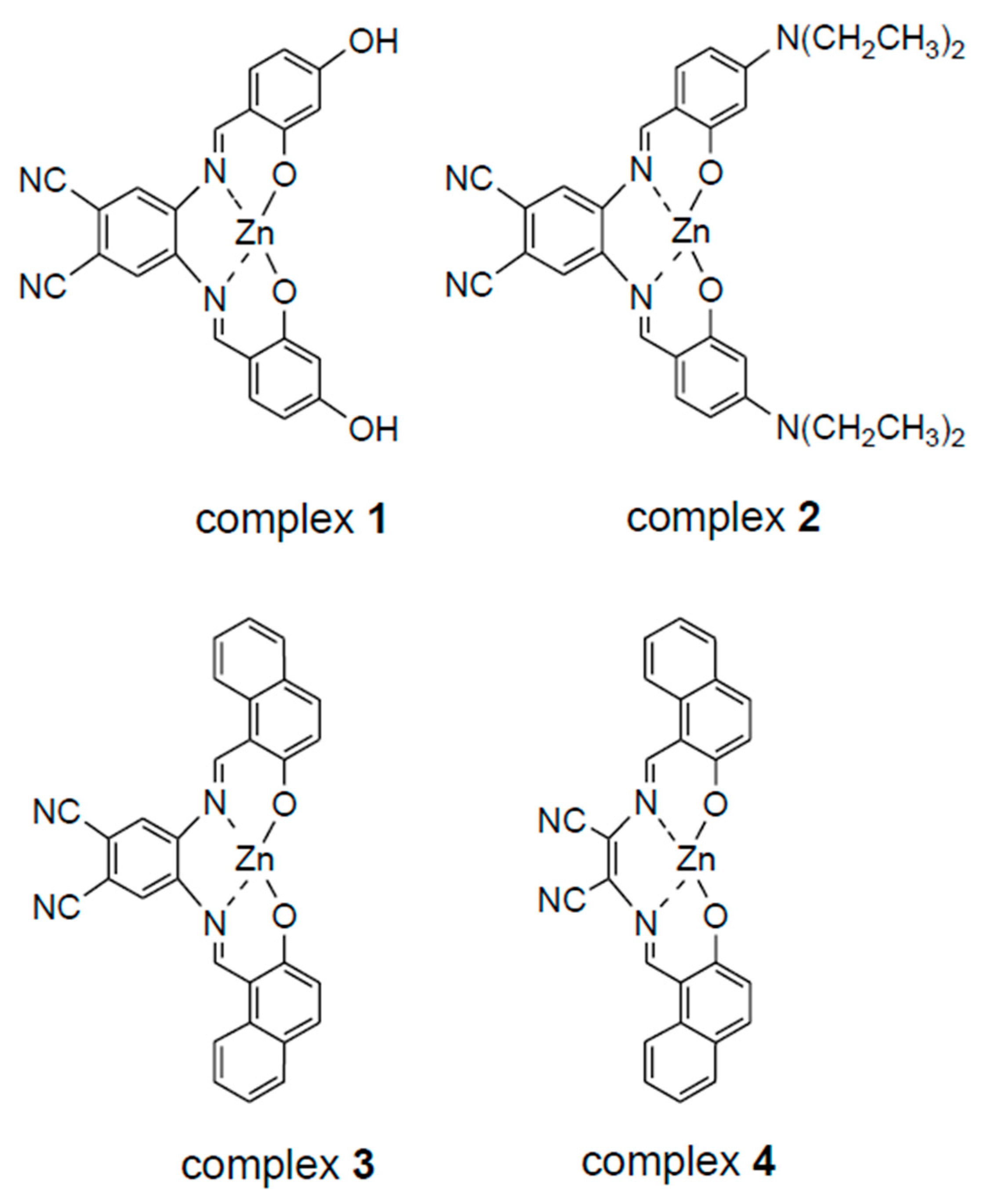
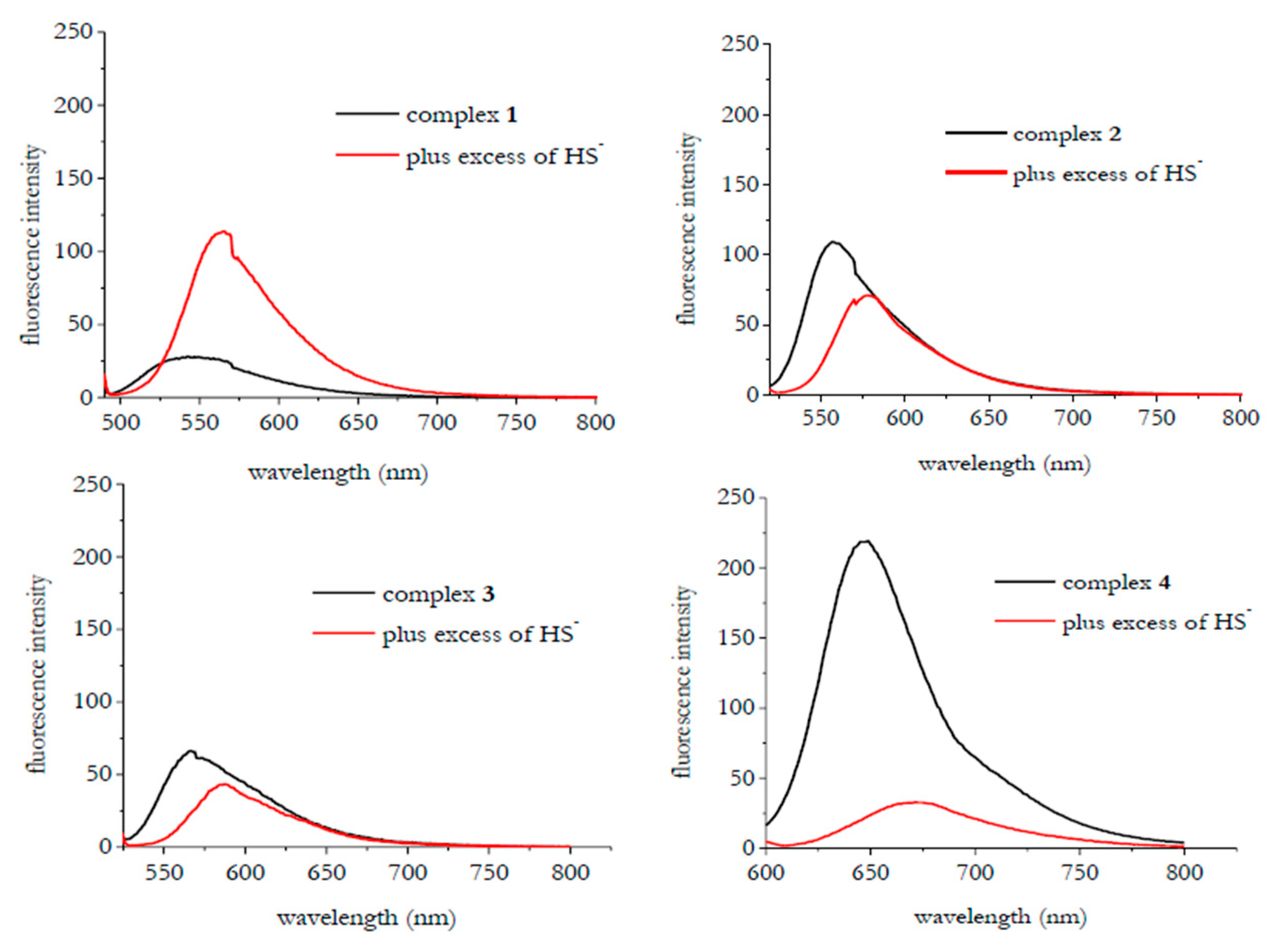
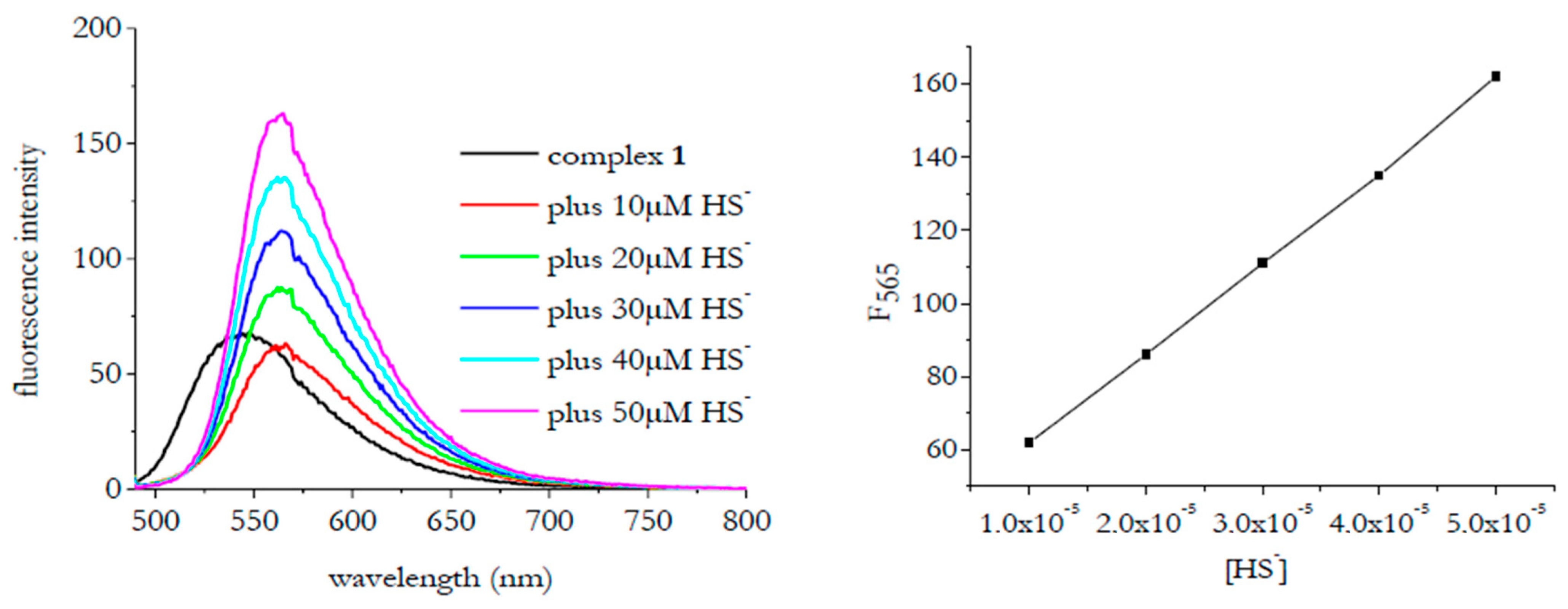
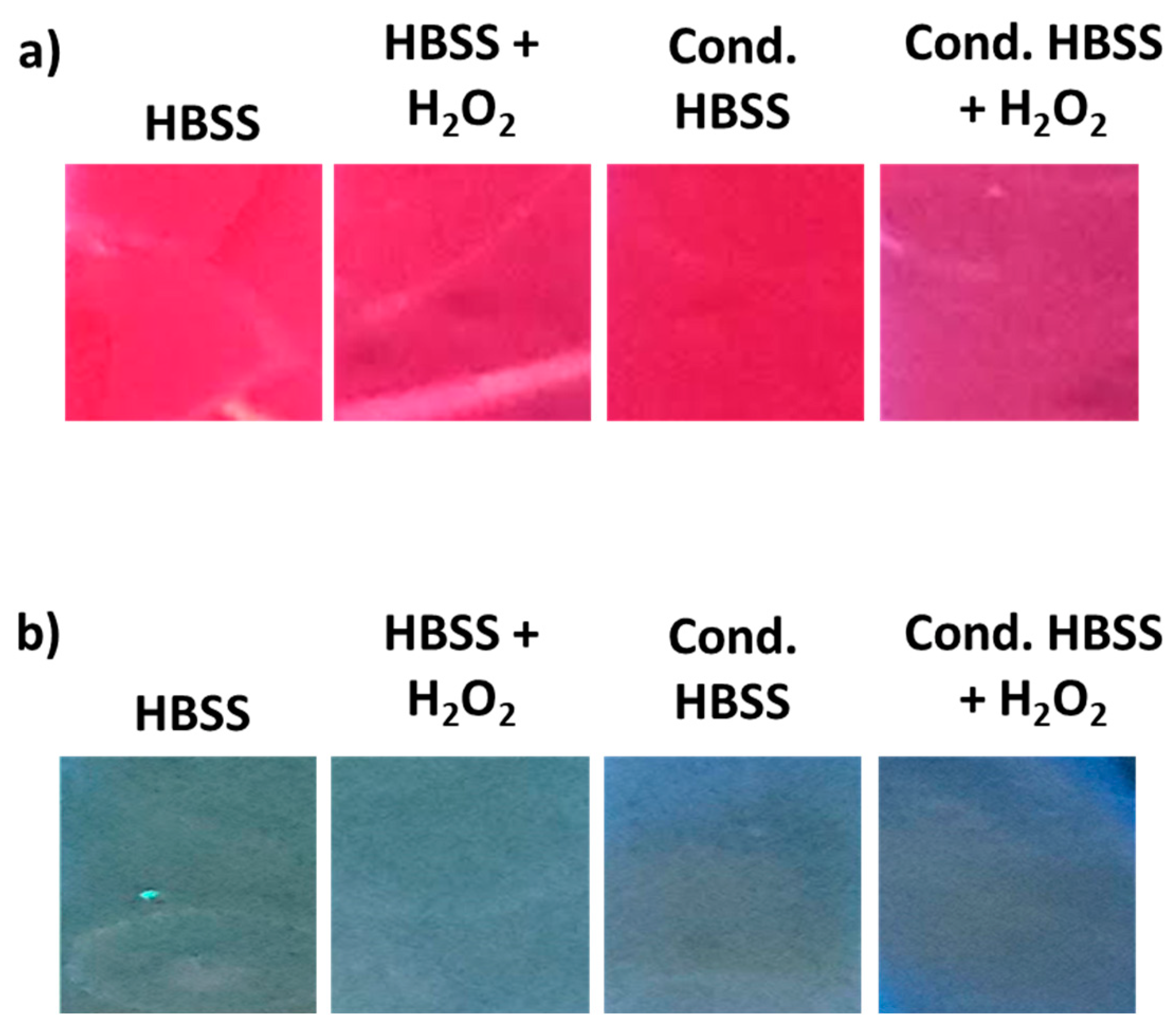
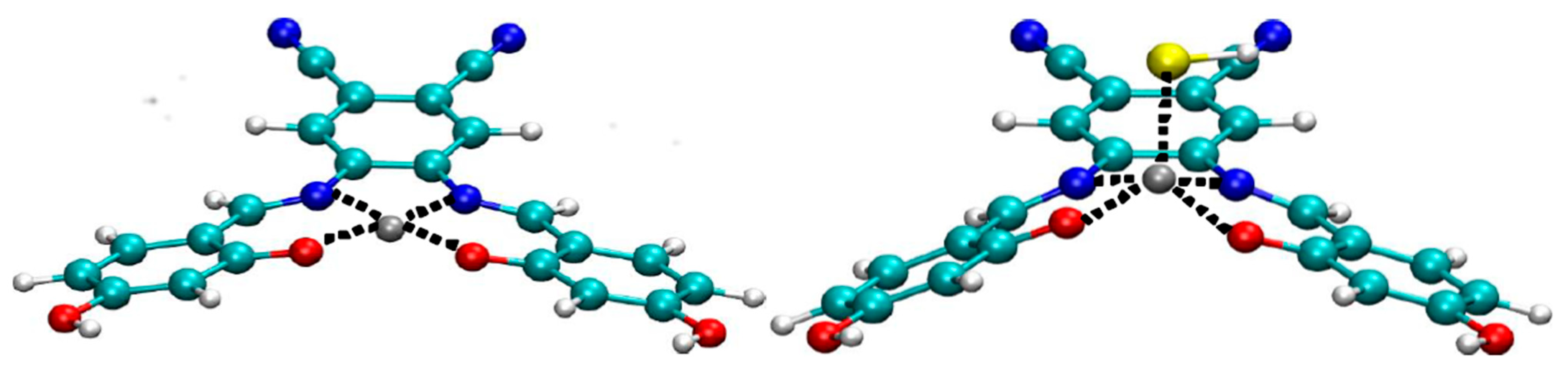
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Signaling Molecules: Hydrogen Sulfide and Polysulfide. Antioxid. Redox Signal 2014, 22, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Moore, P.K. An overview of the biological significance of endogenous gases: New roles for old molecules. Biochem. Soc. Trans. 2007, 35 Pt 5, 1138–1141. [Google Scholar] [CrossRef]
- Li, L.; Moore, P.K. Putative biological roles of hydrogen sulfide in health and disease: A breath of not so fresh air? Trends Pharmacol. Sci. 2008, 29, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Chen, C.Q.; Xin, H.; Zhu, Y.Z. Hydrogen sulfide: Third gaseous transmitter, but with great pharmacological potential. Acta Pharmacol. Sin. 2007, 28, 1709–1716. [Google Scholar] [CrossRef]
- Chandrika, D.; Ma, E.S.; Rettig, S.J.; James, B.R.; Cullen, W.R. Synthesis and X-ray Structure of an H2S Complex, RuCl2(P-N)(P(p-tolyl)3)(SH2) (P-N=o-(Diphenylphosphino)-N,N-dimethylaniline). Inorg. Chem. 1997, 36, 5426–5427. [Google Scholar] [CrossRef]
- Pleus, J.; Waden, H.; Saak, W.; Haase, D.; Pohl, S. Preparation of the first sulfur-containing cobalt and nickel complexes stabilised by the macrocyclic cyclam ligand; observation of S-H bond activation. J. Chem. Soc. Dalton Trans. 1999, 2601–2610. [Google Scholar] [CrossRef]
- Galardon, E.; Tomas, A.; Roussel, P.; Artaud, I. New fluorescent zinc complexes: Towards specific sensors for hydrogen sulfide in solution. Dalton Trans. 2009, 42, 9126–9130. [Google Scholar] [CrossRef]
