A Method for Quantifying Back Flexion/Extension from Three Inertial Measurement Units Mounted on a Horse’s Withers, Thoracolumbar Region, and Pelvis
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
2.2. Data Acquisition
2.3. Data Processing
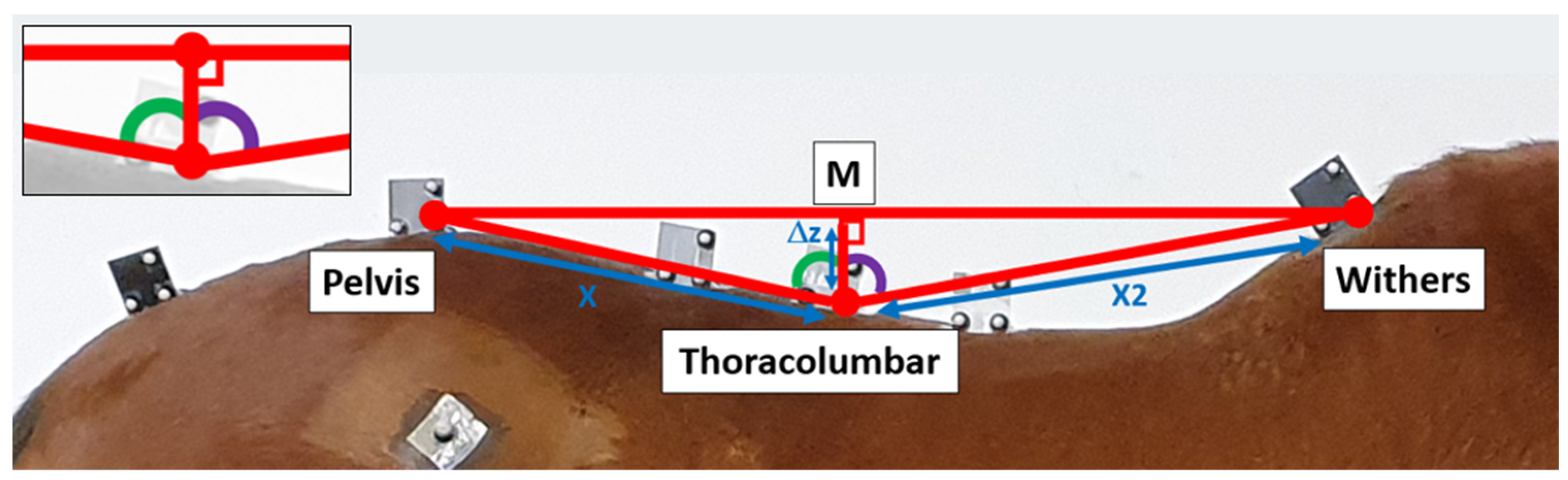
2.3.1. Motion Capture Data Processing
2.3.2. IMU Data Processing
2.3.3. Synchronization and Homogenization of Data Sets
2.3.4. Determination of the Range of Flexion and Extension
2.4. Statistical Analysis
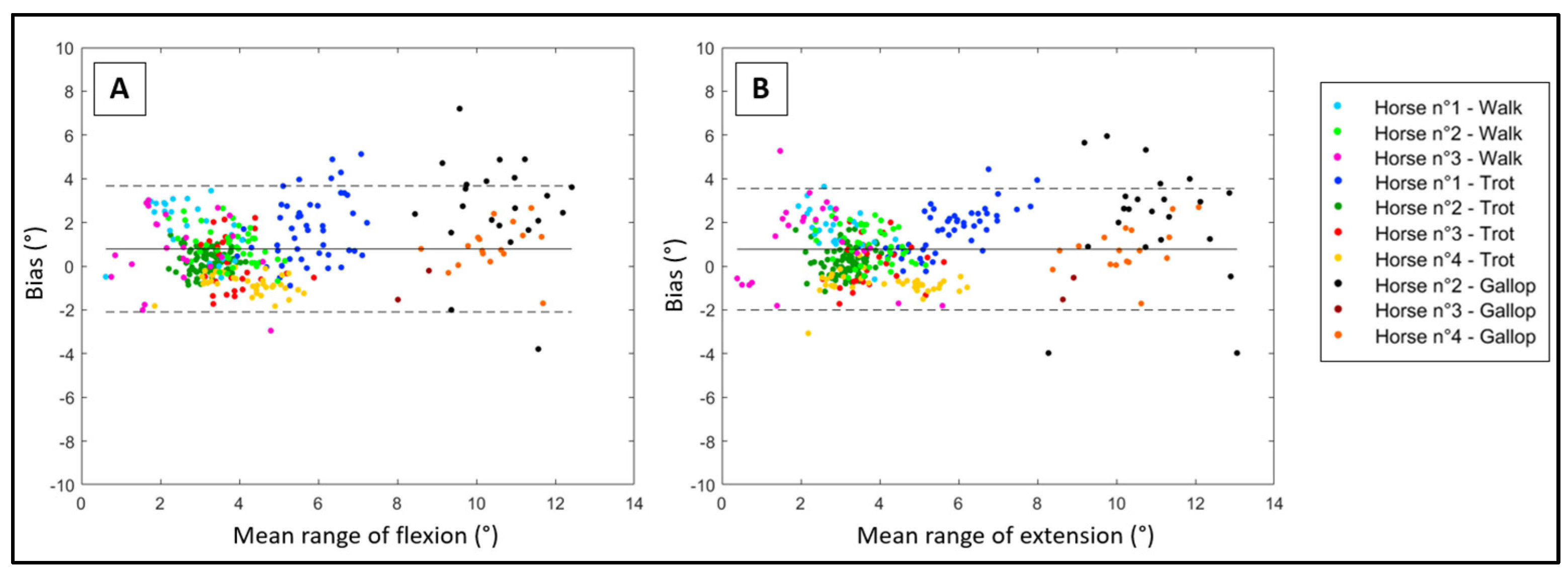
2.4.1. Bland-Altman’s Method
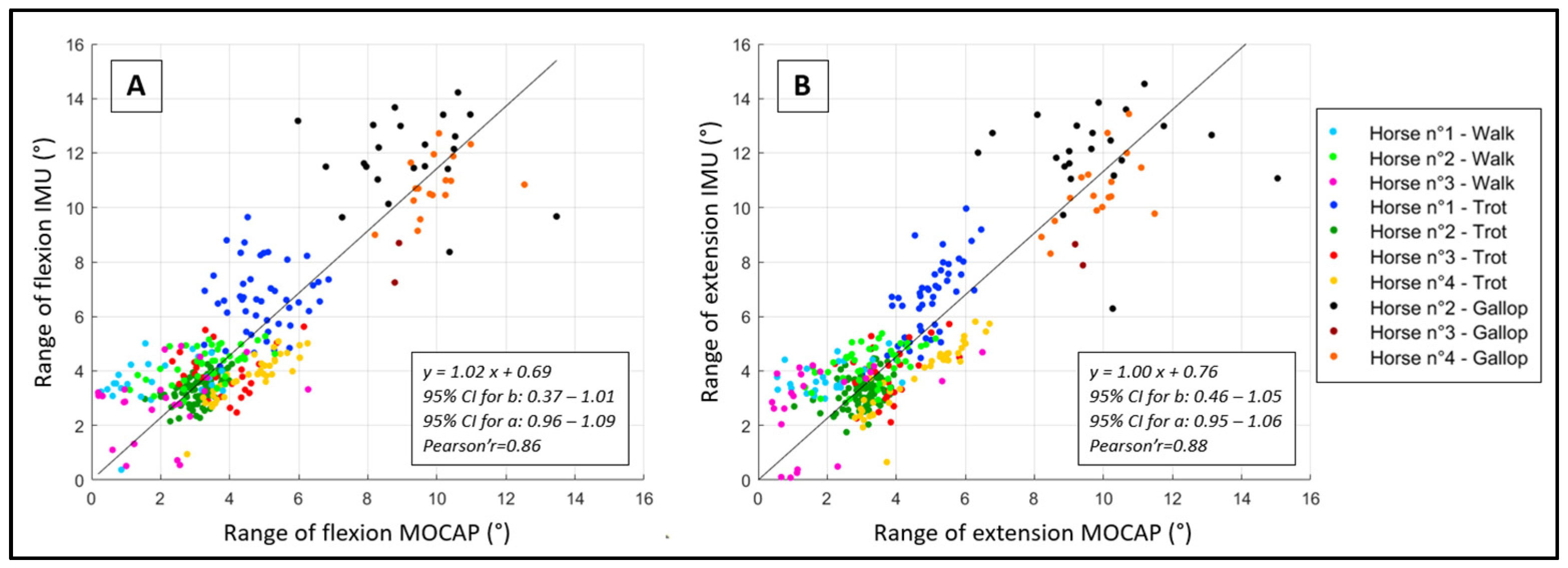
2.4.2. Ordinary Least Product Regression
2.4.3. Correlation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Back, W.; Clayton, H. Equine Locomotion, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-7020-5293-4. [Google Scholar]
- Ridgway, K.; Harman, J. Equine Back Rehabilitation. Vet. Clin. North Am. Equine Pract. 1999, 15, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Burgaud, D.I.; Genoux, N. Fonctionnement du Dos du Cheval. Available online: https://equipedia.ifce.fr/equitation/disciplines-olympiques/planification-de-lentrainement/fonctionnement-du-dos-du-cheval (accessed on 29 November 2023).
