Instrumental and Non-Instrumental Measurements in Patients with Peripheral Vestibular Dysfunctions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
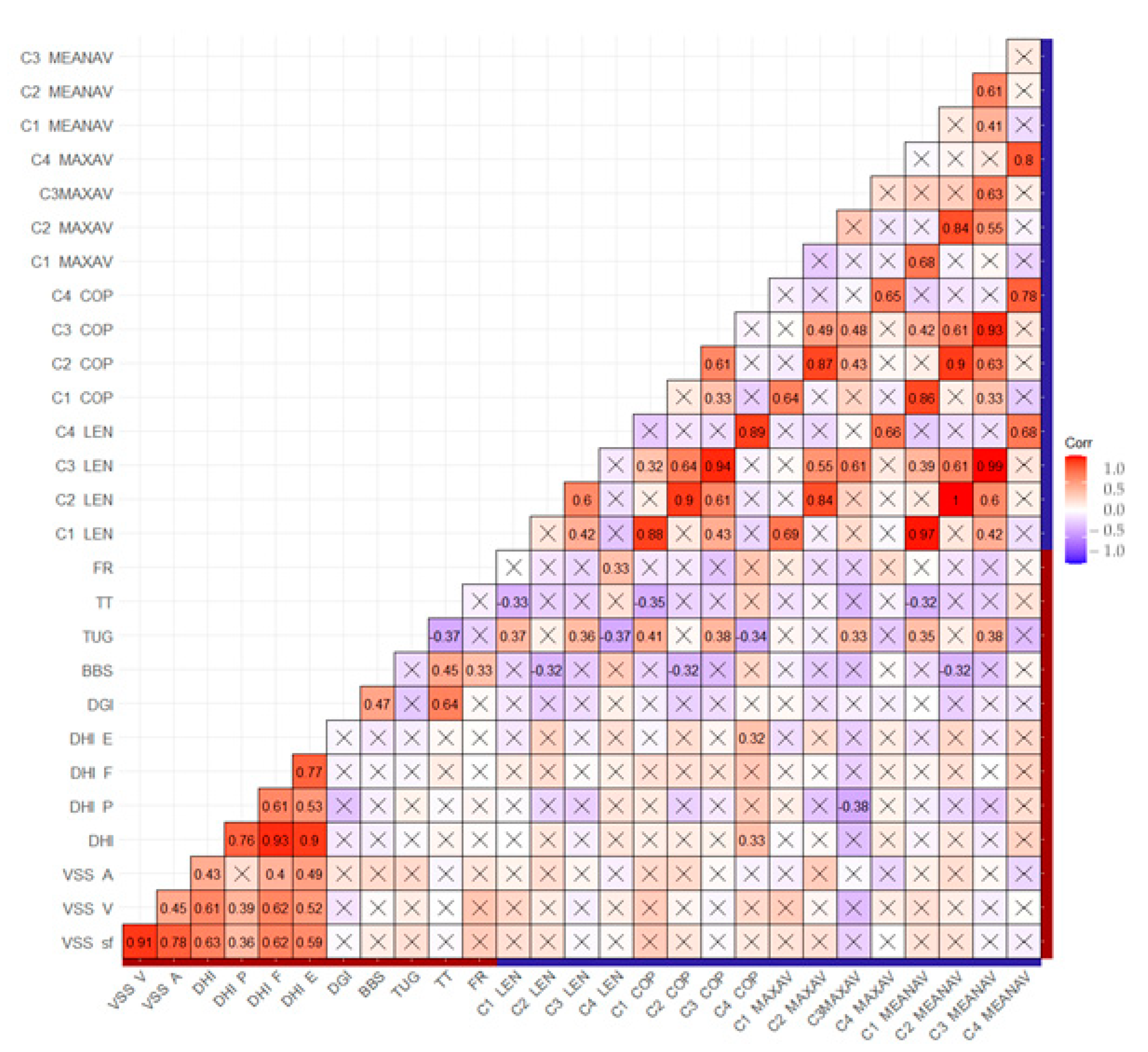
3.1. Statistical Analysis
3.2. Results of the Non-Instrumental Measurements
3.2.1. Questionnaires
3.2.2. Clinical Tests
3.3. Results of the Instrumental Measurements
MediPost Posturography
3.4. Correlation of Improvement between Instrumental and Non-Instrumental Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mancini, M.; Horak, F.B. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur. J. Phys. Rehabil. Med. 2010, 46, 239–248. [Google Scholar] [PubMed]
- Alonso, A.C.; Luna, N.M.; Dionísio, F.N.; Speciali, D.S.; Leme, L.E.; Greve, J.M. Functional balance assessment: Review. Medicalexpress 2014, 1, 298–301. [Google Scholar] [CrossRef]
- Agrawal, Y.; Pineault, K.G.; Semenov, Y.R. Health-related quality of life and economic burden of vestibular loss in older adults. Laryngoscope Investig. Otolaryngol. 2017, 15, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.; Wang, X.; Grill, E. Economic burden of vertigo: A systematic review. Health Econ. Rev. 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Lacour, M.; Bernard-Demanze, L. Interaction between Vestibular Compensation Mechanisms and Vestibular Rehabilitation Therapy: 10 Recommendations for Optimal Functional Recovery. Front Neurol. 2015, 5, 285. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.D.; Herdman, S.J.; Whitney, S.L.; Anson, E.R.; Carender, W.J.; Hoppes, C.W.; Cass, S.P.; Christy, J.B.; Cohen, H.S.; Fife, T.D.; et al. Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Updated Clinical Practice Guideline from the Academy of Neurologic Physical Therapy of the American Physical Therapy Association. J. Neurol. Phys. Ther. 2022, 46, 118–177. [Google Scholar] [CrossRef]
- Ratajczak, A.; Sobczyk, K.; Budnicki, D.; Gos, E.; Skarżyński, P.H. Przegląd wybranych kwestionariuszy stosowanych do oceny zawrotów głowy i zaburzeń równowagi. Nowa. Audiofonologia. 2019, 8, 60–71. [Google Scholar]
- Duracinsky, M.; Mosnier, I.; Bouccara, D.; Sterkers, O.; Chassany, O. Working Group of the Société Française d’Oto-Rhino-Laryngologie (ORL). Literature review of questionnaires assessing vertigo and dizziness, and their impact on patients’ quality of life. Value Health 2007, 10, 273–284. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, X. Effects of resistance training combined with balance training on physical function among older adults: A protocol for a randomised controlled trial. BMJ Open. 2022, 12, e062486. [Google Scholar] [CrossRef]
- Taguchi, C.K.; Menezes, P.L.; Melo, A.C.S.; Santana, L.S.; Conceição, W.R.S.; Souza, G.F.; Araújo, B.C.L.; Silva, A.R.D. Frailty syndrome and risks for falling in the elderly community. Codas 2022, 34, 062486. [Google Scholar] [CrossRef]
