The Influence of Mechanical Bowel Preparation on Volatile Organic Compounds for the Detection of Gastrointestinal Disease—A Systematic Review
Abstract
1. Introduction
1.1. Non-Invasive Biomarkers for Colorectal Cancer and Other Gastrointestinal Diseases
1.2. Faecal Occult Blood Testing (FOBT) and Faecal Immunohistochemical Testing (FIT)
1.3. Carcinoembryonic Antigen (CEA)
1.4. DNA Methylation and ctDNA Methylation—mSEPT9
1.5. MicroRNAs (miRNAs)
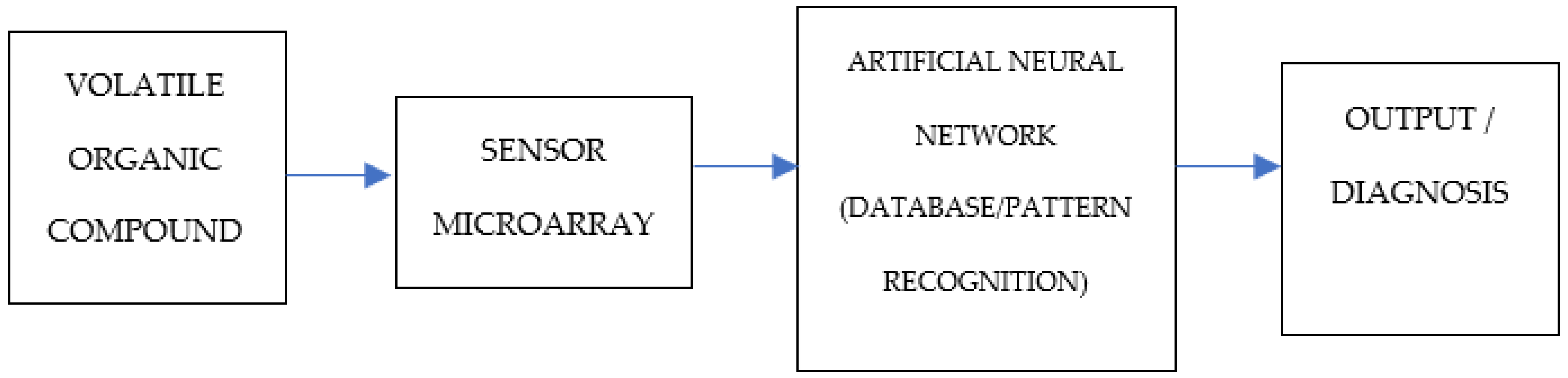
1.6. Volatile Organic Compounds
1.7. VOC Detection Techniques
- Gas Chromatography-Mass Spectrometry (GC-MS)
- Field Asymmetric Ion Mobility Spectrometry (FAIMS)
- Selected Ion Flow Tube Mass Spectrometry (SIFT-MS)
- Gas-Chromatography coupled to Time-of-Flight Mass Spectrometry (GC-TOF-MS)
- Electronic—Nose/“E-Nose” Technology
1.8. Mechanical Bowel Preparation and VOCs
1.9. Different Categories of Bowel Preparation
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Inclusion Criteria
- Studies comparing breath/urine/faecal VOC results in human patients before and after mechanical bowel preparation.
- Statistical analysis of the results and a conclusion drawn from this.
2.1.2. Exclusion Criteria
- VOC Samples solely taken before bowel preparation/colonoscopy.
- Papers not in English.
- No statistical analysis/inadequate numbers for conclusive results.
- Conference abstracts.