- Galardon, E.; Tomas, A.; Selkti, M.; Roussel, P.; Artaud, I. Synthesis, Characterization, and Reactivity of Alkyldisulfanido Zinc Complexes. Inorg. Chem. 2009, 48, 5921–5927. [Google Scholar] [CrossRef]
- Pavlik, J.W.; Noll, B.C.; Oliver, A.G.; Schulz, C.E.; Scheidt, W.R. Hydrosulfide (HS-) Coordination in Iron Porphyrinates. Inorg. Chem. 2010, 49, 1017–1026. [Google Scholar] [CrossRef]
- Galardon, E.; Roger, T.; Deschamps, P.; Pascal, R.; Tomas, A.; Artaud, I. Synthesis of a FeIISH complex stabilized by an intramolecular N-H⋯S hydrogen bond, which acts as a H2S donor. Inorg. Chem. 2012, 51, 10068–10070. [Google Scholar] [CrossRef]
- Meininger, D.J.; Arman, H.D.; Tonzetich, Z.J. Synthesis, characterization, and binding affinity of hydrosulfide complexes of synthetic iron(II) porphyrinates. J. Inorg. Biochem. 2017, 167, 142–149. [Google Scholar] [CrossRef]
- Meininger, D.J.; Chee-Garza, M.; Arman, H.D.; Tonzetich, Z.J. Gallium(III) Tetraphenylporphyrinates Containing Hydrosulfide and Thiolate Ligands: Structural Models for Sulfur-Bound Iron(III) Hemes. Inorg. Chem. 2016, 55, 2421–2426. [Google Scholar] [CrossRef]
- Hartle, M.D.; Prell, J.S.; Pluth, M.D. Spectroscopic investigations into the binding of hydrogen sulfide to synthetic picket-fence porphyrins. Dalton Trans. 2016, 45, 4843–4853. [Google Scholar] [CrossRef]
- Tonzetich, Z.J. H2S and Bioinorganic Metal Complexes. In Hydrogen Sulfide; Wiley: Hoboken, NJ, USA, 2022; pp. 103–141. [Google Scholar]
- Strianese, M.; Varriale, A.; Staiano, M.; Pellecchia, C.; D’Auria, S. Absorption into fluorescence. A method to sense biologically relevant gas molecules. Nanoscale 2011, 3, 298–302. [Google Scholar] [CrossRef]
