Evaluating the Performance of Algorithms in Axillary Microwave Imaging towards Improved Breast Cancer Staging
Abstract
1. Introduction
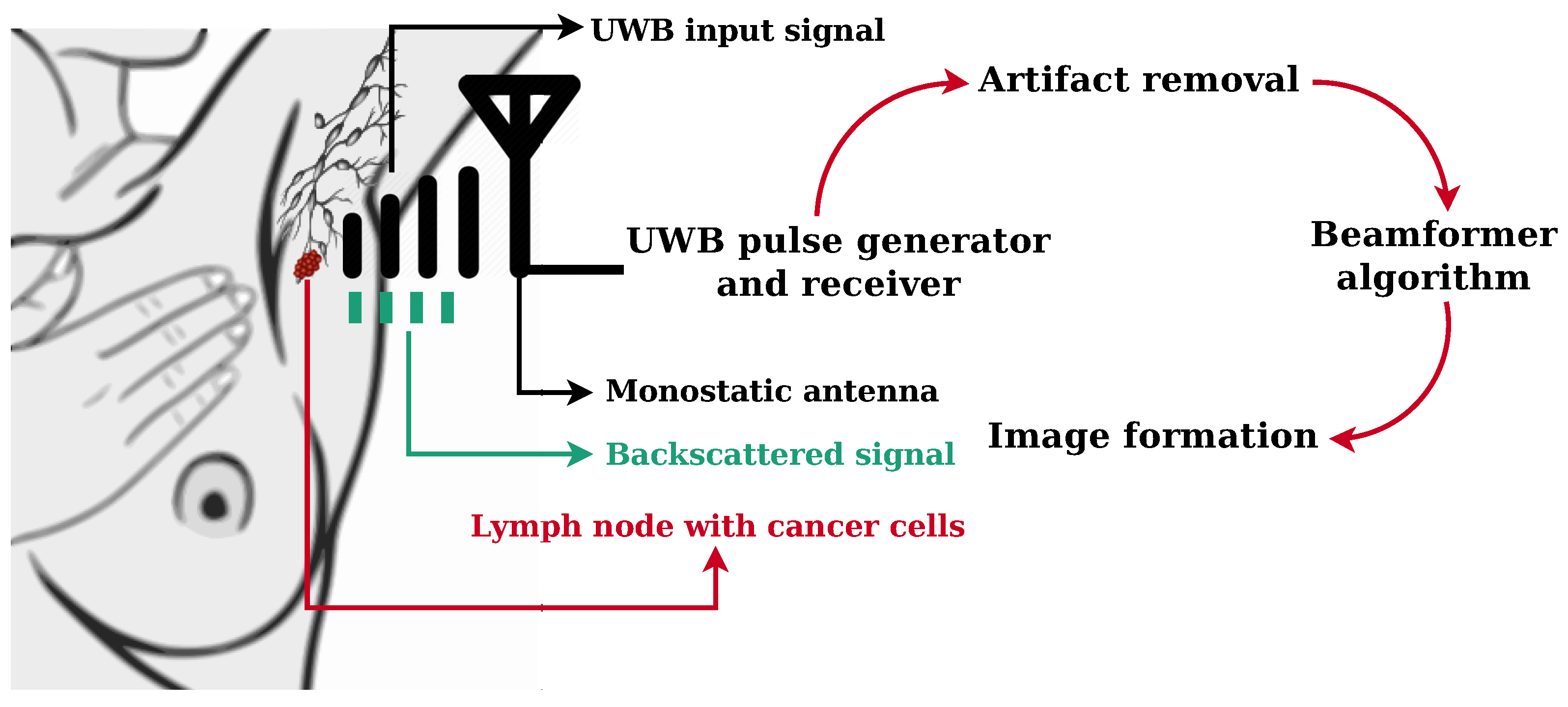
2. Materials and Methods
2.1. Electromagnetic Simulation of an UWB Microwave Imaging Radar System
2.2. Microwave Imaging
2.2.1. Skin Artifact Removal Algorithms
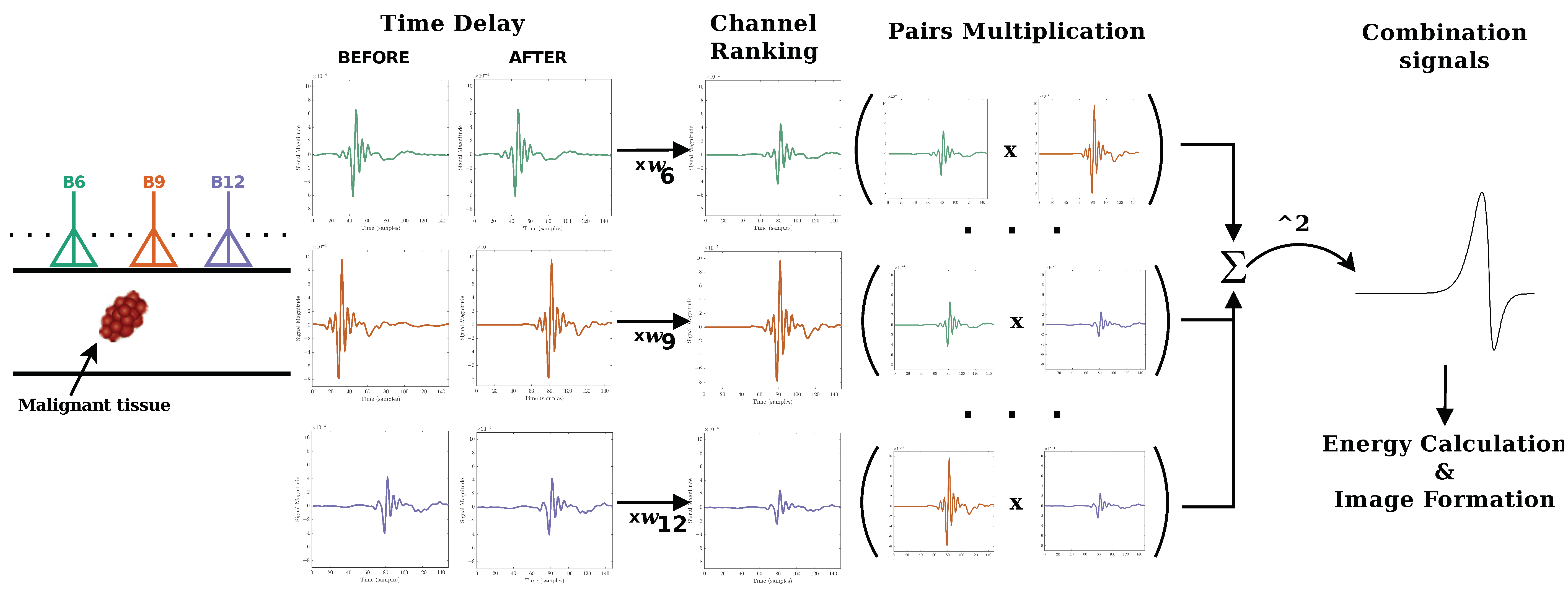
2.2.2. Beamforming Algorithms
2.3. Performance Metrics
3. Results
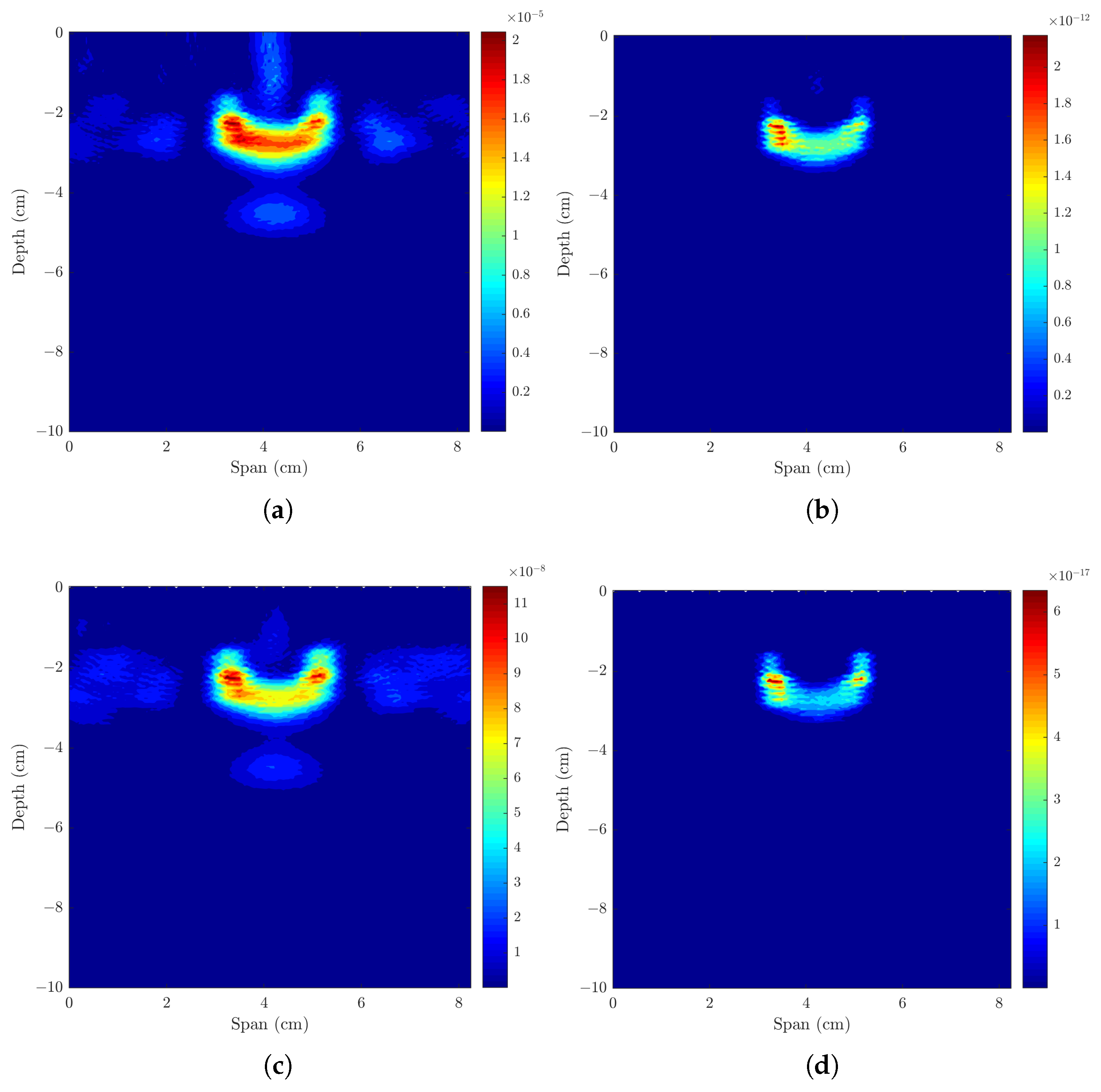
3.1. Imaging One Lymph Node
3.1.1. Axillary Models with One ALN, Uniform or Sinusoidal Skin Layer
3.1.2. Axillary Models with One ALN and Subcutaneous Adipose Tissue
3.1.3. Axillary Models ALNs at Different Depths and Muscle Layer
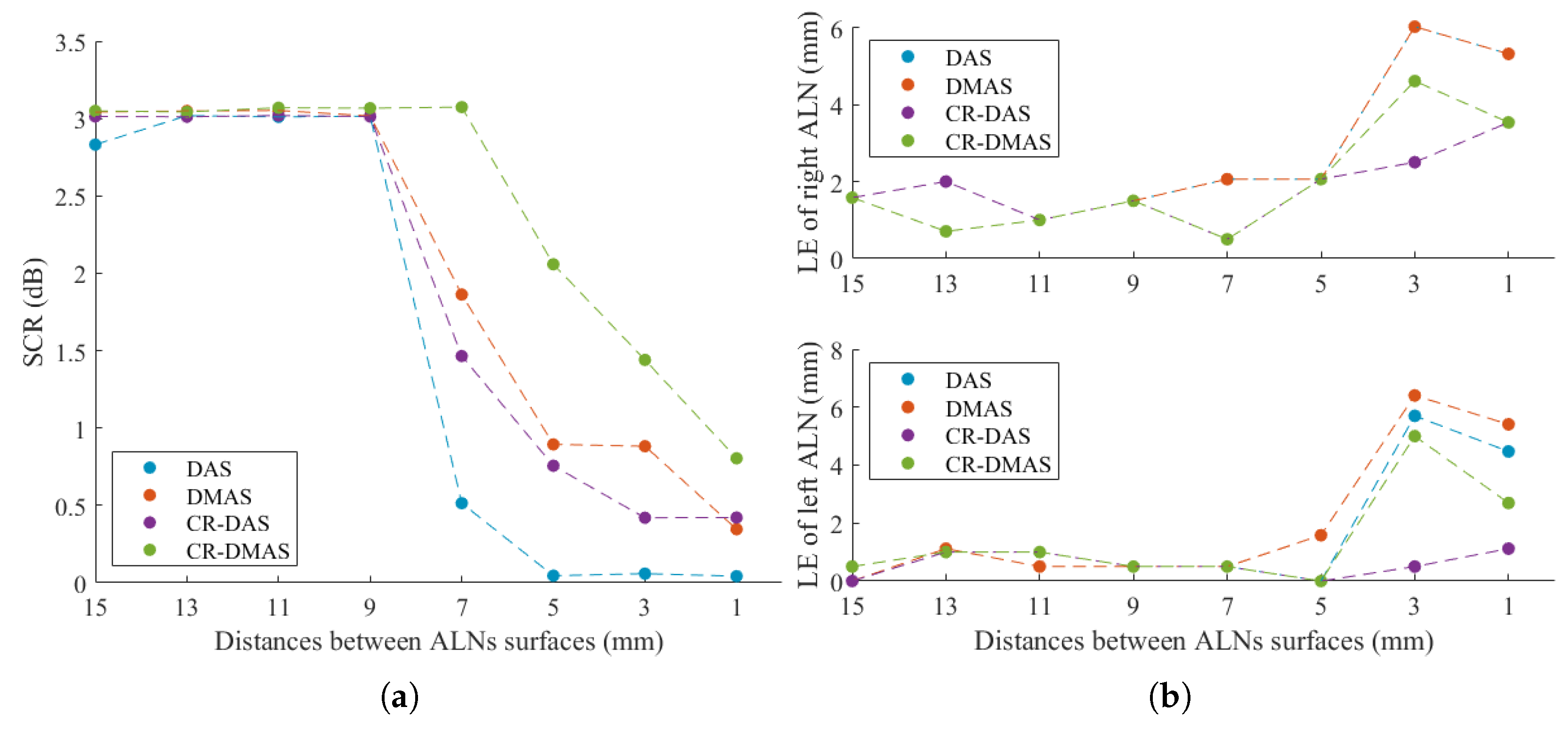
3.2. Imaging Two Axillary Lymph Nodes
3.2.1. Axillary Model with Two Metastasised ALNs
3.2.2. Axillary Model with One Healthy and One Metastasized ALNs
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALNs | Axillary Lymph Nodes |
| CR-DAS | Channel-Ranked Delay-And-Sum |
| CR-DMAS | Channel-Ranked Delay-Multiply-And-Sum |
| DAS | Delay And Sum |
| DMAS | Delay-Multiply-And-Sum |
| FDTD | Finite-Difference Time-Domain |
| FWHM | Full Width Half Maximum |
| LE | Localization Error |
| MMR | Max-to-Max Ratio |
| MWI | Microwave Imaging |
| SCR | Signal-to-Clutter Ratio |
| SMR | Signal-to-Mean Ratio |
| UWB | Ultra-Wide-Band |
References
- The Global Cancer Observatory—World Health Organization. Breast Cancer Fact Sheets. 2020. Available online: http://gco.iarc.fr/today (accessed on 20 December 2022).