- Denoix, J.-M.D. Spinal Biomechanics and Functional Anatomy. Vet. Clin. North Am. Equine Pract. 1999, 15, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Hardeman, A.M.; Byström, A.; Roepstorff, L.; Swagemakers, J.H.; van Weeren, P.R.; Serra Bragança, F.M. Range of Motion and between-Measurement Variation of Spinal Kinematics in Sound Horses at Trot on the Straight Line and on the Lunge. PLoS ONE 2020, 15, e0222822. [Google Scholar] [CrossRef]
- Jeffcott, L.B.; Dalin, G.; Drevemo, S.; Fredricson, I.; Björne, K.; Bergquist, A. Effect of Induced Back Pain on Gait and Performance of Trotting Horses. Equine Vet. J. 1982, 14, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Barrey, E. Methods, Applications and Limitations of Gait Analysis in Horses. Vet. J. 1999, 157, 7–22. [Google Scholar] [CrossRef][Green Version]
- Jeffcott, L.B. Disorders of the Thoracolumbar Spine of the Horse—A Survey of 443 Cases. Equine Vet. J. 1980, 12, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Riccio, B.; Fraschetto, C.; Villanueva, J.; Cantatore, F.; Bertuglia, A. Two Multicenter Surveys on Equine Back-Pain 10 Years a Part. Front. Vet. Sci. 2018, 5, 195. [Google Scholar] [CrossRef]
- Henson, F.M.D. Equine Back Pathology: Diagnosis and Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Coudry, V.; Thibaud, D.; Riccio, B.; Audigié, F.; Didierlaurent, D.; Denoix, J.-M. Efficacy of Tiludronate in the Treatment of Horses with Signs of Pain Associated with Osteoarthritic Lesions of the Thoracolumbar Vertebral Column. Am. J. Vet. Res. 2007, 68, 329–337. [Google Scholar] [CrossRef]
- Spoormakers, T.J.P.; Graat, E.A.M.; Serra Bragança, F.M.; van Weeren, P.R.; Brommer, H. Rater Agreement for Assessment of Equine Back Mobility at Walk and Trot Compared to Quantitative Gait Analysis. PLoS ONE 2021, 16, e0252536. [Google Scholar] [CrossRef]
- Wennerstrand, J.; Johnston, C.; Roethlisberger-Holm, K.; Erichsen, C.; Eksell, P.; Drevemo, S. Kinematic Evaluation of the Back in the Sport Horse with Back Pain. Equine Vet. J. 2010, 36, 707–711. [Google Scholar] [CrossRef]
- Audigié, F.; Pourcelot, P.; Degueurce, C.; Denoix, J.-M.; Geiger, D. Kinematics of the Equine Back: Flexion-Extension Movements in Sound Trotting Horses. Equine Vet. J. 1999, 30, 210–213. [Google Scholar] [CrossRef]
- Egenvall, A.; Engström, H.; Byström, A. Back Motion in Unridden Horses in Walk, Trot and Canter on a Circle. Vet. Res. Commun. 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.K.; Thomsen, M.H.; Pfau, T.; Olsen, E. Accuracy and Precision of Gait Events Derived from Motion Capture in Horses during Walk and Trot. J. Biomech. 2014, 47, 1220–1224. [Google Scholar] [CrossRef]
- Marin, F. Human and Animal Motion Tracking Using Inertial Sensors. Sensors 2020, 20, 6074. [Google Scholar] [CrossRef] [PubMed]
- Sapone, M.; Martin, P.; Ben Mansour, K.; Château, H.; Marin, F. Comparison of Trotting Stance Detection Methods from an Inertial Measurement Unit Mounted on the Horse’s Limb. Sensors 2020, 20, 2983. [Google Scholar] [CrossRef] [PubMed]
- Sapone, M.; Martin, P.; Ben Mansour, K.; Chateau, H.; Marin, F. The Protraction and Retraction Angles of Horse Limbs: An Estimation during Trotting Using Inertial Sensors. Sensors 2021, 21, 3792. [Google Scholar] [CrossRef] [PubMed]
- Macaire, C.; Hanne-Poujade, S.; De Azevedo, E.; Denoix, J.-M.; Coudry, V.; Jacquet, S.; Bertoni, L.; Tallaj, A.; Audigié, F.; Hatrisse, C.; et al. Investigation of Thresholds for Asymmetry Indices to Represent the Visual Assessment of Single Limb Lameness by Expert Veterinarians on Horses Trotting in a Straight Line. Animals 2022, 12, 3498. [Google Scholar] [CrossRef] [PubMed]
