Identification and Quantification of Activities Common to Intensive Care Patients; Development and Validation of a Dual-Accelerometer-Based Algorithm
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Participants
2.3. Activity Protocols
- (1)
- Protocol natural: activities were performed without further instructions in order to induce natural variation in how participants performed the activities,
- (2)
- Protocol healthcare provider: activities were performed with the assistance of a healthcare provider in order to reflect the (passive) status of an IC patient, especially during the transition from one activity to another,
- (3)
- Protocol strict: activities were performed strictly based on instructions on how to do so and with additional instructions if they were not performed correctly, and
- (4)
- Protocol bed cycling: active bed-biking on a bed-cycle ergometer (MOTO-MED) at a self-selected speed.
2.4. Interrater Reliability of the Video Observations
2.5. Accelerometer Locations
2.6. Processing of Accelerometer Data
2.7. Development and Optimization of the Algorithm
2.8. Statistical Analyses
3. Results
3.1. Participants and Protocols
3.2. Interrater Reliability of the Video Observations
3.3. Development and Optimization of the Algorithm
3.4. Validity of the Developed Algorithm
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IC | Intensive Care |
| ICC | Intra Class Correlation Coefficient |
| MCL-TR | midclavicular line left to right thigh |
| MCR-TL | midclavicular line right to left thigh |
| SMA | Signal Magnitude Area |
| %Agr | Percentage Agreement |
| MCL | Midclavicular line Left |
| MCR | Midclavicular line Right |
| TL | Thigh Left |
| TR | Thigh Right |
| CI | Confidence Interval |
| GA | gravitation component |
Appendix A
| Protocol Naturally | t (s) | Protocol Strict | t (s) |
|---|---|---|---|
| lying in bed (supine) | 30 | lying in bed (supine) | 30 |
| transition supine-side lying left | transition supine-side lying left | ||
| side lying left | 30 | side lying left | 30 |
| Transition side lying left-side lying right | transition side lying left-supine | ||
| side lying right | 30 | lying in bed (supine) | 30 |
| transition side lying right-prone lying | transition supine-side lying right | ||
| prone lying | 30 | side lying right | 30 |
| transition prone lying-semi sit | transition side lying right-supine | ||
| semi sit | 30 | lying in bed (supine) | 30 |
| transition semi sit-semi lying left side | transition supine-prone lying | ||
| semi lying left side | 30 | prone lying | 30 |
| transition semi lying left-semi lying right | transition prone lying-supine | ||
| semi lying right side | 30 | lying in bed (supine) | 30 |
| transition semi lying right- edge of bed | transition supine-semi sit | ||
| edge of bed | 30 | semi sit | 30 |
| transition edge of bed-sitting in a chair | transition semi sit-semi lying left side | ||
| sitting in a chair | 30 | semi lying left side | 30 |
| transition sitting in a chair-standing | transition semi lying left-semi lying right | ||
| standing | 30 | semi lying right side | 30 |
| transition standing-walking | transition semi lying right- supine | ||
| walking for 2 m, turn and return | lying in bed (supine) | 30 | |
| transition supine-edge of bed | |||
| edge of bed | 30 | ||
| protocol healthcare | t (s) | transition edge of bed-sitting in a chair | |
| lying in bed (supine) | 30 | sitting in a chair | 30 |