- Cameron, T.R.; Okoniewski, M.; Fear, E.C. A Preliminary Study of the Electrical Properties of Healthy and Diseased Lymph Nodes. In Proceedings of the International Symposium on Antenna Technology and Applied Electromagnetics & the American Electromagnetics Conference (ANTEM-AMEREM), Ottawa, ON, Canada, 5–8 July 2010. [Google Scholar]
- Patani, N.; Dwek, M.; Douek, M. Predictors of axillary lymph node metastasis in breast cancer: A systematic review. Eur. J. Surg. Oncol. 2007, 33, 409–419. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. Breast Cancer. In AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2018; pp. 588–636. [Google Scholar]
- Houssami, N.; Ciatto, S.; Turner, R.M.; Cody, H.S., III; Macaskill, P. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: Meta-analysis of its accuracy and utility in staging the axilla. Ann. Surg. 2011, 254, 243–251. [Google Scholar] [CrossRef]
- Lu, Q.; Hua, J.; Kassir, M.M.; Delproposto, Z.; Dai, Y.; Sun, J.; Haacke, M.; Hu, J. Imaging lymphatic system in breast cancer patients with magnetic resonance lymphangiography. PLoS ONE 2013, 8, e69701. [Google Scholar] [CrossRef]
- Koolen, B.B.; Valdés Olmos, R.A.; Elkhuizen, P.H.M.; Vogel, W.V.; Vrancken Peeters, M.J.T.F.D.; Rodenhuis, S.; Rutgers, E.J.T. Locoregional lymph node involvement on 18F-FDG PET/CT in breast cancer patients scheduled for neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2012, 135, 231–240. [Google Scholar] [CrossRef]
- Sapienza, M.T.; Tavares, M.G.; Endo, I.S.; Campos Neto, G.C.; Lopes, M.M.; Nakagawa, S.; Belfort, F.A.; Soares, J., Jr.; Lewin, S.; Marone, M. The role of sentinel node mapping in malignant melanoma: Experience with 99mTc-phytate and a review of the literature. An. Bras. Dermatol. 2004, 79, 181–191. [Google Scholar] [CrossRef]
