A Novel Fluorescent Aptamer Sensor with DNAzyme Signal Amplification for the Detection of CEA in Blood
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Serum Sample Pretreatment
2.4. Fluorescence Measurements
2.5. Feasibility Study
2.6. Fluorometric Detection of CEA
2.7. Gel Electrophoresis Measurement
3. Results and Discussion
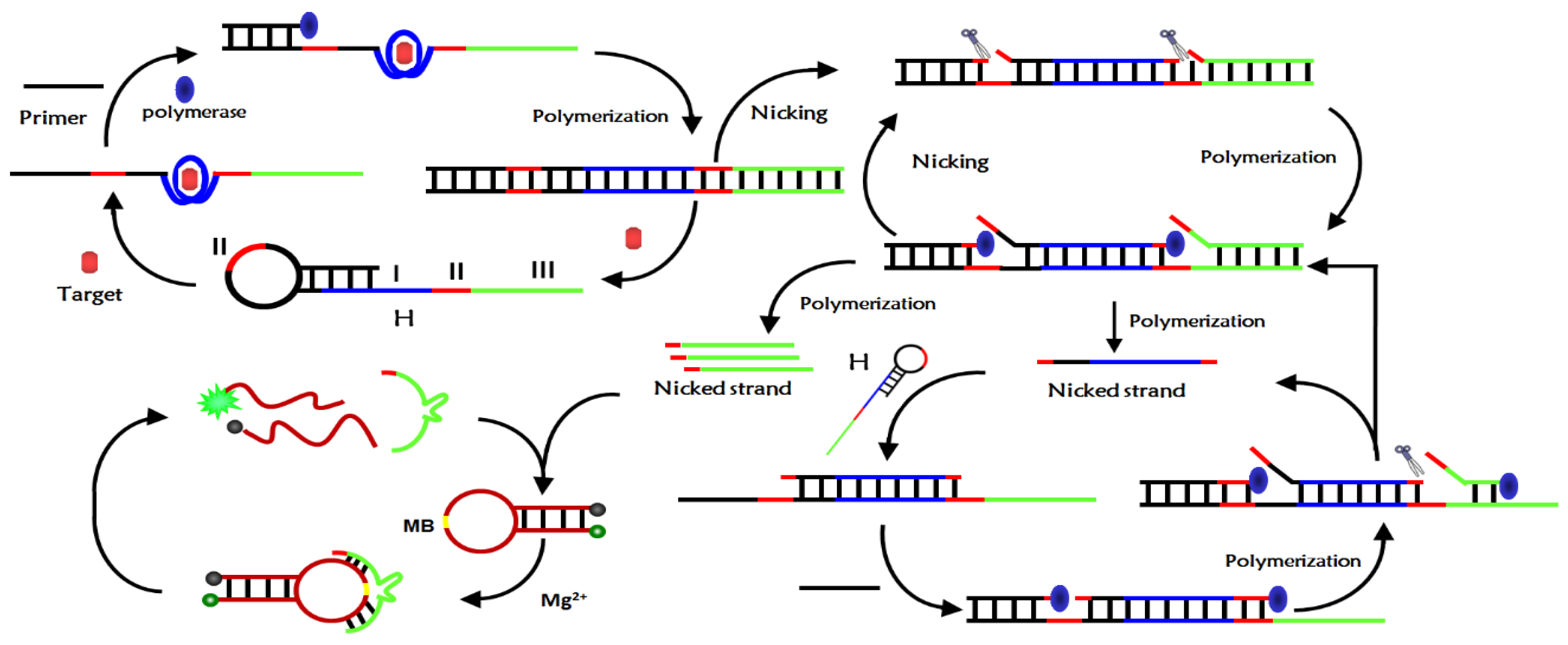
3.1. Analysis of CEA Detection Principle
3.2. Feasibility Verification of CEA Detection
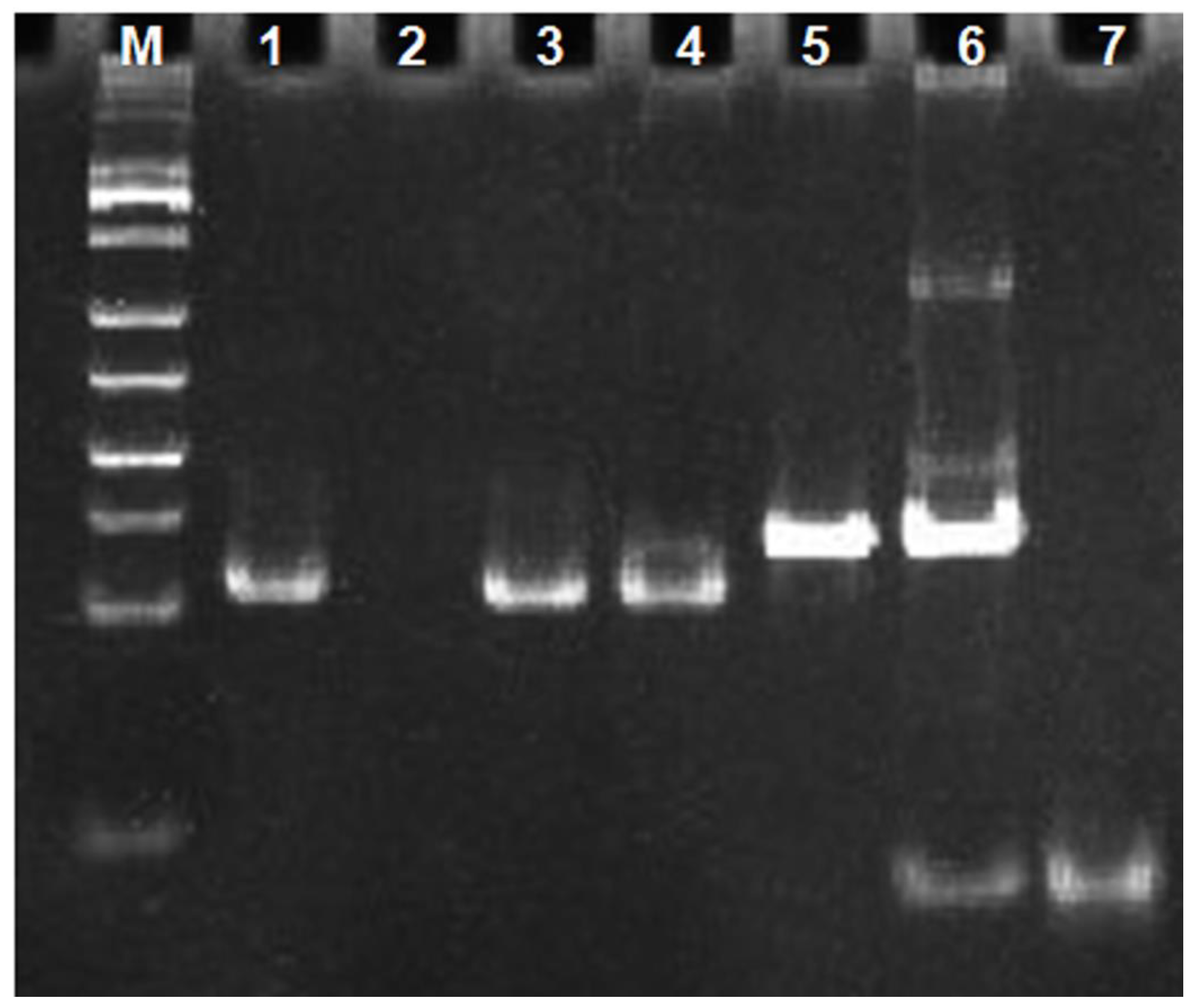
3.3. Gel Electrophoresis Characterization
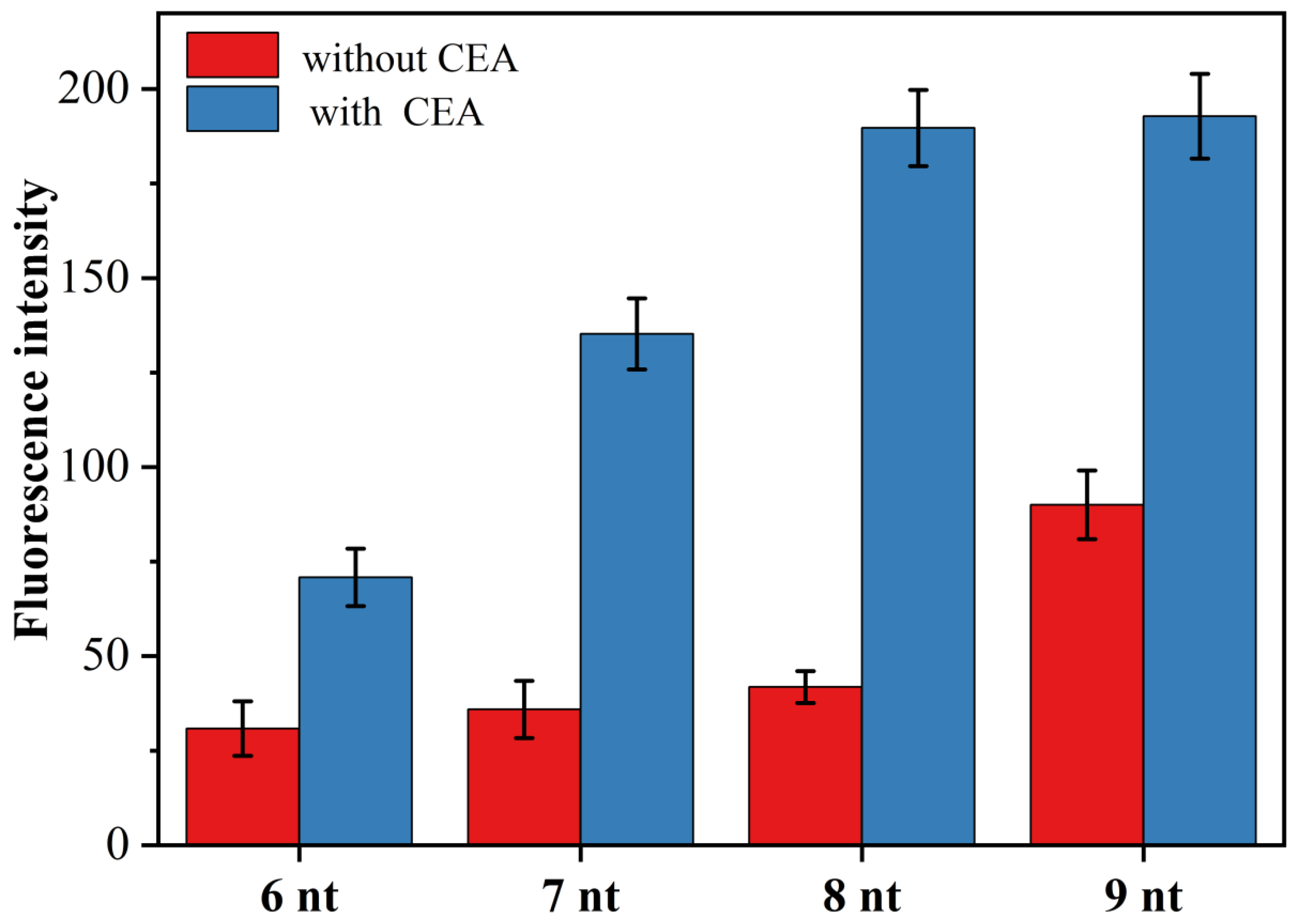
3.4. The Effect of Primer Chain Length on CEA Detection
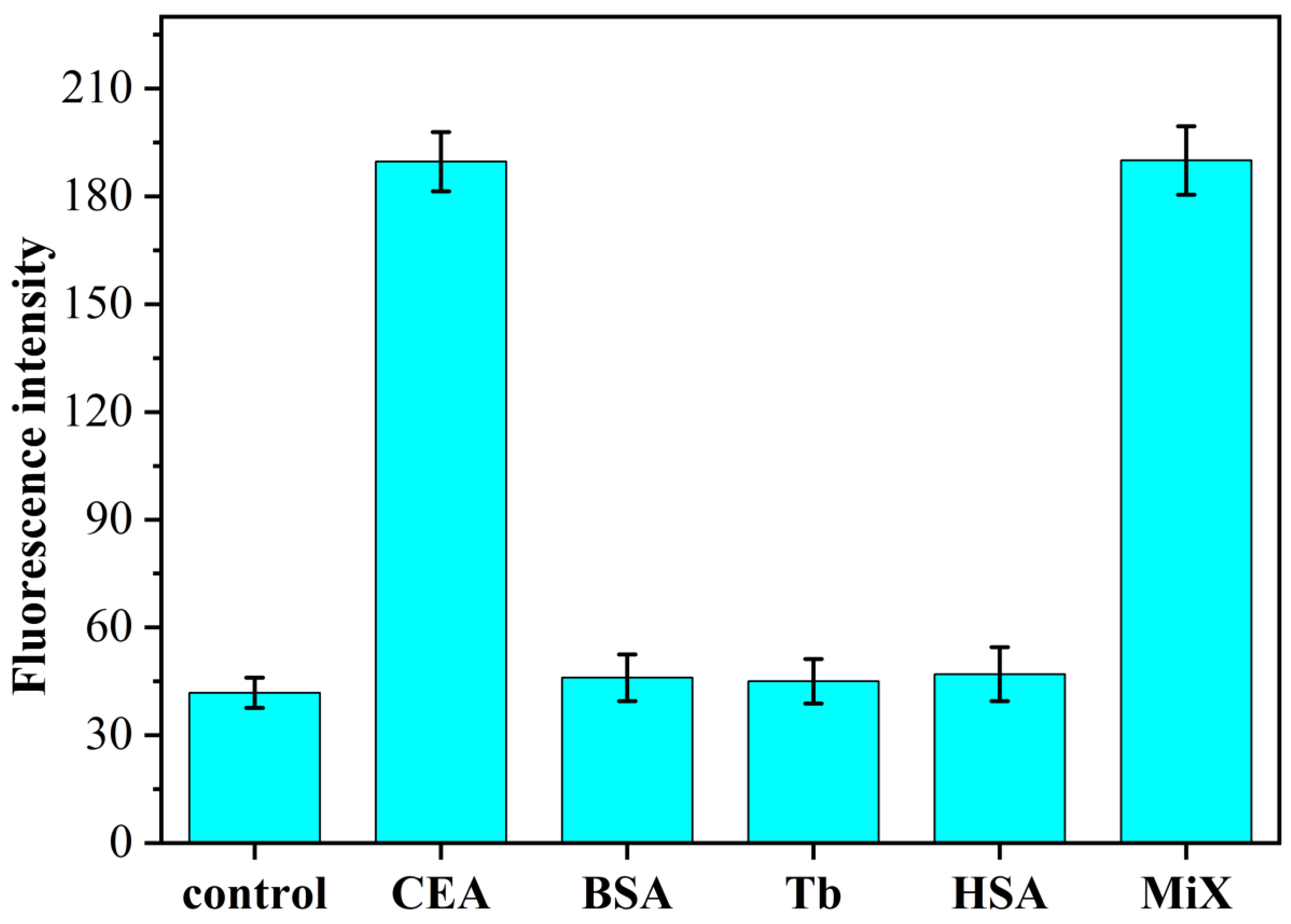
3.5. Study on the Specificity of CEA Identification
3.6. Range of Linearity and Limit of Detection (LOD)
3.7. Sensor for Human Serum Sample Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Xu, S.; Yan, H.; Li, X.; Yazd, H.S.; Li, X.; Huang, T.; Cui, C.; Jiang, J.; Tan, W. Nucleic acid aptamers for molecular diagnostics and therapeutics: Advances and perspectives. Angew. Chem. Int. Edit. 2021, 60, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yue, T.; Huang, C.; Wang, H. A magnified aptamer fluorescence sensor based on the metal organic frameworks adsorbed DNA with enzyme catalysis amplification for ultra-sensitive determination of ATP and its logic gate operation. Bioorg. Chem. 2021, 114, 105020. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, F.; Liu, R.; Liu, M.; Sang, Y.; Wang, S.; Wang, X. A novel colorimetric nano aptasensor for ultrasensitive detection of aflatoxin b1 based on the exonuclease iii-assisted signal amplification approach. Foods 2021, 10, 2568. [Google Scholar] [CrossRef]