| transition supine-side lying left | transition sitting in a chair-standing | ||
| side lying left | 30 | standing | 30 |
| transition side lying left-side lying right | transition standing-walking | ||
| side lying right | 30 | walking for 2 m, turn and return | |
| transition side lying right-semi sit | |||
| semi sit | 30 | ||
| transition semi sit-edge of bed | protocol bed cycling | t (s) | |
| edge of bed | 30 | bed cycling with the head of bed on 10 degrees | 120 |
| transition edge of bed-sitting in a chair | head of bed till 30 degrees | ||
| sitting in a chair | 30 | bed cycling with the head of bed on 30 degrees | 120 |
Appendix B
| Different Activities | Assumptions | |
|---|---|---|
| T phi > 0 B phi < 0 T psi < 40 T psi > −40 B psi < 40 B psi > −40 T theta > −40 T theta > 5 |  |
| T phi > 0 B phi < 0 T psi > 40 T psi < −40 B psi > 40 B psi < −40 T theta > −15 T theta < −40 T psi < −15 B psi < −50 |  |
| T phi > 0 B phi < 0 T psi > 40 T psi < −40 B psi > 40 B psi < −40 T theta > −15 T theta < −40 T psi > 10 B psi > 50 |  |
| T phi < 0 B phi < 0 | 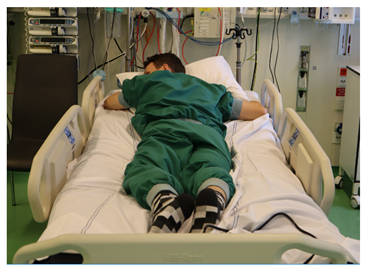 |
| T phi > 0 B phi < 0 T psi < 40 T psi > −40 B psi < 40 B psi > −40 T theta > −40 T theta < 5 T theta > −40 | 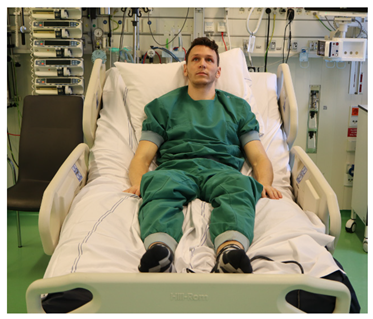 |
| T phi > 0 B phi < 0 T psi > 40 T psi < −40 B psi > 40 B psi < −40 T theta < −15 T theta > −40 T psi < −15 B psi < −45 | 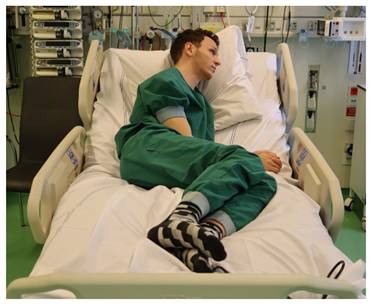 |
| T phi > 0 B phi < 0 T psi > 40 T psi < −40 B psi > 40 B psi < −40 T theta < −15 T theta > −40 T psi > 15 B psi > 45 |  |
| T phi > 0 B phi < 0 T psi < 40 T psi > −40 B psi < 40 B psi > −40 T theta <−40 B theta >−50 |  |
| - | 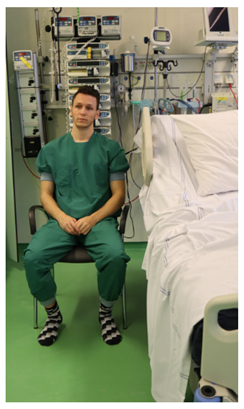 |
| T phi > 0 B phi < 0 T psi < 40 T psi > −40 B psi < 40 B psi > −40 T theta <−40 B theta <−50 |  |
| T SMA > 0.04 T theta <−50 B theta <−40 | |
| B SMA > 0.03 T theta > 5 B theta > 25 Previous 10 values either 12 or 13 |  |
| B SMA > 0.03 T theta < 5 B theta > 25 Previous 10 values either 12 or 13 |  |
| T SMA > 0.04 B theta > −40 |
Appendix C
| Protocol Natural | |||
|---|---|---|---|
| Supine lying | 98.5 | 96.5 | 97.0 |
| Side-semi lying left | 100.0 | 97.6 | 98.2 |
| Side-semi lying right | 99.6 | 96.9 | 98.1 |
| Prone lying | 100.0 | 97.1 | 97.6 |
| Semi sit | 99.5 | 74.3 | 80.7 |
| Sitting (edge of bed or chair) | 99.6 | 85.0 | 97.3 |
| Standing | 99.0 | 78.4 | 97.7 |
| Walking | 94.5 | 83.9 | 81.1 |
| Transitions | 86.9 | 83.1 | 84.1 |
| Protocol Strict | |||
| Supine lying | 99.8 | 96.4 | 97.1 |
| Side-semi lying left | 99.4 | 96.2 | 96.8 |
| Side-semi lying right | 89.7 | 96.0 | 96.9 |
| Prone lying | 99.9 | 95.3 | 95.9 |
| Semi sit | 99.8 | 96.6 | 96.5 |
| Sitting (edge of bed or chair) | 99.4 | 89.9 | 92.1 |
| Standing | 99.9 | 97.5 | 97.7 |
| Walking | 95.1 | 77.0 | 81.3 |
| Transitions | 92.8 | 80.0 | 80.3 |
| Protocol Healthcare Provider | |||
| Supine lying | 99.7 | 99.5 | 100.0 |
| Side lying left | 99.7 | 99.7 | 99.6 |
| Side lying right | 98.3 | 98.7 | 99.6 |
| Prone lying | - | - | - |
| Semi sit | 99.8 | 99.0 | 97.8 |
| Sitting (edge of bed or chair) | 99.8 | 98.3 | 81.8 |
| Standing | - | - | - |
| Walking | - | - | - |
| Transitions | 92.0 | 17.9 | 14.7 |
| Protocol Bed Cycling | |||
| Bed cycling | 99.6 | 75.7 | 73.0 |
| Bed cycling semi sit | 99.9 | 65.4 | 57.3 |
References
- Definition of Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/rehabilitation (accessed on 29 November 2022).