- Saleiro, S.; Rodrigues, A.C.; Frutuoso, C.; Guerra, C.; Coimbra, C.; Amaral, N.; Oliveira, C.F. Analysis of the Results from Sentinel Lymph Node Biopsy in Breast Cancer. Acta Obstet. Ginecol. Port. 2008, 2, 117–123. [Google Scholar]
- Lucci, A.; McCall, L.M.; Beitsch, P.D.; Whitworth, P.W.; Reintgen, D.S.; Blumencranz, P.W.; Leitch, A.M.; Saha, S.; Hunt, K.K.; Giuliano, A.E. Surgical Complications Associated With Sentinel Lymph Node Dissection (SLND) Plus Axillary Lymph Node Dissection Compared with SLND Alone in the American College of Surgeons Oncology Group Trial Z0011. J. Clin. Oncol. 2007, 25, 3657–3663. [Google Scholar] [CrossRef]
- Rahbar, H.; Partridge, S.C.; Javid, S.H.; Lehman, C.D. Imaging axillary lymph nodes in patients with newly diagnosed breast cancer. Curr. Probl. Diagn Radiol. 2012, 41, 149–158. [Google Scholar] [CrossRef]
- Cancer Research UK. Lymphoedema after Breast Cancer Treatment. Available online: https://www.cancerresearchuk.org/about-cancer/breast-cancer/living-with/lymphoedema-after-treatment (accessed on 20 December 2022).
- Conceição, R.C.; Mohr, J.J.; O’Halloran, M. An Introduction to Microwave Imaging for Breast Cancer Detection; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Crocco, L.; Karanasiou, I.; James, M.; Conceição, R.C. Emerging Electromagnetic Technologies for Brain Diseases Diagnostics, Monitoring and Therapy; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Eleutério, R.; Conceição, R.C. Initial Study for Detection of Multiple Lymph Nodes in the Axillary Region Using Microwave Imaging. In Proceedings of the 9th European Conference on Antennas and Propagation (EuCAP 2015), Lisbon, Portugal, 12–17 April 2015; pp. 1–3. [Google Scholar]