- Strianese, M.; Milione, S.; Bertolasi, V.; Pellecchia, C.; Grassi, A. Heteroscorpionate-Based Co2+, Zn2+, and Cu2+ Complexes: Coordination Behavior, Aerobic Oxidation, and Hydrogen Sulfide Detection. Inorg. Chem. 2011, 50, 900–910. [Google Scholar] [CrossRef]
- Strianese, M.; De Martino, F.; Pellecchia, C.; Ruggiero, G.; D’Auria, S. Myoglobin as a new fluorescence probe to sense H2S. Protein Pept. Lett. 2011, 18, 282–286. [Google Scholar] [CrossRef]
- Strianese, M.; Palm, G.J.; Milione, S.; Kuhl, O.; Hinrichs, W.; Pellecchia, C. A FRET enzyme-based probe for monitoring hydrogen sulfide. Inorg. Chem 2012, 51, 11220–11222. [Google Scholar] [CrossRef]
- Strianese, M.; Mirra, S.; Bertolasi, V.; Milione, S.; Pellecchia, C. Organometallic sulfur complexes: Reactivity of the hydrogen sulfide anion with cobaloximes. New J. Chem. 2015, 39, 4093–4099. [Google Scholar] [CrossRef]
- Strianese, M.; Lamberti, M.; Pellecchia, C. Chemically reversible binding of H2S to a zinc porphyrin complex: Towards implementation of a reversible sensor via a “coordinative-based approach”. Dalton Trans. 2017, 46, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Mirra, S.; Lamberti, M.; Pellecchia, C. Zinc (II) porphyrins as viable scaffolds to stabilize hydrogen sulfide binding at the metal center. Inorg. Chim. Acta 2017, 466, 426–431. [Google Scholar] [CrossRef]
- Strianese, M.; Palm, G.J.; Kohlhause, D.; Ndamba, L.A.; Tabares, L.C.; Pellecchia, C. Azurin and HS-: Towards Implementation of a Sensor for HS- Detection. Eur. J. Inorg. Chem. 2019, 2019, 885–891. [Google Scholar] [CrossRef]