- Eggmann, S.; Irincheeva, I.; Luder, G.; Verra, M.L.; Moser, A.; Bastiaenen, C.H.G.; Jakob, S.M. Cardiorespiratory response to early rehabilitation in critically ill adults: A secondary analysis of a randomised controlled trial. PLoS ONE 2022, 17, e0262779. [Google Scholar] [CrossRef]
- González-Seguel, F.; Mayer, K.P.; Leppe, J.; Camus-Molina, A.; Leiva-Corvalán, M. ORIGINAL STUDY Uninterrupted Actigraphy Recording to Quantify Physical Activity and Sedentary Behaviors in Mechanically Ventilated Adults: A Feasibility Prospective Observational Study. J. Acute Care Phys. Ther. 2022, 13, 190–197. [Google Scholar] [CrossRef]
- Impellizzeri, F.M.; Marcora, S.M.; Coutts, A.J. Internal and external training load: 15 years on. Int. J. Sport. Physiol. Perform. 2019, 14, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Schwab, K.E.; To, A.Q.; Chang, J.; Ronish, B.; Needham, D.M.; Martin, J.L.; Kamdar, B.B. Actigraphy to Measure Physical Activity in the Intensive Care Unit: A Systematic Review. J. Intensive Care Med. 2020, 35, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Stocking, J.; Kuhn, B.; Doroy, A.; Blackmon, E.; Young, H.M.; Adams, J.Y. How much do hospitalized adults move? A systematic review and meta-analysis. Appl. Nurs. Res. 2020, 51, 151189. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.M.; Knight, L.D.; Connolly, B.; Baldwin, C.; Puthucheary, Z.; Morris, P.; Mortimore, J.; Hart, N.; Denehy, L.; Granger, C.L. Factors influencing physical activity and rehabilitation in survivors of critical illness: A systematic review of quantitative and qualitative studies. Intensive Care Med. 2017, 43, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Doroy, A.; Da Marto, N.; Taylor, S.; Anderson, N.; Young, H.M.; Adams, J.Y. Quantifying Mobility in the ICU: Comparison of Electronic Health Record Documentation and Accelerometer-Based Sensors to Clinician-Annotated Video. Crit. Care Explor. 2020, 2, e0091. [Google Scholar] [CrossRef]
- Verceles, A.C.; Hager, E.R. Use of accelerometry to monitor physical activity in critically ill subjects: A systematic review. Respir. Care 2015, 60, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Green, A.J.; Yoward, L.S.; Hall, H.K. Validity and reliability of accelerometry in identification of lying, sitting, standing or purposeful activity in adult hospital inpatients recovering from acute or critical illness: A systematic review. Clin. Rehabil. 2018, 32, 233–242. [Google Scholar] [CrossRef]
- Baldwin, C.E.; Rowlands, A.V.; Fraysse, F.; Johnston, K.N. The sedentary behaviour and physical activity patterns of survivors of a critical illness over their acute hospitalisation: An observational study. Aust. Crit. Care 2020, 33, 272–280. [Google Scholar] [CrossRef]