- Ma, G.; Huo, L.; Tong, Y.; Wang, Y.; Li, C.; Jia, H. Label-free and sensitive MiRNA detection based on turn-on fluorescence of DNA-templated silver nanoclusters coupled with duplex-specific nuclease-assisted signal amplification. Microchim. Acta 2021, 188, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Emrani, A.S.; Danesh, N.M.; Ramezani, M.; Taghdisid, S.M.; Abnous, K. A novel fluorescent aptasensor based on hairpin structure of complementary strand of aptamer and nanoparticles as a signal amplification approach for ultrasensitive detection of cocaine. Biosens. Bioelectron. 2016, 79, 288–293. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wu, Z.; Liu, S.J.; Chu, X. Structure-switching aptamer triggering hybridization chain reaction on the cell surface for activatable theranostics. Anal. Chem. 2015, 87, 6470–6474. [Google Scholar] [CrossRef]
- Tang, Y.N.; Lin, Y.W.; Yang, X.L.; Wang, Z.X.; Le, X.C.; Li, F. Universal strategy to engineer catalytic DNA hairpin assemblies for protein analysis. Anal. Chem. 2015, 87, 8063–8066. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Zhou, H. Visual detection of cucumber green mottle mosaic virus based on terminal deoxynucleotidyl transferase coupled with DNAzymes amplification. Sensors 2019, 19, 1298. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, L.; Li, B. Exponential amplification reaction-based fluorescent sensor for the sensitive detection of tumor biomarker flap endonuclease 1. Sens. Actuators B Chem. 2021, 346, 130457. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, D.; Huang, T.; Yang, Z.; Liu, B.; Sun, M.; Chen, J.X.; Dai, Z.; Zou, X. Isothermal self-primer exponential amplification reaction (SPEXPAR) for highly sensitive detection of single-stranded nucleic acids and proteins. Anal. Chem. 2021, 93, 12707–12713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.; Sun, L.; Duan, X.; Li, Z. Lab on a single microbead: An ultrasensitive detection strategy enabling microRNA analysis at the single-molecule level. Chem. Sci. 2015, 6, 6213–6218. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Liu, C.; Wang, H.; Li, Z. A three-way junction structure-based isothermal exponential amplification strategy for sensitive detection of 3′-terminal 2′-O-methylated plant microRNA. Chem. Commun. 2017, 53, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tian, T.; Zhang, Y.; Ding, L.; Yu, J.; Yan, M. Sensitive and rapid detection of microRNAs using hairpin probes-mediated exponential isothermal amplification. Biosen. Bioelectron. 2017, 89, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, D.; Cheng, W.; Hu, R.; Sang, Y.; Yin, Y.; Ding, S.; Ju, H. Chemiluminescence imaging for microRNA detection based on cascade exponential isothermal amplification machinery. Anal. Chim. Acta 2016, 936, 229–235. [Google Scholar] [CrossRef]