- Strianese, M.; Brenna, S.; Ardizzoia, G.; Guarnieri, D.; Lamberti, M.; D’Auria, I.; Pellecchia, C. Imidazo-pyridine-based zinc(II) complexes as fluorescent hydrogen sulfide probes. Dalton Trans. 2021, 50, 17075–17085. [Google Scholar] [CrossRef]
- Pluth, M.D.; Tonzetich, Z.J. Hydrosulfide complexes of the transition elements: Diverse roles in bioinorganic, cluster, coordination, and organometallic chemistry. Chem. Soc. Rev. 2020, 49, 4070–4134. [Google Scholar] [CrossRef]
- Hartle, M.D.; Tillotson, M.R.; Prell, J.S.; Pluth, M.D. Spectroscopic investigation of the reaction of metallo-protoporphyrins with hydrogen sulfide. J. Inorg. Biochem. 2017, 173, 152–157. [Google Scholar] [CrossRef]
- Hartle, M.D.; Sommer, S.K.; Dietrich, S.R.; Pluth, M.D. Chemically Reversible Reactions of Hydrogen Sulfide with Metal Phthalocyanines. Inorg. Chem. 2014, 53, 7800–7802. [Google Scholar] [CrossRef]
- Hartle, M.D.; Delgado, M.; Gilbertson, J.D.; Pluth, M.D. Stabilization of a Zn(II) hydrosulfido complex utilizing a hydrogen-bond accepting ligand. Chem. Commun. 2016, 52, 7680–7682. [Google Scholar] [CrossRef]
- Strianese, M.; Zauner, G.; Tepper, A.W.; Bubacco, L.; Breukink, E.; Aartsma, T.J.; Canters, G.W.; Tabares, L.C. A protein-based oxygen biosensor for high-throughput monitoring of cell growth and cell viability. Anal. Biochem. 2009, 385, 242–248. [Google Scholar] [CrossRef]
- Dulac, M.; Melet, A.; Galardon, E. Reversible Detection and Quantification of Hydrogen Sulfide by Fluorescence Using the Hemoglobin I from Lucina pectinata. ACS Sens. 2018, 3, 2138–2144. [Google Scholar] [CrossRef]