- Tamura, S.; Miyata, K.; Kobayashi, S.; Takeda, R.; Iwamoto, H. Development of Cut-off Values on the Berg Balance Scale for Predicting Walking Independence in Older Adults with Hip Fracture. Prog. Rehabil. Med. 2022, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, E.; Fioretti, S.; Ghetti, G.G.; Rabini, R.A.; Burattini, L.; Mercante, O.; Di Nardo, F. The surface electromyographic evaluation of the Functional Reach in elderly subjects. J. Electromyogr. Kinesiol. 2016, 26, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Chiari, L. Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Parkinsonism. Relat. Disord. 2011, 17, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Leirós-Rodríguez, R.; García-Soidán, J.L.; Romo-Pérez, V. Analyzing the Use of Accelerometers as a Method of Early Diagnosis of Alterations in Balance in Elderly People: A Systematic Review. Sensors 2019, 19, 3883. [Google Scholar] [CrossRef] [PubMed]
- Kingma, H.; Gauchard, G.C.; de Waele, C.; van Nechel, C.; Bisdorff, A.; Yelnik, A.; Magnusson, M.; Perrin, P.P. Stocktaking on the Development of Posturography for Clinical Use. J. Vestib. Res. Equilib. Orientat. 2011, 21, 117–125. [Google Scholar] [CrossRef]
- Kotas, R.; Janc, M.; Kaminski, M.; Marciniak, P.; Zamyslowska-Szmytke, E.; Tylman, W. Evaluation of Agreement Between Static Posturography Methods Employing Tensometers and Inertial Sensors. IEEE Access 2019, 7, 164120–164126. [Google Scholar] [CrossRef]
- Gawronska, A.; Pajor, A.; Zamyslowska-Szmytke, E.; Rosiak, O.; Jozefowicz-Korczynska, M. Usefulness of Mobile Devices in the Diagnosis and Rehabilitation of Patients with Dizziness and Balance Disorders: A State of the Art Review. Clin. Interv. Aging 2020, 15, 2397–2406. [Google Scholar] [CrossRef]
- Ghislieri, M.; Gastaldi, L.; Pastorelli, S.; Tadano, S.; Agostini, V. Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review. Sensors 2019, 20, 4075. [Google Scholar] [CrossRef]
- Mancini, M.; Salarian, A.; Carlson-Kuhta, P.; Zampieri, C.; King, L.; Chiari, L.; Horak, F.B. ISway: A sensitive, valid and reliable measure of postural control. J. Neuroeng. Rehabil. 2012, 9, 59. [Google Scholar] [CrossRef]
- Szostek-Rogula, S.; Zamysłowska-Szmytke, E. Validation of the Polish version of the Dizziness Handicap Inventory. Med. Pr. 2019, 5, 529–534. [Google Scholar] [CrossRef]
- Yardley, L.; Masson, E.; Verschuur, C.; Haacke, N.; Luxon, L. Symptoms, Anxiety and Handicap in Dizzy Patients: Development of the Vertigo Symptom Scale. J. Psychosom. Res. 1992, 36, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsen, K.; Strand, L.I.; Nordahl, S.H.G.; Eide, G.E.; Ljunggren, A.E. Psychometric properties of the Vertigo symptom scale—Short form. BMC Ear Nose Throat Disord. 2008, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed "Up & Go": A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Shumway-Cook, A.; Baldwin, M.; Polissar, N.L.; Gruber, W. Predicting the probability for falls in community-dwelling older adults. Phys. Ther. 1997, 77, 812–819. [Google Scholar] [CrossRef]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83, S7–S11. [Google Scholar]