- Bassett, D.R.; John, D.; Conger, S.A.; Rider, B.C.; Passmore, R.M.; Clark, J.M. Detection of lying down, sitting, standing, and stepping using two activPAL monitors. Med. Sci. Sport. Exerc. 2014, 46, 2025–2029. [Google Scholar] [CrossRef]
- Lugade, V.; Fortune, E.; Morrow, M.; Kaufman, K. Validity of using tri-axial accelerometers to measure human movement-Part I: Posture and movement detection. Med. Eng. Phys. 2014, 36, 169–176. [Google Scholar] [CrossRef]
- Culhane, K.M.; Lyons, G.M.; Hilton, D.; Grace, P.A.; Lyons, D. Long-term mobility monitoring of older adults using accelerometers in a clinical environment. Clin. Rehabil. 2004, 18, 335–343. [Google Scholar] [CrossRef]
- Hartley, P.; Keevil, V.L.; Westgate, K.; White, T.; Brage, S.; Romero-Ortuno, R.; Deaton, C. Using accelerometers to measure physical activity in older patients admitted to hospital. Curr. Gerontol. Geriatr. Res. 2018, 2018, 3280240. [Google Scholar] [CrossRef]
- Rauen, K.; Schaffrath, J.; Pradhan, C.; Schniepp, R.; Jahn, K. Accelerometric trunk sensors to detect changes of body positions in immobile patients. Sensors 2018, 18, 3272. [Google Scholar] [CrossRef] [PubMed]
- van Dijk-Huisman, H.C.; Bijnens, W.; Senden, R.; Essers, J.M.N.; Meijer, K.; Aarts, J.; Lenssen, A.F. Optimization and validation of a classification algorithm for assessment of physical activity in hospitalized patients. Sensors 2021, 21, 1652. [Google Scholar] [CrossRef]
- Valkenet, K.; Veenhof, C. Validity of three accelerometers to investigate lying, sitting, standing and walking. PLoS ONE 2019, 14, e0217545. [Google Scholar] [CrossRef] [PubMed]
- Aminian, K.; Robert, P.; Buchser, E.E.; Hayoz, D.; Depairon, M.; Rutschmann, B. Physical activity monitoring based on accelerometry: Validation and comparison with video observation. Med. Biol. Eng. Comput. 1999, 37, 304–308. [Google Scholar] [CrossRef]
- Veltink, P.H.; Bussmann, H.B.J.; De Vries, W.; Martens, W.L.J.; Van Lummel, R.C. Detection of Static and Dynamic Activities Using Uniaxial Accelerometers. IEEE Trans. Rehabil. Eng. 1996, 4, 375–385. [Google Scholar] [CrossRef]
- M Morais, C.L.; D Santos, M.C.; G Lima, K.M.; Martin, F.L. Data and text mining Improving data splitting for classification applications in spectrochemical analyses employing a random-mutation Kennard-Stone algorithm approach. Bioinformatics 2019, 35, 5257–5263. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.J.n.d. Using an Accelerometer for Inclination Sensing. Available online: www.analog.com (accessed on 4 October 2021).