- Watanabe, K.; Suzuki, T.; Kitagishi, H.; Kano, K. Reaction between a haemoglobin model compound and hydrosulphide in aqueous solution. Chem. Commun. 2015, 51, 4059–4061. [Google Scholar] [CrossRef]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Purrello, R.; Di Bella, S. Supramolecular Aggregation/Deaggregation in Amphiphilic Dipolar Schiff-Base Zinc(II) Complexes. Inorg. Chem. 2010, 49, 5134–5142. [Google Scholar] [CrossRef]
- Consiglio, G.; Failla, S.; Oliveri, I.P.; Purrello, R.; Di Bella, S. Controlling the molecular aggregation. An amphiphilic Schiff-base zinc(II) complex as supramolecular fluorescent probe. Dalton Trans. 2009, 47, 10426–10428. [Google Scholar] [CrossRef]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Bella, S.D. Aggregation properties of bis(salicylaldiminato)zinc(II) Schiff-base complexes and their Lewis acidic character. Dalton Trans. 2012, 41, 387–395. [Google Scholar] [CrossRef]
- Consiglio, G.; Oliveri, I.P.; Failla, S.; Di Bella, S. On the Aggregation and Sensing Properties of Zinc(II) Schiff-Base Complexes of Salen-Type Ligands. Molecules 2019, 24, 2514. [Google Scholar] [CrossRef]
- Dumur, F.; Contal, E.; Wantz, G.; Gigmes, D. Photoluminescence of Zinc Complexes: Easily Tunable Optical Properties by Variation of the Bridge Between the Imido Groups of Schiff Base Ligands. Eur. J. Inorg. Chem. 2014, 2014, 4186–4198. [Google Scholar] [CrossRef]
- Xie, D.; Jing, J.; Cai, Y.B.; Tang, J.; Chen, J.J.; Zhang, J.L. Construction of an orthogonal ZnSalen/Salophen library as a colour palette for one- and two-photon live cell imaging. Chem. Sci. 2014, 5, 2318–2327. [Google Scholar] [CrossRef]