- Chen, X.; Feng, Y.; Chen, H.; Zhang, Y.; Wang, X.; Zhou, N. Fluorescent aptasensor for highly specific detection of atp using a newly screened aptamer. Sensors 2022, 22, 2425. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ren, M.; Zhang, Q.; Tang, B.; Zhang, C. Excision repair-initiated enzyme-assisted bicyclic cascade signal amplification for ultrasensitive detection of uracil-DNA glycosylase. Anal. Chem. 2017, 89, 4488–4494. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, D.W.; Tie, C.; Zhou, Y.L.; Zhang, X.X. G-quadruplex based two-stage isothermal exponential amplification reaction for label-free DNA colorimetric detection. Biosens. Bioelectron. 2014, 56, 237–242. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Liu, L.J.; Qiao, W.P.; Zhang, S.S. Ultrasensitive SERS detection of lysozyme by a target-triggering multiple cycle amplification strategy based on a gold substrate. Chem. Eur. J. 2013, 19, 7452–7460. [Google Scholar] [CrossRef]
- Silverman, S.K. In vitro selection, characterization, and application of deoxyribozymes that cleave RNA. Nucleic Acids Res. 2005, 33, 6151–6163. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Jiang, B.; Yuan, R.; Xiang, Y. Metallo-toehold-activated catalytic hairpin assembly formation of three-way DNAzyme junctions for amplified fluorescent detection of Hg2+. ACS Appl. Mater. Inter. 2017, 9, 5733–5738. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Ding, H.; Wu, Y.; Wu, Z.Y.; Shen, G.L.; Yu, R.Q. Label-free liquid crystal biosensor for L-histidine: A DNAzyme-based platform for small molecule assay. Biosens. Bioelectron. 