2.2. Information Sources
2.3. Search Strategy
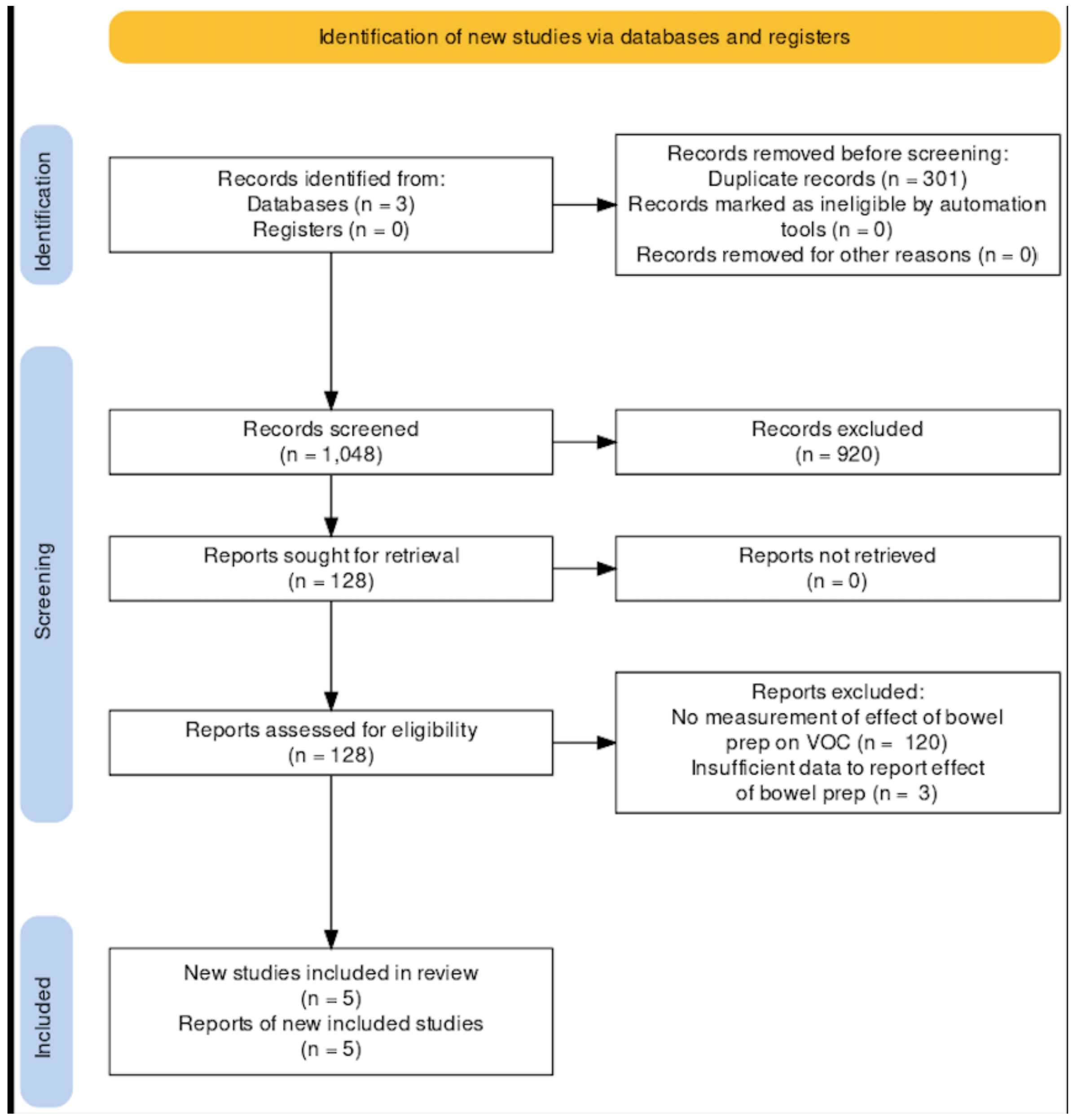
2.4. Selection Process
3. Results
4. Discussion
The Exact Origin of VOCs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.; Hilsden, R.J.; Martel, M.; Ruan, Y.; Dube, C.; Rostom, A.; Shorr, R.; Menard, C.; Brenner, D.R.; Barkun, A.N.; et al. Association Between Time to Colonoscopy After Positive Fecal Testing and Colorectal Cancer Outcomes: A Systematic Review. Clin. Gastroenterol. Hepatol. 2021, 19, 1344–1354.e8. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Manigrasso, M.; Cantore, G.; Maione, R.; Milone, M.; Maione, F.; De Palma, G.D. Can Computed Tomography Colonography Replace Optical Colonoscopy in Detecting Colorectal Lesions? State of the Art. Clin. Endosc. 2022, 55, 183. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.; Mustafee, N. Strategic resource planning of endoscopy services using hybrid modelling for future demographic and policy change. J. Oper. Res. Soc. 2022, 1–14. [Google Scholar] [CrossRef]
- Pellino, G.; Gallo, G.; Pallante, P.; Capasso, R.; De Stefano, A.; Maretto, I.; Malapelle, U.; Qiu, S.; Nikolaou, S.; Barina, A.; et al. Noninvasive Biomarkers of Colorectal Cancer: Role in Diagnosis and Personalised Treatment Perspectives. Gastroenterol. Res. Pract. 2018, 2018, 2397863. [Google Scholar] [CrossRef]
- Ferrari, A.; Neefs, I.; Hoeck, S.; Peeters, M.; Van Hal, G. Towards Novel Non-Invasive Colorectal Cancer Screening Methods: A Comprehensive Review. Cancers 2021, 13, 1820. [Google Scholar] [CrossRef]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 171–181. [Google Scholar] [CrossRef]
- Tinmouth, J.; Lansdorp-Vogelaar, I.; Allison, J.E. Faecal immunochemical tests versus guaiac faecal occult blood tests: What clinicians and colorectal cancer screening programme organisers need to know. Gut 2015, 64, 1327–1337. [Google Scholar] [CrossRef]
- Monahan, K.J.; Davies, M.M.; Abulafi, M.; Banerjea, A.; Nicholson, B.D.; Arasaradnam, R.; Barker, N.; Benton, S.; Booth, R.; Burling, D.; et al. Faecal immunochemical testing (FIT) in patients with signs or symptoms of suspected colorectal cancer (CRC): A joint guideline from the Association of Coloproctology of Great Britain and Ireland (ACPGBI) and the British Society of Gastroenterology (BSG). Gut 2022, 71, 1939–1962. [Google Scholar] [CrossRef]