- Savazzi, M.; Abedi, S.; Ištuk, N.; Joachimowicz, N.; Roussel, H.; Porter, E.; O’ Halloran, M.; Costa, J.R.; Fernandes, C.A.; Felício, J.M.; et al. Development of an anthropomorphic phantom of the axillary region for microwave imaging assessment. Sensors 2020, 20, 4968. [Google Scholar] [CrossRef]
- Godinho, D.M.; Felício, J.M.; Fernandes, C.A.; Conceição, R.C. Experimental Evaluation of an Axillary Microwave Imaging System to Aid Breast Cancer Staging. IEEE J. Electromagn. RF Microwaves Med. Biol. 2022, 6, 68–76. [Google Scholar] [CrossRef]
- Godinho, D.M.; Silva, C.; Baleia, C.; Felício, J.M.; Castela, T.; Silva, N.A.; Orvalho, M.L.; Fernandes, C.A.; Conceição, R.C. Modelling Level I Axillary Lymph Nodes Depth for Diagnostic Imaging Technologies. Phys. Med. 2022, 104, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Vispa, A.; Sani, L.; Paoli, M.; Bigotti, A.; Raspa, G.; Ghavami, N.; Caschera, S.; Ghavami, M.; Duranti, M.; Tiberi, G. UWB device for breast microwave imaging: Phantom and clinical validations. Measurement 2019, 146, 582–589. [Google Scholar] [CrossRef]
- Li, X.; Hagness, S.C. A confocal microwave imaging algorithm for breast cancer detection. IEEE Microw. Wirel. Components Lett. 2001, 11, 130–132. [Google Scholar]
- Benchakroun, H.; O’Halloran, M.; O’Loughlin, D. Impact of rotational artefact removal on microwave breast images. In Proceedings of the 15th European Conference on Antennas and Propagation (EuCAP), Dusseldorf, Germany, 22–26 March 2021; pp. 1–4. [Google Scholar]
- Bond, E.J.; Li, X.; Hagness, S.C.; Van Veen, B.D. Microwave imaging via space-time beamforming for early detection of breast cancer. IEEE Trans. Antennas Propag. 2003, 51, 1690–1705. [Google Scholar] [CrossRef]