2016, 79, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, C.; Gong, H.; Wang, L. Programmable Mg2+-dependent DNAzyme switch by the catalytic hairpin DNA assembly for dual-signal amplification toward homogeneous analysis of protein and DNA. Chem. Commun. 2015, 51, 7364–7367. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dou, B.; Yuan, R.; Xiang, Y. Proximity binding and metal ion-dependent DNAzyme cyclic amplification-integrated aptasensor for label-free and sensitive electrochemical detection of thrombin. Anal. Chem. 2016, 88, 8218–8223. [Google Scholar] [CrossRef] [PubMed]
- Lilienthal, S.; Klein, M.; Orbach, R.; Willner, I.; Remacleab, F.; Levine, R.D. Continuous variables logic via coupled automata using a DNAzyme cascade with feedback. Chem. Sci. 2017, 8, 2161–2168. [Google Scholar] [PubMed]
- Hu, R.; Liu, T.; Zhang, X.B.; Yang, Y.; Chen, T.; Wu, C.; Liu, Y.; Zhu, G.; Huan, S.; Fu, T.; et al. DLISA: A DNAzyme-based ELISA for protein enzyme-free immunoassay of multiple analytes. Anal. Chem. 2015, 87, 7746–7753. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, Y.; Lei, J.; Zhang, L.; Ju, H. Design and biosensing of Mg2+-dependent DNAzyme-triggered ratiometric electrochemiluminescence. Analy. Chem. 2014, 86, 5158–5163. [Google Scholar]
- Wei, L.; Wang, X.; Wu, D.; Li, C.; Yin, Y.; Li, G. Proximity ligation-induced assembly of DNAzymes for simple and cost-effective colourimetric detection of proteins with high sensitivity. Chem. Commun. 2016, 52, 5633–5636. [Google Scholar]


| Oligonucleotides | Sequences |
|---|---|