- Clackett, W.; Barclay, S.T.; Stanley, A.J.; Cahill, A. The Value of Quantitative Faecal Immunochemical Testing as a Prioritisation Tool for the Endoscopic Investigation of Patients with Iron Deficiency. Front. Med. 2021, 8, 700753. [Google Scholar] [CrossRef]
- Goldstein, M.J.; Mitchell, E.P. Carcinoembryonic Antigen in the Staging and Follow-up of Patients with Colorectal Cancer. Cancer Investig. 2005, 23, 338–351. [Google Scholar] [CrossRef]
- Duffy, M.J. Carcinoembryonic antigen as a marker for colorectal cancer: Is it clinically useful? Clin. Chem. 2001, 47, 624–630. [Google Scholar] [CrossRef]
- Sun, J.; Fei, F.; Zhang, M.; Li, Y.; Zhang, X.; Zhu, S.; Zhang, S. The role of mSEPT9 in screening, diagnosis, and recurrence monitoring of colorectal cancer. BMC Cancer 2019, 19, 450. [Google Scholar] [CrossRef]
- Hariharan, R.; Jenkins, M. Utility of the methylated SEPT9 test for the early detection of colorectal cancer: A systematic review and meta-analysis of diagnostic test accuracy. BMJ Open Gastroenterol. 2020, 7, e000355. [Google Scholar] [CrossRef]
- Setti, G.; Pezzi, M.E.; Viani, M.V.; Pertinhez, T.A.; Cassi, D.; Magnoni, C.; Bellini, P.; Musolino, A.; Vescovi, P.; Meleti, M. Salivary MicroRNA for Diagnosis of Cancer and Systemic Diseases: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 907. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Fabiani, S.; Stefanelli, G.; Longo, S.; Viscido, A.; Latella, G. Are Volatile Organic Compounds Accurate Markers in the Assessment of Colorectal Cancer and Inflammatory Bowel Diseases? A Review. Cancers 2021, 13, 2361. [Google Scholar] [CrossRef]
- Di Lena, M.; Porcelli, F.; Altomare, D.F. Volatile organic compounds as new biomarkers for colorectal cancer: A review. Color. Dis. 2016, 18, 654–663. [Google Scholar] [CrossRef]
- De Vietro, N.; Aresta, A.; Rotelli, M.T.; Zambonin, C.; Lippolis, C.; Picciariello, A.; Altomare, D.F. Relationship between cancer tissue derived and exhaled volatile organic compound from colorectal cancer patients. Preliminary results. J. Pharm. Biomed. Anal. 2020, 180, 113055. [Google Scholar] [CrossRef]
- Xiang, L.; Wu, S.; Hua, Q.; Bao, C.; Liu, H. Volatile Organic Compounds in Human Exhaled Breath to Diagnose Gastrointestinal Cancer: A Meta-Analysis. Front. Oncol. 2021, 11, 269. [Google Scholar] [CrossRef]
- Altomare, D.F.; Picciariello, A.; Rotelli, M.T.; De Fazio, M.; Aresta, A.; Zambonin, C.G.; Vincenti, L.; Trerotoli, P.; De Vietro, N. Chemical signature of colorectal cancer: Case-control study for profiling the breath print. BJS Open 2020, 4, 1189–1199. [Google Scholar] [CrossRef]
