Ultrasonic High-Resolution Imaging and Acoustic Tweezers Using Ultrahigh Frequency Transducer: Integrative Single-Cell Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. UHF Transducers
2.2. Experimental Setup
2.3. PC-3 Preparation
3. Results
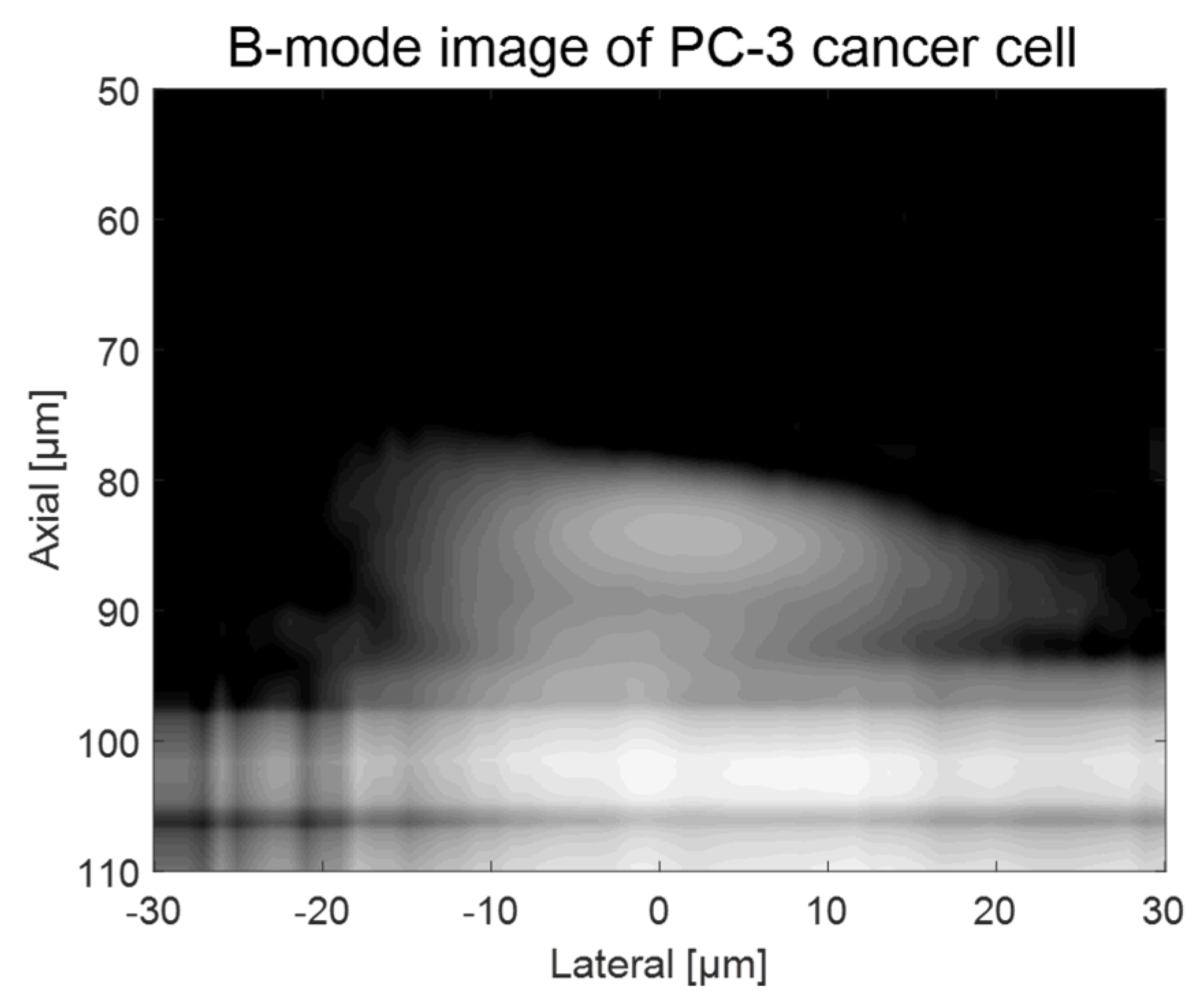
3.1. B-Mode Imaging of a Single Cell and Particle Using UHF Transducers
3.2. A Sinlge-Cell and Particle Trapping Using Acoustic Tweezers
4. Discussion
4.1. A Single-Cell B-Mode Imaging
4.2. Acoustic Tweezer of a Sinlge Cell
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, B.; Czarnota, G.; Porter, T.; Gallippi, C. Advances in Ultrasound Imaging Technology. Med. Phys. 2015, 42, 3683–3684. [Google Scholar] [CrossRef]
- Huang, Q.; Zeng, Z. A Review on Real-Time 3D Ultrasound Imaging Technology. BioMed Res. Int. 2017, 2017, 6027029. [Google Scholar] [CrossRef] [PubMed]
- Bamber, J.C. Ultrasound elasticity imaging: Definition and technology. Eur. Radiol. 1999, 9, S327–S330. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.C.; Kinger, N.; Mittal, P.K.; Spivey, J.; Baumgarten, D.A.; Duszak, R. Ultrasound Elastography with Imaging: Overcoming Emerging Technology Reimbursement Challenges. J. Am. Coll. Radiol. 2017, 14, 1426–1428. [Google Scholar] [CrossRef]
- Forsberg, F. Ultrasonic biomedical technology; marketing versus clinical reality. Ultrasonics 2004, 42, 17–27. [Google Scholar] [CrossRef]
- Holland, M.; Byram, B.; Thomenius, K.; Insana, M. Advances in Ultrasound Imaging Technology. Med. Phys. 2017, 44, 3253. [Google Scholar]
- Qiu, W.; Bouakaz, A.; Konofagou, E.E.; Zheng, H. Special Issue on: Recent Advances in Ultrasound Technology for Brain Imaging and Therapy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2019, 66, 1827. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Du, M.; Chen, Z.-Y. Ultrasound Technology for Molecular Imaging: From Contrast Agents to Multimodal Imaging. ACS Biomater. Sci. Eng. 2018, 4, 2716–2728. [Google Scholar] [CrossRef]
- Lemons, R.A.; Quate, C.F. Acoustic microscopy: Biomedical applications. Science 1975, 188, 905–911. [Google Scholar] [CrossRef]
- Che, S.; Guduru, P.; Nurmikko, A.; Maris, H. A scanning acoustic microscope based on picosecond ultrasonics. Ultrasonics 2015, 56, 153–159. [Google Scholar] [CrossRef]
- Laux, D.; de Weerd, W.; Papaioannou, D.; Kitajima, S.; Rondinella, V.; Despaux, G. Scanning acoustic microscope for mechanical characterization and density estimation of irradiated nuclear fuel. Prog. Nucl. Energy 2014, 72, 63–66. [Google Scholar] [CrossRef]
- Miura, K. Application of Scanning Acoustic Microscope to evaluate lymph node lesions. Virchows 2012, 461, S132. [Google Scholar]