| H | ATGCTTGGTACATGGGTGATCGCTGTCGGTATTCCTCAGCCAATA CCAGCTTATTCAATTCCAGCTCCTCAGCGTATACTGGAATTGAATC |
| MB | FAM-CACCACTACAAATTATGCTTGGTTrAGGTCGGTATACGAGC GTGTGGTG-DABCYL |
| DNA-1 | TGAGGAATACCGACAGCGATCACCCATGTACCAAGCAT |
| Primer-1 | GATTCA |
| Primer-2 | GATTCAA |
| Primer-3 | GATTCAAT |
| Primer-4 | GATTCAATT |
| Sample | Present Method (ng/mL) | ELISA (ng/mL) | Relative Deviation (%) |
|---|---|---|---|
| 1 | 5.64 | 5.40 | −4.4 |
| 2 | 3.58 | 3.32 | −7.8 |
| 3 | 4.72 | 4.58 | −3.1 |
| 4 | 206.20 | 203.00 | −1.6 |
| 5 | 186.55 | 188.34 | 1.0 |
| 6 | 292.85 | 289.75 | −1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Huang, H.; Wang, S.; Liu, F.; Xu, J.; Luo, Z. A Novel Fluorescent Aptamer Sensor with DNAzyme Signal Amplification for the Detection of CEA in Blood. Sensors 2023, 23, 1317. https://doi.org/10.3390/s23031317
Wei Q, Huang H, Wang S, Liu F, Xu J, Luo Z. A Novel Fluorescent Aptamer Sensor with DNAzyme Signal Amplification for the Detection of CEA in Blood. Sensors. 2023; 23(3):1317. https://doi.org/10.3390/s23031317
Chicago/Turabian StyleWei, Qingmin, Huakui Huang, Shulong Wang, Fa Liu, Jiayao Xu, and Zhihui Luo. 2023. "A Novel Fluorescent Aptamer Sensor with DNAzyme Signal Amplification for the Detection of CEA in Blood" Sensors 23, no. 3: 1317. https://doi.org/10.3390/s23031317
APA StyleWei, Q., Huang, H., Wang, S., Liu, F., Xu, J., & Luo, Z. (2023). A Novel Fluorescent Aptamer Sensor with DNAzyme Signal Amplification for the Detection of CEA in Blood. Sensors, 23(3), 1317. https://doi.org/10.3390/s23031317