- Strianese, M.; Lamberti, M.; Pellecchia, C. Interaction of monohydrogensulfide with a family of fluorescent pyridoxal-based Zn(ii) receptors. Dalton Trans. 2018, 47, 17392–17400. [Google Scholar] [CrossRef]
- Strianese, M.; Lamberti, M.; Persico, A.; Pellecchia, C. Reactivity of monohydrogensulfide with a suite of pyridoxal-based complexes: A combined NMR, ESI-MS, UV-visible and fluorescence study. Inorg. Chim. Acta 2019, 501, 119235. [Google Scholar] [CrossRef]
- Strianese, M.; Guarnieri, D.; Lamberti, M.; Landi, A.; Peluso, A.; Pellecchia, C. Fluorescent salen-type Zn(II) Complexes As Probes for Detecting Hydrogen Sulfide and Its Anion: Bioimaging Applications. Inorg. Chem. 2020, 59, 15977–15986. [Google Scholar] [CrossRef]
- Strianese, M.; Vykhovanets, V.; Blal, N.; Guarnieri, D.; Landi, A.; Lamberti, M.; Peluso, A.; Pellecchia, C. Paper-Strip-Based Sensors for H2S Detection: A Proof-of-Principle Study. Sensors 2022, 22, 3173. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; D’Auria, G.J.; Lamberti, M.; Landi, A.; Peluso, A.; Varriale, A.; D’Auria, S.; Pellecchia, C. Salen, salan and salalen zinc(ii) complexes in the interaction with HS-: Time-resolved fluorescence applications. Dalton Trans. 2023, 52, 1357–1365. [Google Scholar] [CrossRef]
- Strianese, M.; Pellecchia, C. Fluorescent Probes for H2S Detection: Metal-Based Approaches. In Hydrogen Sulfide; Wiley: Hoboken, NJ, USA, 2022; pp. 203–233. [Google Scholar]
- Ellerby, L.M.; Nishida, C.R.; Nishida, F.; Yamanaka, S.A.; Dunn, B.; Valentine, J.S.; Zink, J.I. Encapsulation of proteins in transparent porous silicate glasses prepared by the sol-gel method. Science 1992, 255, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Cramer, C.J.; Truhlar, D.G. Density functional theory for transition metals and transition metal chemistry. Phys. Chem. Chem. Phys. 2009, 11, 10757–10816. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Bai, S.; Fazzi, D.; Niehaus, T.; Barbatti, M.; Thiel, W. Evaluation of Spin-Orbit Couplings with Linear-Response Time-Dependent Density Functional Methods. J. Chem. Theory Comput. 2017, 13, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Velardo, A.; Borrelli, R.; Capobianco, A.; Landi, A.; Peluso, A. Disentangling Electronic and Vibrational Effects in the Prediction of Band Shapes for Singlet-Triplet Transitions. J. Phys. Chem. C 2019, 123, 14173–14179. [Google Scholar] [CrossRef]
- Landi, A.; Capobianco, A.; Peluso, A. Coherent Effects in Charge Transport in Molecular Wires: Toward a Unifying Picture of Long-Range Hole Transfer in DNA. J. Phys. Chem. Lett. 2020, 11, 7769–7775. [Google Scholar] [CrossRef]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys 1981, 55, 117–129. [Google Scholar] [CrossRef]
- La Cort, A.; Mandolini, L.; Pasquini, C.; Rissanen, K.; Russo, L.; Schiaffino, L. Zinc-salophen complexes as selective receptors for tertiary amines. New J. Chem. 2007, 31, 1633–1638. [Google Scholar] [CrossRef]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef]
- Sanokawa-Akakura, R.; Ostrakhovitch, E.A.; Akakura, S.; Goodwin, S.; Tabibzadeh, S. A H2S-Nampt Dependent Energetic Circuit Is Critical to Survival and Cytoprotection from Damage in Cancer Cells. PLoS ONE 2014, 9, e108537. [Google Scholar] [CrossRef]
- Rombach, M.; Vahrenkamp, H. Pyrazolylborate-Zinc-Hydrosulfide Complexes and Their Reactions. Inorg. Chem. 2001, 40, 6144–6150. [Google Scholar] [CrossRef]