- Tinetti, M.E.; Williams, T.F.; Mayewski, R. Fall Risk Index for elderly patients based on number of chronic dis- abilities. Am. J. Med. 1986, 80, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional reach: A new clinical measure of balance. J. Gerontol. A Biol. Sci. 1990, 45, M192–M197. [Google Scholar] [CrossRef]
- McCaslin, D.L. Electronystagmography/Videonystagmography: (ENG/VNG), 2nd ed.; Plural Publishing, Inc.: San Diego, CA, USA, 2013; pp. 177–205. [Google Scholar]
- Rosiak, O.; Gawronska, A.; Janc, M.; Marciniak, P.; Kotas, R.; Zamyslowska-Szmytke, E.; Jozefowicz-Korczynska, M. Utility of the Novel MediPost Mobile Posturography Device in the Assessment of Patients with a Unilateral Vestibular Disorder. Sensors 2022, 22, 2208. [Google Scholar] [CrossRef]
- Madgwick, S.O.H.; Harrison, A.J.L.; Vaidyanathan, R. Estimation of IMU and MARG orientation using a gradient descent algorithm. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; pp. 1–7. [Google Scholar]
- Hall, C.D.; Herdman, S.J.; Whitney, S.L.; Cass, S.P.; Clendaniel, R.A.; Fife, T.D.; Furman, J.M.; Getchius, T.S.; Goebel, J.A.; Shepard, N.T.; et al. Vestibular rehabilitation for peripheral vestibular hypofunction: An evidence-based clinical practice guideline: From the American Physical Therapy Association neurology section. J. Neurol. Phys. Ther. 2016, 40, 124–155. [Google Scholar] [CrossRef]
- Dunlap, P.M.; Holmberg, J.M.; Whitney, S.L. Vestibular Rehabilitation: Advances in Peripheral and Central Vestibular Disorders. Curr. Opin. Neurol. 2019, 32, 137–144. [Google Scholar] [CrossRef]
- Hillier, S.L.; McDonnell, M. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst. Rev. 2011, 2, cd005397. [Google Scholar]
- Herdman, D.; Norton, S.; Pavlou, M.; Murdin, L.; Moss-Morris, R. Vestibular deficits and psychological factors correlating to dizziness handicap and symptom severity. J. Psychosom. Res. 2020, 132, 109969. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Fairhall, N.J.; Wallbank, G.K.; Tiedemann, A.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S.E. Exercise for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2019, 1, cd012424. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.E.; Whitney, S.L.; Marchetti, G.F.; Wrisley, D.M.; Furman, J.M. Physical therapy for central vestibular dysfunction. Arch. Phys. Med. Rehabil. 2006, 87, 76–81. [Google Scholar] [CrossRef]
- Soto-Varela, A.; Gayoso-Diz, P.; Faraldo-García, A.; Rossi-Izquierdo, M.; Vaamonde-Sánchez-Andrade, I.; Del-Río-Valeiras, M.; Lirola-Delgado, A.; Santos-Pérez, S. Optimising costs in reducing rate of falls in older people with the improvement of balance by means of vestibular rehabilitation (ReFOVeRe study): A randomized controlled trial comparing computerised dynamic posturography vs mobile vibrotactile posturography system. BMC Geriatr. 2019, 19, 1. [Google Scholar]
- O’Sullivan, M.; Blake, C.; Cunningham, C.; Boyle, G.; Finucane, C. Correlation of accelerometry with clinical balance tests in older fallers and non-fallers. Age Ageing 2009, 38, 308–313. [Google Scholar] [CrossRef]