- O’Halloran, M.; Glavin, M.; Jones, E. Quasi-Multistatic MIST Beamforming for the Early Detection of Breast Cancer. IEEE Trans. Biomed. Eng. 2010, 57, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Ruvio, G.; Solimene, R.; Cuccaro, A.; Gaetano, D.; Browne, J.E.; Ammann, M.J. Breast cancer detection using interferometric MUSIC: Experimental and numerical assessment. Med. Phys. 2014, 41, 103101. [Google Scholar] [CrossRef] [PubMed]
- Felício, J.M.; Bioucas-Dias, J.M.; Costa, J.R.; Fernandes, C.A. Microwave Breast Imaging using a Dry Setup. IEEE Trans. Comput. Imaging 2020, 6, 167–180. [Google Scholar] [CrossRef]
- Elahi, M.A.; Shahzad, A.; Glavin, M.; Jones, E.; O’Halloran, M. Hybrid Artifact Removal for Confocal Microwave Breast Imaging. IEEE Antennas Wirel. Propag. Lett. 2014, 13, 149–152. [Google Scholar] [CrossRef]
- Reimer, T.; Solis-Nepote, M.; Pistorius, S. The Application of an Iterative Structure to the Delay-and-Sum and the Delay-Multiply-and-Sum Beamformers in Breast Microwave Imaging. Diagnostics 2020, 10, 411. [Google Scholar] [CrossRef]
- Taflove, A.; Hagness, S.C. Computational Electrodynamics: The Finite-Difference Time-Domain Method; Artech House: Norwood, MA, USA, 2005. [Google Scholar]
- O’Halloran, M.; Conceicao, R.C.; Byrne, D.; Glavin, M.; Jones, E. FDTD modeling of the breast: A review. Prog. Electromagn. Res. 2009, 18, 1–24. [Google Scholar] [CrossRef]