- Miura, K.; Yamamoto, S. A scanning acoustic microscope discriminates cancer cells in fluid. Sci. Rep. 2015, 5, 15243. [Google Scholar] [CrossRef]
- Chiao, R.; Lee, H. Scanning tomographic acoustic microscopy. IEEE Trans. Image Process. 1995, 4, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Grill, W.; Hillmann, K.; Kim, T.; Lenkeit, O.; Ndop, J.; Schubert, M. Scanning acoustic microscopy with vector contrast. Phys. B Condens. Matter 1999, 263-264, 553–558. [Google Scholar] [CrossRef]
- Miura, K.; Tsuchida, T.; Yamamoto, S. A Scanning Acoustic Microscope—A Novel Tool for Cytological Discrimination. FASEB J. 2015, 29, LB459. [Google Scholar] [CrossRef]
- Nelson, T.; Smith, R. Scanning acoustic microscopy. Adv. Mater. Process. 2004, 162, 29–32. [Google Scholar]
- White, G.; Barr, R.; Shaw, L.; Harris, F. Scanning acoustic microscopy of human tissue. Lab. Investig. 1990, 62, A106. [Google Scholar]
- Ghorayeb, S.; Lord, W.; Udpa, S. Application of a beamforming technique to ultrasound imaging in nondestructive testing. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1994, 41, 199–208. [Google Scholar] [CrossRef]
- Fritsch, C.; Parrilla, M.; Ibanez, A.; Giacchetta, R.C.; Martinez, O. The progressive focusing correction technique for ultrasound beamforming. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2006, 53, 1820–1831. [Google Scholar] [CrossRef]
- Taki, H.; Nagatani, Y.; Matsukawa, M.; Mizuno, K.; Sato, T. Fast characterization of two ultrasound longitudinal waves in cancellous bone using an adaptive beamforming technique. J. Acoust. Soc. Am. 2015, 137, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Shamsian, S.E.; Sakhaei, S.M. Fast adaptive beamforming through a cascade structure for ultrasound imaging. J. Med. Ultrason. 2019, 46, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zeng, L.; Song, J.; Zhou, L.; Ding, M.; Yuchi, M. Coherence Factor-Like Beamforming for Ultrasound Computed Tomography. J. Med. Imaging Health Inform. 2020, 10, 672–676. [Google Scholar] [CrossRef]
- Zhuang, B.; Rohling, R.; Abolmaesumi, P. Region-of-Interest-Based Closed-Loop Beamforming for Spinal Ultrasound Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2019, 66, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Lindner, J.R. Molecular imaging with targeted contrast ultrasound. Curr. Opin. Biotechnol. 2007, 18, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lindner, J.R. Molecular imaging with contrast ultrasound and targeted microbubbles. J. Nucl. Cardiol. 2004, 11, 215–221. [Google Scholar] [CrossRef]