- de Vet, H.C.W.; Terwee, C.B.; Knol, D.L.; Bouter, L.M. When to use agreement versus reliability measures. J. Clin. Epidemiol. 2006, 59, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Halfwerk, F.R.; van Haaren, J.H.L.; Klaassen, R.; van Delden, R.W.; Veltink, P.H.; Grandjean, J.G. Objective quantification of in-hospital patient mobilization after cardiac surgery using accelerometers: Selection, use, and analysis. Sensors 2021, 21, 1979. [Google Scholar] [CrossRef]
- Sommers, J.; Klooster, E.; Zoethout, S.B.; van den Oever, H.L.A.; Nollet, F.; Tepaske, R.; Horn, J.; Engelbert, R.H.H.; van der Schaaf, M. Feasibility of Exercise Testing in Patients Who Are Critically Ill: A Prospective, Observational Multicenter Study. Arch. Phys. Med. Rehabil. 2019, 100, 239–246. [Google Scholar] [CrossRef]
- Fuller, D.; Buote, R.; Stanley, K. A glossary for big data in population and public health: Discussion and commentary on terminology and research methods. J. Epidemiol. Community Health 2017, 71, 1113–1117. [Google Scholar] [CrossRef]
- Narayanan, A.; Desai, F.; Stewart, T.; Duncan, S.; MacKay, L. Application of raw accelerometer data and machine-learning techniques to characterize human movement behavior: A systematic scoping review. J. Phys. Act. Health 2020, 17, 360–383. [Google Scholar] [CrossRef] [PubMed]
- Bidargaddi, N.; Klingbeil, L.; Sarela, A.; Boyle, J.; Cheung, V.; Yelland, C.; Karunanithi, M.; Gray, L. Wavelet based approach for posture transition estimation using a waist worn accelerometer. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 1884–1887. [Google Scholar]



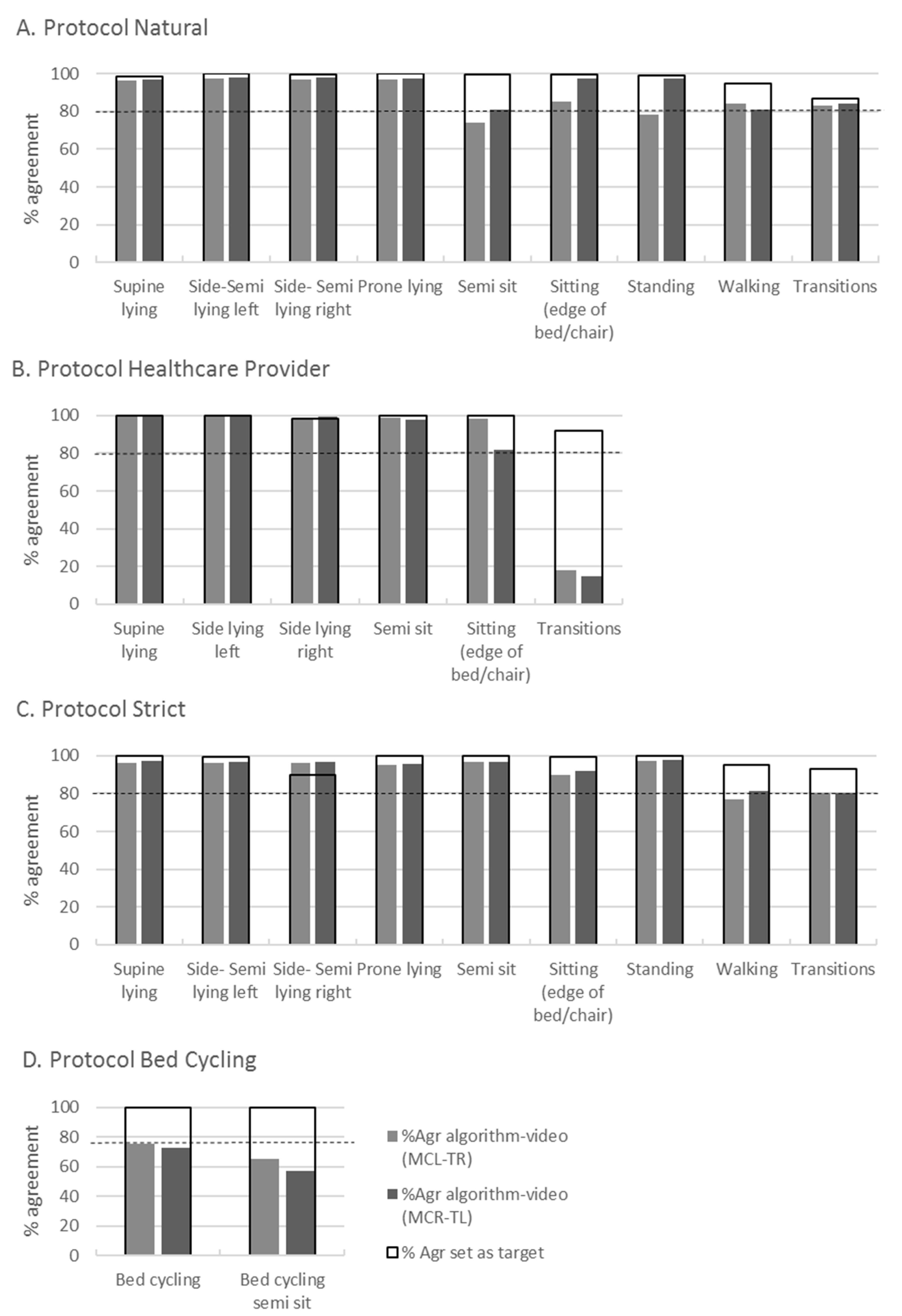
| Duration of Activities in Seconds with ICC and CI Between Two Raters (Mean and SD) | %Agr between Two Raters on Coded Video Data | %Agr between Algorithm and Video | |||||
|---|---|---|---|---|---|---|---|
| Protocol Natural | Rater 1 (s) | Rater 2 (s) | ICC | 95% CI | %Agr * | %Agr MCL-TR (n = 4) | %Agr MCR-TL (n = 4) |
| Supine lying | 37.12 (7.74) | 36.74 (7.60) | 0.98 | 0.93–0.99 | 98.5 | 99.1 | 96.7 |
| Side lying left | 31.4 (2.35) | 31.92 (2.40) | 0.92 | 0.68–0.98 | 99.8 | 72.8 | 73.4 |