- Bosch, S.; Berkhout, D.J.; Ben Larbi, I.; de Meij, T.G.; de Boer, N.K. Fecal volatile organic compounds for early detection of colorectal cancer: Where are we now? J. Cancer Res. Clin. Oncol. 2019, 145, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Sukaram, T.; Tansawat, R.; Apiparakoon, T.; Tiyarattanachai, T.; Marukatat, S.; Rerknimitr, R.; Chaiteerakij, R. Exhaled volatile organic compounds for diagnosis of hepatocellular carcinoma. Sci. Rep. 2022, 12, 5326. [Google Scholar] [CrossRef] [PubMed]
- Bannaga, A.; Tyagi, H.; Daulton, E.; Covington, J.; Arasaradnam, R. Exploratory Study Using Urinary Volatile Organic Compounds for the Detection of Hepatocellular Carcinoma. Molecules 2021, 26, 2447. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Aggio, R.; Staudacher, H.M.; Lomer, M.C.; Lindsay, J.O.; Irving, P.; Probert, C.; Whelan, K. Volatile Organic Compounds in Feces Associate with Response to Dietary Intervention in Patients with Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2018, 16, 385–391.e1. [Google Scholar] [CrossRef] [PubMed]
- Couch, R.D.; Dailey, A.; Zaidi, F.; Navarro, K.; Forsyth, C.B.; Mutlu, E.; Engen, P.A.; Keshavarzian, A. Alcohol Induced Alterations to the Human Fecal VOC Metabolome. PLoS ONE 2015, 10, e0119362. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Bindels, L.B.; Porporato, P.E.; Dewulf, E.M.; Verrax, J.; Neyrinck, A.; Martin, J.C.; Scott, K.P.; Calderon, P.B.; Feron, O.; Muccioli, G.; et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer 2012, 107, 1337–1344. [Google Scholar] [CrossRef]
- Sagar, N.M.; Cree, I.A.; Covington, J.A.; Arasaradnam, R.P. The Interplay of the Gut Microbiome, Bile Acids, and Volatile Organic Compounds. Gastroenterol. Res. Pract. 2015, 2015, 398585. [Google Scholar] [CrossRef]
- Chandrapalan, S.; Bosch, S.; Cubiella, J.; Guardiola, J.; Kimani, P.; Mulder, C.; Persaud, K.; de Meij, T.G.J.; Altomare, D.F.; Brenner, H.; et al. Systematic review with meta-analysis: Volatile organic compound analysis to improve faecal immunochemical testing in the detection of colorectal cancer. Aliment. Pharmacol. Ther. 2021, 54, 14–23. [Google Scholar] [CrossRef]
- de Swart, J.; van Gaal, N.; Berkhout, D.J.; de Meij, T.G.; de Boer, N.K. Smoking Influences Fecal Volatile Organic Compounds Composition. Clin. Gastroenterol. Hepatol. 2018, 16, 1168–1169. [Google Scholar] [CrossRef]