- Zastrow, E.; Davis, S.K.; Lazebnik, M.; Kelcz, F.; Veen, B.D.V.; Hagness, S.C. Development of anatomically realistic numerical breast phantoms with accurate dielectric properties for modeling microwave interactions with the human breast. IEEE Trans. Biomed. Eng. 2008, 55, 2792–2800. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Okoniewski, M.; Booske, J.H.; Hagness, S.C. Highly accurate Debye models for normal and malignant breast tissue dielectric properties at microwave frequencies. IEEE Microw. Wirel. Compon. Lett. 2007, 17, 822–824. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [PubMed]
- Godinho, D.M.; Felício, J.M.; Castela, T.; Silva, N.A.; Orvalho, M.L.; Fernandes, C.A.; Conceição, R.C. Extracting Dielectric Properties for MRI-based Phantoms for Axillary Microwave Imaging Device. In Proceedings of the 14th European Conference on Antennas and Propagation (EuCAP), Copenhagen, Denmark, 15–20 March 2020; pp. 1–4. [Google Scholar]
- Godinho, D.M.; Felício, J.M.; Castela, T.; Silva, N.A.; Orvalho, M.L.; Fernandes, C.A.; Conceição, R.C. Development of MRI-based axillary numerical models and estimation of axillary lymph node dielectric properties for microwave imaging. Med. Phys. 2021, 48, 5974–5990. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093. [Google Scholar] [CrossRef] [PubMed]
- Eleiwa, M.A.; Elsherbeni, A.Z. Debye Constants for Biological Tissues from 30 Hz to 20 GHz. Appl. Comput. Electromagn. Soc. J. 2001, 16, 202–213. [Google Scholar]
- Ludescher, B.; Rommel, M.; Willmer, T.; Fritsche, A.; Schick, F.; MacHann, J. Subcutaneous adipose tissue thickness in adults—Correlation with BMI and recommendations for pen needle lengths for subcutaneous self-injection. Clin. Endocrinol. 2011, 75, 786–790. [Google Scholar] [CrossRef]
- Weisstein, E.W. Bean Curve. Available online: http://mathworld.wolfram.com/BeanCurve.html (accessed on 20 December 2022).