| Side lying right | 31.62 (1.83) | 32.02 (2.07) | 0.96 | 0.69–0.99 | 99.8 | 73.6 | 98.3 |
| Prone lying | 32.76 (1.96) | 33.08 (2.02) | 0.97 | 0.80–0.99 | 100 | 98.0 | 98.2 |
| Semi sit | 33.52 (1.94) | 33.98 (2.11) | 0.95 | 0.62–0.99 | 99.5 | 80.1 | 70.1 |
| Semi lying left | 31.62 (4.02) | 32.04 (4.12) | 0.97 | 0.90–0.99 | 99.7 | 99.0 | 74.4 |
| Semi lying right | 33.30 (2.38) | 33.72 (1.90) | 0.94 | 0.74–0.98 | 99.6 | 74.7 | 50.3 |
| Sitting (edge of bed or chair) | 32.98 (1.72) | 33.37 (1.48) | 0.86 | 0.65–0.95 | 99.6 | 99.1 | 98.3 |
| Standing | 36.11 (5.69) | 36.44 (5.90) | 0.99 | 0.95–0.99 | 99.0 | 98.3 | 99.4 |
| Walking | 6.50 (0.79) | 6.46 (1.17) | 0.76 | 0.27–0.94 | 94.6 | 85.4 | 89.9 |
| Transitions | 3.39 (1.85) | 3.35 (1.97) | 0.87 | 0.81–0.95 | 86.9 | 85.1 | 86.9 |
| Protocol Strict | |||||||
| Supine lying | 33.06 (2.59) | 33.14 (2.56) | 0.99 | 0.99–0.99 | 99.8 | 98.3 | 97.7 |
| Side lying left | 31.06 (1.43) | 31.34 (1.45) | 0.96 | 0.73–0.99 | 99.8 | 99.2 | 98.2 |
| Side lying right | 31.82 (1.87) | 32.26 (1.72) | 0.88 | 0.59–0.97 | 99.5 | 72.4 | 96.7 |
| Prone lying | 32.72 (1.74) | 33.18 (1.63) | 0.91 | 0.56–0.98 | 99.9 | 99.1 | 96.4 |
| Semi sit | 32.76 (2.79) | 33.50 (3.32) | 0.94 | 0.62–0.99 | 99.8 | 98.7 | 73.1 |
| Semi lying left | 33.04 (2.38) | 33.52 (1.81) | 0.89 | 0.62–0.97 | 99.1 | 96.2 | 94.6 |
| Semi lying right | 33.28 (4.90) | 33.56 (5.01) | 0.99 | 0.96–0.99 | 99.9 | 74.6 | 74.5 |
| Sitting (edge of bed or chair) | 33.24 (2.58) | 34.02 (2.37) | 0.80 | 0.51–0.92 | 99.4 | 99.4 | 97.9 |
| Standing | 34.54 (4.55) | 35.36 (4.25) | 0.97 | 0.71–0.99 | 99.9 | 98.4 | 98.9 |
| Walking | 6.16 (0.83) | 6.36 (0.98) | 0.30 | −0.41–0.77 | 95.1 | 83.6 | 88.9 |
| Transitions | 2.95 (1.38) | 3.26 (1.43) | 0.81 | 0.72–0.87 | 92.8 | 84.5 | 82.9 |
| Protocol Healthcare Provider | |||||||
| Supine lying | 38.90 (5.13) | 39.02 (5.00) | 0.99 | 0.98–0.99 | 99.7 | 88.0 | 100.0 |
| Side lying left | 32.82 (2.13) | 33.04 (2.09) | 0.98 | 0.91–0.99 | 99.7 | 99.7 | 88.0 |
| Side lying right | 33.64 (2.31) | 33.32 (2.86) | 0.80 | 0.40–0.95 | 98.3 | 96.4 | 98.7 |
| Prone lying | - | - | - | - | - | - | - |
| Semi sit | 37.42 (3.89) | 37.84 (3.88) | 0.98 | 0.91–0.99 | 99.8 | 100 | 99.9 |