- Bosch, S.; Lemmen, J.P.M.; de Menezes, R.X.; van der Hulst, R.; Kuijvenhoven, J.; Stokkers, P.C.F.; de Meij, T.G.J.; de Boer, N.K. The influence of lifestyle factors on fecal volatile organic compound composition as measured by an electronic nose. J. Breath Res. 2019, 13, 046001. [Google Scholar] [CrossRef]
- Lubes, G.; Goodarzi, M. GC–MS based metabolomics used for the identification of cancer volatile organic compounds as biomarkers. J. Pharm. Biomed. Anal. 2018, 147, 313–322. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; McFarlane, M.J.; Ryan-Fisher, C.; Westenbrink, E.; Hodges, P.; Thomas, M.G.; Chambers, S.; O’Connell, N.; Bailey, C.; Harmston, C.; et al. Detection of Colorectal Cancer (CRC) by Urinary Volatile Organic Compound Analysis. PLoS ONE 2014, 9, e108750. [Google Scholar] [CrossRef]
- Perkins, M.; Langford, V. Application of Routine Analysis Procedures to a Direct Mass Spectrometry Technique: Selected Ion Flow Tube Mass Spectrometry (SIFT-MS). Rev. Sep. Sci. 2021, 3, e21003. [Google Scholar] [CrossRef]
- Song, S.M.; Marriott, P.; Kotsos, A.; Drummer, O.; Wynne, P. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry (GC × GC-TOFMS) for drug screening and confirmation. Forensic Sci. Int. 2004, 143, 87–101. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Liu, B.; Libanori, A.; Zhou, Y.; Xiao, X.; Xie, G.; Zhao, X.; Su, Y.; Wang, S.; Yuan, Z.; Duan, Z.; et al. Simultaneous Biomechanical and Biochemical Monitoring for Self-Powered Breath Analysis. ACS Appl. Mater. Interfaces 2022, 14, 7301–7310. [Google Scholar] [CrossRef]
- van der Sar, I.G.; Wijbenga, N.; Nakshbandi, G.; Aerts, J.G.J.V.; Manintveld, O.C.; Wijsenbeek, M.S.; Hellemons, M.E.; Moor, C.C. The smell of lung disease: A review of the current status of electronic nose technology. Respir. Res. 2021, 22, 246. [Google Scholar] [CrossRef]
- Altomare, D.F.; Porcelli, F.; Picciariello, A.; Pinto, M.; Di Lena, M.; Iambrenghi, O.C.; Ugenti, I.; Guglielmi, A.; Vincenti, L.; De Gennaro, G. The use of the PEN3 e-nose in the screening of colorectal cancer and polyps. Technol. Coloproctol. 2016, 20, 405–409. [Google Scholar] [CrossRef]
- The British Society of Gastroenterology. UK Key Performance Indicators and Quality Assurance Standards for Colonoscopy. Available online: https://www.bsg.org.uk/clinical-resource/uk-key-performance-indicators-and-quality-assurance-standards-for-colonoscopy/ (accessed on 15 December 2022).