- Hagness, S.C.; Taflove, A.; Bridges, J.E. Two-dimensional FDTD analysis of a pulsed microwave confocal system for breast cancer detection: Fixed-focus and antenna-array sensors. IEEE Trans. Biomed. Eng. 1998, 45, 1470–1479. [Google Scholar] [CrossRef]
- Lim, H.B.; Nhung, N.T.T.; Li, E.P.; Thang, N.D. Confocal microwave imaging for breast cancer detection: Delay-multiply-and-sum image reconstruction algorithm. IEEE Trans. Biomed. Eng. 2008, 55, 1697–1704. [Google Scholar] [PubMed]
- O’Halloran, M.; Glavin, M.; Jones, E. Channel-ranked beamformer for the early detection of breast cancer. Prog. Electromagn. Res. 2010, 103, 153–168. [Google Scholar] [CrossRef]
- Fear, E.C.; Li, X.; Hagness, S.C.; Stuchly, M.A. Confocal microwave imaging for breast cancer detection: Localization of tumors in three dimensions. IEEE Trans. Biomed. Eng. 2002, 49, 812–822. [Google Scholar] [CrossRef]
- Conceição, R.C. The Development of Ultra Wideband Scanning Techniques for Detection and Classification of Breast Cancer. Ph.D. Thesis, National University of Ireland Galway, Galway, Ireland, 2010. [Google Scholar]
- Godinho, D.M.; Felício, J.M.; Fernandes, C.A.; Conceição, R.C. Optimisation of Artefact Removal Algorithm for Microwave Imaging of the Axillary Region Using Experimental Prototype Signals. In Proceedings of the 15th European Conference on Antennas and Propagation (EuCAP), Dusseldorf, Germany, 22–26 March 2021; pp. 1–5. [Google Scholar]
- Conceição, R.C.; O’Halloran, M.; Glavin, M.; Jones, E. Support Vector Machines for the Classification of Early-Stage Breast Cancer Based on Radar Target Signatures. Prog. Electromagn. Res. B 2010, 23, 311–327. [Google Scholar] [CrossRef]
- McGinley, B.; O’Halloran, M.; Conceicao, R.C.; Morgan, F.; Glavin, M.; Jones, E. Spiking Neural Networks for Breast Cancer Classification Using Radar Target Signatures. Prog. Electromagn. Res. C 2010, 17, 79–94. [Google Scholar] [CrossRef]
- Reimer, T.; Pistorius, S. The Diagnostic Performance of Machine Learning in Breast Microwave Sensing on an Experimental Dataset. IEEE J. Electromagn. RF Microw. Med. Biol. 2022, 6, 139–145. [Google Scholar] [CrossRef]
- Conceição, R.C.; Medeiros, H.; O’Halloran, M.; Rodriguez-Herrera, D.; Flores-Tapia, D.; Pistorius, S. Initial classification of breast tumour phantoms using a UWB radar prototype. In Proceedings of the International Conference on Electromagnetics in Advanced Applications (ICEAA), Torino, Italy, 9–13 September 2013; pp. 720–723. [Google Scholar]
- Oliveira, B.L.; Godinho, D.; O’Halloran, M.; Glavin, M.; Jones, E.; Conceição, R.C. Diagnosing Breast Cancer with Microwave Technology: Remaining challenges and potential solutions with machine learning. Diagnostics 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Conceição, R.C.; Medeiros, H.; Godinho, D.M.; O’Halloran, M.; Rodriguez-Herrera, D.; Flores-Tapia, D.; Pistorius, S. Classification of breast tumor models with a prototype microwave imaging system. Med. Phys. 2020, 47, 1860–1870. [Google Scholar] [CrossRef]





| Tissues | |||||
|---|---|---|---|---|---|
| Skin | 13 | ||||
| Adipose Tissue | |||||
| Muscle | |||||
| Healthy Lymph Nodes | Surface | ||||
| Cross-Section | |||||
| Metastasized Lymph Nodes | Surface | ||||