- Capobianco, A.; Borrelli, R.; Landi, A.; Velardo, A.; Peluso, A. Absorption Band Shapes of a Push-Pull Dye Approaching the Cyanine Limit: A Challenging Case for First Principle Calculations. J. Phys. Chem. A 2016, 120, 5581–5589. [Google Scholar] [CrossRef]






| Vertical | Adiabatic | Oscillator Strength | |
|---|---|---|---|
| 1 | 3.22 | 2.64 | 0.57 |
| 1 + HS− | 3.13 | 2.67 | 0.42 |
| 4 | 2.61 | 2.21 | 0.96 |
| 4 + HS− | 2.42 | 2.11 | 0.80 |
| SOC T2-S1 | SOC T3-S1 | SOC T4-S1 | |
|---|---|---|---|
| 1 | 1.23 | 10.0 | 14.1 |
| 1 + HS− | 45.0 | 29.2 | 10.5 |
| 4 | 16.59845 | 5.06933 | 2.80429 |
| 4 + HS− | 95.8 | 240 | 71.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strianese, M.; Ferrara, G.; Vykhovanets, V.; Blal, N.; Guarnieri, D.; Landi, A.; Lamberti, M.; Peluso, A.; Pellecchia, C. Sol-Gel Dipping Devices for H2S Visualization. Sensors 2023, 23, 2023. https://doi.org/10.3390/s23042023
Strianese M, Ferrara G, Vykhovanets V, Blal N, Guarnieri D, Landi A, Lamberti M, Peluso A, Pellecchia C. Sol-Gel Dipping Devices for H2S Visualization. Sensors. 2023; 23(4):2023. https://doi.org/10.3390/s23042023
Chicago/Turabian StyleStrianese, Maria, Giovanni Ferrara, Viktoriia Vykhovanets, Naym Blal, Daniela Guarnieri, Alessandro Landi, Marina Lamberti, Andrea Peluso, and Claudio Pellecchia. 2023. "Sol-Gel Dipping Devices for H2S Visualization" Sensors 23, no. 4: 2023. https://doi.org/10.3390/s23042023
APA StyleStrianese, M., Ferrara, G., Vykhovanets, V., Blal, N., Guarnieri, D., Landi, A., Lamberti, M., Peluso, A., & Pellecchia, C. (2023). Sol-Gel Dipping Devices for H2S Visualization. Sensors, 23(4), 2023. https://doi.org/10.3390/s23042023