| Semi lying left | - | - | - | - | - | - | - |
| Semi lying right | - | - | - | - | - | - | - |
| Sitting (edge of bed or chair) | 33.85 (2.80) | 34.35 (3.02) | 0.97 | 0.80–0.99 | 99.8 | 97.9 | 88.5 |
| Standing | - | - | - | - | - | - | - |
| Walking | - | - | - | - | - | - | - |
| Transitions | 1.85 (1.36) | 2.37 (1.52) | 0.79 | 0.45–0.91 | 92.0 | 23.9 | 18.5 |
| Protocol Bed Cycling | |||||||
| Bed cycling | 130.36 (3.55) | 130.46 (3.94) | 0.98 | 0.94–0.99 | 99.6 | 61.1 | 91.1 |
| Bed cycling semi sit | 135.44 (13.36) | 137.05 (13.55) | 0.96 | 0.86–0.99 | 99.9 | 65.1 | 48.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dikkema, Y.; Mouton, N.; Gerrits, K.; Valk, T.; van der Steen-Diepenrink, M.; Eshuis, H.; Houdijk, H.; van der Schans, C.; Niemeijer, A.; Nieuwenhuis, M. Identification and Quantification of Activities Common to Intensive Care Patients; Development and Validation of a Dual-Accelerometer-Based Algorithm. Sensors 2023, 23, 1720. https://doi.org/10.3390/s23031720
Dikkema Y, Mouton N, Gerrits K, Valk T, van der Steen-Diepenrink M, Eshuis H, Houdijk H, van der Schans C, Niemeijer A, Nieuwenhuis M. Identification and Quantification of Activities Common to Intensive Care Patients; Development and Validation of a Dual-Accelerometer-Based Algorithm. Sensors. 2023; 23(3):1720. https://doi.org/10.3390/s23031720
Chicago/Turabian StyleDikkema, Yvonne, Noor Mouton, Koen Gerrits, Tim Valk, Mariëlle van der Steen-Diepenrink, Hans Eshuis, Han Houdijk, Cees van der Schans, Anuschka Niemeijer, and Marianne Nieuwenhuis. 2023. "Identification and Quantification of Activities Common to Intensive Care Patients; Development and Validation of a Dual-Accelerometer-Based Algorithm" Sensors 23, no. 3: 1720. https://doi.org/10.3390/s23031720
APA StyleDikkema, Y., Mouton, N., Gerrits, K., Valk, T., van der Steen-Diepenrink, M., Eshuis, H., Houdijk, H., van der Schans, C., Niemeijer, A., & Nieuwenhuis, M. (2023). Identification and Quantification of Activities Common to Intensive Care Patients; Development and Validation of a Dual-Accelerometer-Based Algorithm. Sensors, 23(3), 1720. https://doi.org/10.3390/s23031720