- Anderson, C.R.; Hu, X.; Zhang, H.; Tlaxca, J.; Declèves, A.-E.; Houghtaling, R.; Sharma, K.; Lawrence, M.; Ferrara, K.; Rychak, J.J. Ultrasound Molecular Imaging of Tumor Angiogenesis with an Integrin Targeted Microbubble Contrast Agent. Investig. Radiol. 2011, 46, 215–224. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Zhang, Y.; Zhang, J.; Su, J.; Yang, C. Molecular imaging of atherosclerotic plaque with lipid nanobubbles as targeted ultrasound contrast agents. Colloids Surf. B Biointerfaces 2020, 189, 110861. [Google Scholar] [CrossRef]
- Emelianov, S. TH-B-351-01: Elastography—Real-Time Ultrasound Elasticity Imaging Technique. Med. Phys. 2008, 35, 2965–2966. [Google Scholar] [CrossRef]
- Zhou, H.; Goss, M.; Hernandez, C.; Mansour, J.M.; Exner, A. Validation of Ultrasound Elastography Imaging for Nondestructive Characterization of Stiffer Biomaterials. Ann. Biomed. Eng. 2015, 44, 1515–1523. [Google Scholar] [CrossRef]
- Athanasiou, A.; Tardivon, A. Élastographie par ultrasons en imagerie cancérologique. Oncologie 2010, 12, 208–212. [Google Scholar] [CrossRef]
- Islam, T.; Chaudhry, A.; Unnikrishnan, G.; Reddy, J.N.; Righetti, R. An analytical poroelastic model for ultrasound elastography imaging of tumors. Phys. Med. Biol. 2018, 63, 025031. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Munoz, N.E.; Eliahoo, P.; Wodnicki, R.; Jung, H.; Chiu, C.T.; Williams, J.A.; Kim, H.H.; Zhou, Q.; Shung, K.K. Forward-looking 30-MHz phased-array transducer for peripheral intravascular imaging. Sens. Actuators A Phys. 2018, 280, 145–163. [Google Scholar] [CrossRef]
- Sung, J.; Jeong, J. High-frequency ultrasound transducer by using inversion layer technique for intravascular ultrasound imaging. Electron. Lett. 2016, 52, 1003–1005. [Google Scholar] [CrossRef]
- Zemp, R.J.; Bitton, R.; Li, M.-L.; Shung, K.K.; Stoica, G.; Wang, L.V. Photoacoustic imaging of the microvasculature with a high-frequency ultrasound array transducer. J. Biomed. Opt. 2007, 12, 010501. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Han, Z.; Liu, P.; Xu, J.; Li, Z.; Li, P.; Shao, W.; Cui, Y. A High Frequency Geometric Focusing Transducer Based on 1-3 Piezocomposite for Intravascular Ultrasound Imaging. BioMed Res. Int. 2017, 2017, 9327270. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.; Ahn, J.; Han, M.; Lim, H.G.; Lee, K.J.; Lee, J.; Kim, C.; Kim, H.H. Detection of micro inclusions in steel sheets using high-frequency ultrasound speckle analysis. Sci. Rep. 2021, 11, 20416. [Google Scholar] [CrossRef]