- Allum, J.H.J.; Carpenter, M.G.; Adkin, A.L. Balance control analysis as a method for screening and identifying balance deficits. Ann. N. Y. Acad. Sci. 2001, 942, 413–427. [Google Scholar] [CrossRef]
- Lacour, M.; Tardivet, L.; Thiry, A. Posture Deficits and Recovery After Unilateral Vestibular Loss: Early Rehabilitation and Degree of Hypofunction Matter. Front Hum. Neurosci. 2022, 15, 776970. [Google Scholar] [CrossRef]
- Basta, D.; Rossi-Izquierdo, M.; Soto-Varela, A.; Ernst, A. Mobile posturography: Posturographic analysis of daily-life mobility. Otol. Neurotol. 2013, 34, 288–297. [Google Scholar] [CrossRef]
- Janc, M.; Śliwińska-Kowalska, M.; Jozefowicz-Korczynska, M.; Marciniak, P.; Rosiak, O.; Kotas, R.; Szmytke, Z.; Grodecka, J.; Zamysłowska-Szmytke, E. A comparison of head movements tests in force plate and accelerometer based posturography in patients with balance problems due to vestibular dysfunction. Sci. Rep. 2021, 11, 19094. [Google Scholar] [CrossRef]
- Rossi-Izquierdo, M.; Santos-Pérez, S.; Del-Río-Valeiras, M.; Lirola-Delgado, A.; Faraldo-García, A.; Vaamonde-Sánchez-Andrade, I.; Gayoso-Diz, P.; Soto-Varela, A. Is there a relationship between objective and subjective assessment of balance in elderly patients with instability? Eur. Arch. Otorhinolaryngol. 2015, 272, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Mbongo, F.; Tran Ba Huy, P.; Vidal, P.P.; de Waele, C. Relationship between dynamic balance and self- reported handicap in patients who have unilateral peripheral vestibular loss. Otol. Neurotol. 2007, 28, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.W.; Strupp, M. The Dizziness Handicap Inventory does not correlate with vestibular function tests: A prospective study. J. Neurol. 2018, 265, 1210–1218. [Google Scholar] [CrossRef]
- Sawacha, Z.; Carraro, E.; Contessa, P.; Guiotto, A.; Masiero, S.; Cobelli, C. Relationship between clinical and instrumental balance assessments in chronic post-stroke hemiparesis subjects. J. Neuroeng. Rehabil. 2013, 13, 95. [Google Scholar] [CrossRef]
- Zampogna, A.; Mileti, I.; Palermo, E.; Celletti, C.; Paoloni, M.; Manoni, A.; Mazzetta, I.; Dalla Costa, G.; Pérez-López, C.; Camerota, F.; et al. Fifteen Years of Wireless Sensors for Balance Assessment in Neurological Disorders. Sensors 2020, 20, 3247. [Google Scholar] [CrossRef]


| Clinical Test | Purpose | Number of Tasks | Score | Total | Interpretation | References |
|---|---|---|---|---|---|---|
| The Timed Up and Go Test (TUG) | Dynamic balance, fall risk | 1 | Time (seconds) | - | >12 s * | [2,23] |
| Dynamic Gait Index (DGI) | Dynamic balance | 8 | 0–3 (points) | 24 | ≤19/24 predictive of falls in the elderly >22/24 safe ambulators | [2,24] |
| Berg Balance Scale (BBS) | Static and dynamic balance | 14 | 0–4 (points) | 56 | 0–20—wheelchair-bound, 21–40—walking with assistance, 41–56, independent | [2,25] |
| The Tinetti test | Static and dynamic balance | 16 (9 balance-, 7 gait-related) | 0–1; 0–2 (points) | 28 | fall risk ≤ 18—high; 19–23—moderate; ≥24—low | [26] |