- Belsey, J.; Epstein, O.; Heresbach, D. Systematic review: Oral bowel preparation for colonoscopy. Aliment. Pharmacol. Ther. 2007, 25, 373–384. [Google Scholar] [CrossRef]
- Wallace, S.K.; Bakkum-Gamez, J.N. Bowel Preparation. In The ERAS® Society Handbook for Obstetrics & Gynecology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 31–39. [Google Scholar] [CrossRef]
- Reumkens, A.; van der Zander, Q.; Winkens, B.; Bogie, R.; Bakker, C.M.; Sanduleanu, S.; Masclee, A.A.M. Electrolyte disturbances after bowel preparation for colonoscopy: Systematic review and meta-analysis. Dig. Endosc. 2022, 34, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; East, J.; Radaelli, F.; Spada, C.; Benamouzig, R.; Bisschops, R.; Bretthauer, M.; Dekker, E.; Dinis-Ribeiro, M.; Ferlitsch, M.; et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2019. Endoscopy 2019, 51, 775–794. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Tohya, M.; Fukuda, S.; Suda, W.; Nishijima, S.; Takeuchi, F.; Ohsugi, M.; Tsujimoto, T.; Nakamura, T.; Shimomura, A.; et al. Effects of bowel preparation on the human gut microbiome and metabolome. Sci. Rep. 2019, 9, 4042. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, M.; Millard, A.; Hall, H.; Savage, R.; Constantinidou, C.; Arasaradnam, R.; Nwokolo, C. Urinary volatile organic compounds and faecal microbiome profiles in colorectal cancer. Color. Dis. 2019, 21, 1259–1269. [Google Scholar] [CrossRef]
- Francis, N.K.; Curtis, N.J.; Salib, E.; Costello, B.D.L.; Lemm, N.M.; Gould, O.; Crilly, L.; Allison, J.; Ratcliffe, N. Feasibility of perioperative volatile organic compound breath testing for prediction of paralytic ileus following laparoscopic colorectal resection. Color. Dis. 2020, 22, 86–94. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Ouaret, N.; Thomas, M.G.; Gold, P.; Quraishi, M.N.; Nwokolo, C.U.; Bardhan, K.D.; Covington, J.A. Evaluation of gut bacterial populations using an electronic e-nose and field asymmetric ion mobility spectrometry: Further insights into ‘fermentonomics’. J. Med. Eng. Technol. 2012, 36, 333–337. [Google Scholar] [CrossRef]
- Leja, M.; Amal, H.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Tolmanis, I.; Vanags, A.; Kupcinskas, J.; Ramonaite, R.; et al. Analysis of the effects of microbiome-related confounding factors on the reproducibility of the volatolomic test. J. Breath Res. 2016, 10, 037101. [Google Scholar] [CrossRef]
- Woodfield, G.; Belluomo, I.; Laponogov, I.; Veselkov, K.; Lin, G.; Myridakis, A.; Ayrton, O.; Španěl, P.; Vidal-Diez, A.; Romano, A.; et al. Diagnostic Performance of a Noninvasive Breath Test for Colorectal Cancer: COBRA1 Study. Gastroenterology 2022, 163, 1447–1449.e8. [Google Scholar] [CrossRef]
- Bosch, S.; Bot, R.; Wicaksono, A.; Savelkoul, E.; Van Der Hulst, R.; Kuijvenhoven, J.; Stokkers, P.; Daulton, E.; Covington, J.A.; De Meij, T.G.; et al. Early detection and follow-up of colorectal neoplasia based on faecal volatile organic compounds. Color. Dis. 2020, 22, 1119–1129. [Google Scholar] [CrossRef]
- Markar, S.R.; Chin, S.; Romano, A.; Wiggins, T.; Antonowicz, S.; Paraskeva, P.; Ziprin, P.; Darzi, A.; Hanna, G.B. Breath Volatile Organic Compound Profiling of Colorectal Cancer Using Selected Ion Flow-tube Mass Spectrometry. Ann. Surg. 2019, 269, 903–910. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Wang, G.; Guo, N.; Liu, D.; Li, D.; Guo, L.; Zheng, X.; Yu, K.; Yu, K.; et al. Release of volatile organic compounds (VOCs) from colorectal cancer cell line LS174T. Anal. Biochem. 2019, 581, 113340. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef]