| Cross- Section | |||||
| Li’s Skin Artifact Removal | Bond’s Skin Artifact Removal | |||||||
|---|---|---|---|---|---|---|---|---|
| Beamformer | SCR (dB) | SMR (dB) | LE (mm) | FWHM (mm) | SCR (dB) | SMR (dB) | LE (mm) | FWHM (mm) |
| ALN with uniform skin | ||||||||
| DAS | 3.03 | 14.62 | 1.80 | 8.50 | 3.03 | 14.88 | 1.80 | 8.50 |
| DMAS | 3.03 | 24.05 | 0.50 | 6.50 | 3.02 | 24.38 | 0.50 | 6.50 |
| CR-DAS | 3.02 | 15.57 | 1.80 | 8.25 | 3.01 | 16.50 | 1.80 | 8.25 |
| CR-DMAS | 3.01 | 24.44 | 0.50 | 7.00 | 3.03 | 25.23 | 0.50 | 7.00 |
| ALN with sinusoidal skin | ||||||||
| DAS | 3.01 | 15.56 | 32.18 | 2.75 | 3.04 | 15.37 | 1.12 | 8.25 |
| DMAS | 3.04 | 19.76 | 32.18 | 1.75 | 3.04 | 24.25 | 1.12 | 6.00 |
| CR-DAS | 3.02 | 16.07 | 40.03 | 2.75 | 3.13 | 16.22 | 1.12 | 8.25 |
| CR-DMAS | 3.04 | 20.60 | 19.70 | 1.00 | 3.02 | 24.18 | 1.12 | 6.00 |
| Beamformer | SCR (dB) | SMR (dB) | LE (mm) | FWHM (mm) |
|---|---|---|---|---|
| ALN with subcutaneous adipose | ||||
| DAS | 3.02 | 13.63 | 3.50 | 9.25 |
| DMAS | 3.07 | 22.73 | 3.50 | 7.50 |
| CR-DAS | 3.01 | 14.32 | 3.50 | 9.25 |
| CR-DMAS | 3.03 | 23.11 | 3.50 | 7.50 |
| Shallowest ALN with muscle | ||||
| DAS | 3.01 | 11.57 | 1.58 | 8.25 |
| DMAS | 3.01 | 19.00 | 1.58 | 6.50 |
| CR-DAS | 3.02 | 13.12 | 1.58 | 8.25 |
| CR-DMAS | 3.07 | 20.78 | 1.58 | 7.25 |
| Deepest ALN with muscle | ||||
| DAS | 3.02 | 16.68 | 0.71 | 9.00 |
| DMAS | 3.03 | 24.86 | 0.71 | 7.25 |
| CR-DAS | 2.54 | 15.34 | 0.71 | 9.00 |
| CR-DMAS | 3.02 | 23.24 | 0.71 | 7.25 |
| Beamformer | SCR (dB) | SMR (dB) | MMR (dB) | LE Left (mm) | LE Right (mm) | FWHM Left (mm) | FWHM Right (mm) |
|---|---|---|---|---|---|---|---|
| Two metastasized ALNs | |||||||
| DAS | 2.83 | 13.39 | 0.18 | 1.58 | 0.00 | 7.00 | 8.00 |
| DMAS | 3.04 | 20.70 | 0.87 | 1.58 | 0.00 | 5.00 | 5.50 |
| CR-DAS | 3.01 | 14.44 | 0.03 | 1.58 | 0.00 | 7.50 | 8.25 |
| CR-DMAS | 3.05 | 21.83 | 0.44 | 1.58 | 0.50 | 5.25 | 6.00 |
| One healthy and one metastasized ALNs | |||||||
| DAS | 3.03 | 14.12 | 3.63 | 1.58 | 1.58 | 6.50 | 8.00 |
| DMAS | 3.01 | 23.17 | 8.37 | 1.12 | 1.58 | 4.50 | 6.75 |
| CR-DAS | 3.01 | 14.64 | 2.85 | 1.58 | 1.58 | 6.50 | 7.75 |
| CR-DMAS | 3.04 | 23.48 | 6.60 | 1.12 | 1.58 | 4.75 | 6.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pato, M.; Eleutério, R.; Conceição, R.C.; Godinho, D.M. Evaluating the Performance of Algorithms in Axillary Microwave Imaging towards Improved Breast Cancer Staging. Sensors 2023, 23, 1496. https://doi.org/10.3390/s23031496
Pato M, Eleutério R, Conceição RC, Godinho DM. Evaluating the Performance of Algorithms in Axillary Microwave Imaging towards Improved Breast Cancer Staging. Sensors. 2023; 23(3):1496. https://doi.org/10.3390/s23031496
Chicago/Turabian StylePato, Matilde, Ricardo Eleutério, Raquel C. Conceição, and Daniela M. Godinho. 2023. "Evaluating the Performance of Algorithms in Axillary Microwave Imaging towards Improved Breast Cancer Staging" Sensors 23, no. 3: 1496. https://doi.org/10.3390/s23031496
APA StylePato, M., Eleutério, R., Conceição, R. C., & Godinho, D. M. (2023). Evaluating the Performance of Algorithms in Axillary Microwave Imaging towards Improved Breast Cancer Staging. Sensors, 23(3), 1496. https://doi.org/10.3390/s23031496