- Lim, H.; Kim, H.; Kim, K.; Park, J.; Kim, Y.; Yoo, J.; Heo, D.; Baik, J.; Park, S.-M.; Kim, H. Thermal Ablation and High-Resolution Imaging Using a Back-to-Back (BTB) Dual-Mode Ultrasonic Transducer: In Vivo Results. Sensors 2021, 21, 1580. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Chiu, C.T.; Chen, X.; Chen, Z.; Ma, J.; Zhu, B.; Shung, K.K.; Zhou, Q. Ultrahigh Frequency (100 MHz–300 MHz) Ultrasonic Transducers for Optical Resolution Medical Imagining. Sci. Rep. 2016, 6, 28360. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kushida, K.; Sugawara, K.; Takeuchi, H. A 100-MHz ultrasonic transducer array using ZnO thin films. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1995, 42, 316–324. [Google Scholar] [CrossRef]
- Cannata, J.M.; Williams, J.A.; Zhou, Q.F.; Sun, L.; Shung, K.K.; Yu, H.; Kim, E.S. Self-focused ZnO transducers for ultrasonic biomicroscopy. J. Appl. Phys. 2008, 103, 084109. [Google Scholar] [CrossRef]
- Briggs, G.A.D.; Wang, J.; Gundle, R. Quantitative acoustic microscopy of individual living human cells. J. Microsc. 1993, 172, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Czarnota, G.J.; Kolios, M. Ultrasound detection of cell death. Imaging Med. 2010, 2, 17–28. [Google Scholar] [CrossRef]
- Strohm, E.M.; Moore, M.J.; Kolios, M.C. High resolution ultrasound and photoacoustic imaging of single cells. Photoacoustics 2016, 4, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Li, Y.; Li, Y.; Lim, H.G.; Zhou, Q.; Shung, K.K. Multifunctional single beam acoustic tweezer for non-invasive cell/organism manipulation and tissue imaging. Sci. Rep. 2016, 6, 37554. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Yoon, C.W.; Lim, H.G.; Park, J.M.; Yoon, S.; Lee, J.; Shung, K.K. Acoustic tweezers for studying intracellular calcium signaling in SKBR-3 human breast cancer cells. Ultrasonics 2015, 63, 94–101. [Google Scholar] [CrossRef]
- Gesellchen, F.; Bernassau, A.L.; Déjardin, T.; Cumming, D.R.S.; Riehle, M.O. Cell patterning with a heptagon acoustic tweezer—Application in neurite guidance. Lab Chip 2014, 14, 2266–2275. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, H.; Kim, Y.; Lim, H.G.; Kim, H.H. Collapse pressure measurement of single hollow glass microsphere using single-beam acoustic tweezer. Ultrason. Sonochem. 2021, 82, 105844. [Google Scholar] [CrossRef]
- Lim, H.G.; Li, Y.; Lin, M.-Y.; Yoon, C.; Lee, C.; Jung, H.; Chow, R.H.; Shung, K.K. Calibration of Trapping Force on Cell-Size Objects from Ultrahigh-Frequency Single-Beam Acoustic Tweezer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016, 63, 1988–1995. [Google Scholar] [CrossRef]
- Lim, H.G.; Kim, H.H.; Yoon, C. Evaluation method for acoustic trapping performance by tracking motion of trapped microparticle. Jpn. J. Appl. Phys. 2018, 57, 057202. [Google Scholar] [CrossRef]