| Study | VOC Medium | Measurement Method | Significant Effect of Bowel Prep on VOCs | Number of Patients Analysed |
|---|---|---|---|---|
| Arasaradnam et al., 2012 [48] | URINE | FAIMS | YES | 23 |
| Leja et al., 2016 [49] | BREATH | GC-MS | YES | 61 |
| Markar et al., 2019 [52] | BREATH | SIFT-MS | NO | 112 |
| Francis et al., 2019 [47] | BREATH | SIFT-MS | NO | 22 |
| Woodfield et al., 2022 [50] | BREATH | GC-MS | NO | 161 |
| Study | Specific VOCs Measured in Study |
|---|---|
| Arasaradnam et al., 2012 [48] | Hydrogen sulphide (Urine) |
| Leja et al., 2016 [49] | Acetone (Breath) |
| Markar et al., 2019 [52] | Propanal (Breath) |
| Francis et al., 2019 [47] | Methane, Hydrogen, Dimethyl sulphide, Acetic acid, Ethanol, Methanol, Ammonia, Hydrogen sulphide (Breath) |
| Woodfield et al., 2022 [50] | Dimethyl sulphide, 1-Penten-3-ol, Heptane, cyclopropane, (Breath) |
| Study | Index Group vs. Control | Cases | Controls | Matched Patients? | Sample Medium | Significant Effect on VOCs? |
|---|---|---|---|---|---|---|
| Arasaradnam et al., 2012 [48] | Picolax + moviprep vs. after | 19 | 19 | YES | Urine | YES |
| Leja et al., 2016 [49] | Before polyethylene glycol vs. after | 61 | 61 | YES | Breath | NO |
| Marker et al., 2019 [52] | Mechanical vs. no bowel prep | 30 | 82 | NO | Breath | NO |
| Frances et al., 2020 [47] | Enema/mechanical vs. no bowel prep | 22 | 25 | NO | Breath | NO |
| Woodfield et al., 2022 [50] | Mechanical vs. no bowel prep | 124 | 37 | NO | Breath | NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnamoorthy, A.; Chandrapalan, S.; Bosch, S.; Bannaga, A.; De Boer, N.K.H.; De Meij, T.G.J.; Leja, M.; Hanna, G.B.; De Vietro, N.; Altomare, D.; et al. The Influence of Mechanical Bowel Preparation on Volatile Organic Compounds for the Detection of Gastrointestinal Disease—A Systematic Review. Sensors 2023, 23, 1377. https://doi.org/10.3390/s23031377
Krishnamoorthy A, Chandrapalan S, Bosch S, Bannaga A, De Boer NKH, De Meij TGJ, Leja M, Hanna GB, De Vietro N, Altomare D, et al. The Influence of Mechanical Bowel Preparation on Volatile Organic Compounds for the Detection of Gastrointestinal Disease—A Systematic Review. Sensors. 2023; 23(3):1377. https://doi.org/10.3390/s23031377
Chicago/Turabian StyleKrishnamoorthy, Ashwin, Subashini Chandrapalan, Sofie Bosch, Ayman Bannaga, Nanne K.H. De Boer, Tim G.J. De Meij, Marcis Leja, George B. Hanna, Nicoletta De Vietro, Donato Altomare, and et al. 2023. "The Influence of Mechanical Bowel Preparation on Volatile Organic Compounds for the Detection of Gastrointestinal Disease—A Systematic Review" Sensors 23, no. 3: 1377. https://doi.org/10.3390/s23031377
APA StyleKrishnamoorthy, A., Chandrapalan, S., Bosch, S., Bannaga, A., De Boer, N. K. H., De Meij, T. G. J., Leja, M., Hanna, G. B., De Vietro, N., Altomare, D., & Arasaradnam, R. P. (2023). The Influence of Mechanical Bowel Preparation on Volatile Organic Compounds for the Detection of Gastrointestinal Disease—A Systematic Review. Sensors, 23(3), 1377. https://doi.org/10.3390/s23031377