| The Functional Reach test (FR) | Dynamic balance | 1 | centimeters | - | ≥25 cm—low risk of falls, 15–24 cm—risk of falling is 2× greater | [2,27] |
| Non-Instrumental Test | Before VRT | After VRT | MD | p-Value | |
|---|---|---|---|---|---|
| DHI (points) | Total | 53.9 ± 18.7 | 36.3 ± 20.6 | −17.7 | *** |
| Physical | 15.5 ± 7 | 11.3 ± 6.9 | −4.2 | *** | |
| Functional | 21.2 ± 7.8 | 16.2 ± 9.7 | −5 | ** | |
| Emotional | 17.3 ± 7.9 | 10.4 ± 7.8 | −7 | *** | |
| VSS-sf (points) | Total | 19.7 ± 9.3 | 6.9 ± 5.1 | −7.9 | *** |
| Vertigo–balance | 12.1 ± 6 | 6.9 ± 5.1 | −5.2 | *** | |
| Autonomic–anxiety | 7.7 ± 5.3 | 5.0 ± 4.2 | −2.7 | *** | |
| TUG (seconds) | 12.4 ± 5 | 8.5 ± 2.5 | −3.9 | ** | |
| DGI (points) | 18.7 ± 4.1 | 21.1 ± 3.9 | 2.4 | ** | |
| BBS (points) | 49.9 ± 5.4 | 52.5 ± 5.5 | 2.6 | ** | |
| Tinetti (points) | 23.7 ± 4.5 | 26.0 ± 3.1 | 2.3 | ** | |
| FR (points) | 29.2 ± 8.4 | 32.8 ± 8.4 | 3.6 | * | |
| Posturography Condition | Before VRT | After VRT | Before VRT | After VRT | Before VRT | After VRT | Before VRT | After VRT |
|---|---|---|---|---|---|---|---|---|
| Posturography Measure | ||||||||
| Length of Trajectory (mm) | Surface of COP (mm2) | Max Angular Velocity (deg/s) | Mean Angular Velocity (deg/s) | |||||
| Condition 1 | 86.7 ± 45.2 | 73 ns ± 23.2 | 151.5 ± 208.6 | 92.1 ns ± 69.6 | 2.8 ± 1.2 | 2.6 * ± 1.3 | 0.5 ± 0.3 | 0.4 ns ± 0.1 |
| Condition 2 | 143.7 ± 146.6 | 114.5 ns ± 82 | 508.8 ± 1435.1 | 198.5 ns ± 245.1 | 3.6 ± 2.7 | 3.3 ns ± 1.5 | 0.8 ± 0.9 | 0.7 ns ± 0.5 |
| Condition 3 | 213.5 ± 130.7 | 169.5 ** ± 82.5 | 1118.6 ± 1712.5 | 759.1 ** ± 1237.7 | 4.5 ± 3.1 | 3.7 ns ± 1.5 | 1.3 ± 0.9 | 1 ** ± 0.5 |
| Condition 4 | 373.9 ± 172.7 | 301.7 ** ± 122.6 | 3931.2 ± 5577.5 | 2381.6 ** ± 2055.8 | 7.8 ± 4 | 6.1 *** ± 3 | 2.6 ± 1.4 | 2 *** ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawronska, A.; Rosiak, O.; Pajor, A.; Janc, M.; Kotas, R.; Kaminski, M.; Zamyslowska-Szmytke, E.; Jozefowicz-Korczynska, M. Instrumental and Non-Instrumental Measurements in Patients with Peripheral Vestibular Dysfunctions. Sensors 2023, 23, 1994. https://doi.org/10.3390/s23041994
Gawronska A, Rosiak O, Pajor A, Janc M, Kotas R, Kaminski M, Zamyslowska-Szmytke E, Jozefowicz-Korczynska M. Instrumental and Non-Instrumental Measurements in Patients with Peripheral Vestibular Dysfunctions. Sensors. 2023; 23(4):1994. https://doi.org/10.3390/s23041994
Chicago/Turabian StyleGawronska, Anna, Oskar Rosiak, Anna Pajor, Magdalena Janc, Rafal Kotas, Marek Kaminski, Ewa Zamyslowska-Szmytke, and Magdalena Jozefowicz-Korczynska. 2023. "Instrumental and Non-Instrumental Measurements in Patients with Peripheral Vestibular Dysfunctions" Sensors 23, no. 4: 1994. https://doi.org/10.3390/s23041994
APA StyleGawronska, A., Rosiak, O., Pajor, A., Janc, M., Kotas, R., Kaminski, M., Zamyslowska-Szmytke, E., & Jozefowicz-Korczynska, M. (2023). Instrumental and Non-Instrumental Measurements in Patients with Peripheral Vestibular Dysfunctions. Sensors, 23(4), 1994. https://doi.org/10.3390/s23041994