- Liu, H.-C.; Gang, E.J.; Na Kim, H.; Lim, H.G.; Jung, H.; Chen, R.; Abdel-Azim, H.; Shung, K.K.; Kim, Y.-M. Characterizing Deformability of Drug Resistant Patient-Derived Acute Lymphoblastic Leukemia (ALL) Cells Using Acoustic Tweezers. Sci. Rep. 2018, 8, 15708. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.G.; Liu, H.-C.; Yoon, C.W.; Jung, H.; Kim, M.G.; Yoon, C.; Kim, H.H.; Shung, K.K. Investigation of cell mechanics using single-beam acoustic tweezers as a versatile tool for the diagnosis and treatment of highly invasive breast cancer cell lines: An in vitro study. Microsyst. Nanoeng. 2020, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.G.; Lee, O.-J.; Shung, K.K.; Kim, J.-T.; Kim, H.H. Classification of Breast Cancer Cells Using the Integration of High-Frequency Single-Beam Acoustic Tweezers and Convolutional Neural Networks. Cancers 2020, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Park, J.; Lim, H.G.; Yoon, S.; Lee, C.; Chang, J.H.; Shung, K.K. Label-free analysis of the characteristics of a single cell trapped by acoustic tweezers. Sci. Rep. 2017, 7, 14092. [Google Scholar] [CrossRef]
- Lee, O.-J.; Lim, H.G.; Shung, K.K.; Kim, J.-T.; Kim, H.H. Automated estimation of cancer cell deformability with machine learning and acoustic trapping. Sci. Rep. 2022, 12, 6891. [Google Scholar] [CrossRef]
- Lim, H.G.; Shung, K.K. Quantification of Inter-Erythrocyte Forces with Ultra-High Frequency (410 MHz) Single Beam Acoustic Tweezer. Ann. Biomed. Eng. 2017, 45, 2174–2183. [Google Scholar] [CrossRef]










| Layer | Material | Thickness | |
|---|---|---|---|
| 110 MHz | 410 MHz | ||
| Piezoelectric layer | LiNbO3 | 26 μm | 7.3 μm |
| Matching layer | Parylene | 5.2 μm | 1.1 μm |
| Backing layer | E-Solder 3022 | 1000 μm | 1000 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.; Shung, K.K.; Lim, H.G. Ultrasonic High-Resolution Imaging and Acoustic Tweezers Using Ultrahigh Frequency Transducer: Integrative Single-Cell Analysis. Sensors 2023, 23, 1916. https://doi.org/10.3390/s23041916
Jung H, Shung KK, Lim HG. Ultrasonic High-Resolution Imaging and Acoustic Tweezers Using Ultrahigh Frequency Transducer: Integrative Single-Cell Analysis. Sensors. 2023; 23(4):1916. https://doi.org/10.3390/s23041916
Chicago/Turabian StyleJung, Hayong, K. Kirk Shung, and Hae Gyun Lim. 2023. "Ultrasonic High-Resolution Imaging and Acoustic Tweezers Using Ultrahigh Frequency Transducer: Integrative Single-Cell Analysis" Sensors 23, no. 4: 1916. https://doi.org/10.3390/s23041916
APA StyleJung, H., Shung, K. K., & Lim, H. G. (2023). Ultrasonic High-Resolution Imaging and Acoustic Tweezers Using Ultrahigh Frequency Transducer: Integrative Single-Cell Analysis. Sensors, 23(4), 1916. https://doi.org/10.3390/s23041916