- Hatrisse, C.; Macaire, C.; Sapone, M.; Hebert, C.; Hanne-Poujade, S.; De Azevedo, E.; Marin, F.; Martin, P.; Chateau, H. Stance Phase Detection by Inertial Measurement Unit Placed on the Metacarpus of Horses Trotting on Hard and Soft Straight Lines and Circles. Sensors 2022, 22, 703. [Google Scholar] [CrossRef]
- Briggs, E.V.; Mazzà, C. Automatic Methods of Hoof-on and -off Detection in Horses Using Wearable Inertial Sensors during Walk and Trot on Asphalt, Sand and Grass. PLoS ONE 2021, 16, e0254813. [Google Scholar] [CrossRef]
- Tijssen, M.; Hernlund, E.; Rhodin, M.; Bosch, S.; Voskamp, J.P.; Nielen, M.; Serra Braganςa, F.M. Automatic Detection of Break-over Phase Onset in Horses Using Hoof-Mounted Inertial Measurement Unit Sensors. PLoS ONE 2020, 15, e0233649. [Google Scholar] [CrossRef]
- Tijssen, M.; Hernlund, E.; Rhodin, M.; Bosch, S.; Voskamp, J.P.; Nielen, M.; Serra Braganςa, F.M. Automatic Hoof-on and -off Detection in Horses Using Hoof-Mounted Inertial Measurement Unit Sensors. PLoS ONE 2020, 15, e0233266. [Google Scholar] [CrossRef]
- Bosch, S.; Serra Bragança, F.; Marin-Perianu, M.; Marin-Perianu, R.; van der Zwaag, B.; Voskamp, J.; Back, W.; van Weeren, R.; Havinga, P. EquiMoves: A Wireless Networked Inertial Measurement System for Objective Examination of Horse Gait. Sensors 2018, 18, 850. [Google Scholar] [CrossRef] [PubMed]
- Audigié, F.; Pourcelot, P.; Degueurce, C.; Geiger, D.; Denoix, J.M. Fourier Analysis of Trunk Displacements: A Method to Identify the Lame Limb in Trotting Horses. J. Biomech. 2002, 35, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Chateau, H.; Pourcelot, P.; Duray, L.; Cheze, L. Comparison Between Inertial Sensors and Motion Capture System to Quantify Flexion-Extension Motion in the Back of a Horse. Equine Vet. J. 2014, 46, 43. [Google Scholar] [CrossRef]
- Warner, S.M.; Koch, T.O.; Pfau, T. Inertial Sensors for Assessment of Back Movement in Horses during Locomotion over Ground: Inertial Sensors for Back Movement. Equine Vet. J. 2010, 42, 417–424. [Google Scholar] [CrossRef]
- MacKechnie-Guire, R.; Pfau, T. Differential Rotational Movement and Symmetry Values of the Thoracolumbosacral Region in High-Level Dressage Horses When Trotting. PLoS ONE 2021, 16, e0251144. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Int. J. Nurs. Stud. 2010, 47, 931–936. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Agreement Between Methods of Measurement with Multiple Observations Per Individual. J. Biopharm. Stat. 2007, 17, 571–582. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. A Note on the Use of the Intraclass Correlation Coefficient in the Evaluation of Agreement between Two Methods of Measurement. Comput. Biol. Med. 1990, 20, 337–340. [Google Scholar] [CrossRef]
- Ludbrook, J. Statistical Techniques for Comparing Measurers and Methods Of Measurement: A Critical Review. Clin. Exp. Pharmacol. Physiol. 2002, 29, 527–536. [Google Scholar] [CrossRef]
- Hart, S.; Drevets, K.; Alford, M.; Salacinski, A.; Hunt, B.E. A Method-Comparison Study Regarding the Validity and Reliability of the Lactate Plus Analyzer. BMJ Open 2013, 3, e001899. [Google Scholar] [CrossRef] [PubMed]
- Berkman, C.; Pereira, M.C.; Nardi, K.B.; Pereira, G.T.; Soares, O.A.B.; Restan, W.A.Z.; Queiroz-Neto, A.; Ferraz, G.C. Agreement between I-STAT and YSI 2300 Devices to Determine Lactate Concentrations in Dogs Undergoing Exercise. CEP 2016, 12, 75–82. [Google Scholar] [CrossRef]
- Restan, A.Z.; Zacche, E.; Da Silva, S.B.; Cerqueira, J.A.; Carfiofi, A.C.; Queiroz-Neto, A.; Camacho, A.A.; Ferraz, G.C. Lactate and Glucose Thresholds and Heart Rate Deflection Points for Beagles during Intense Exercise. AJVR 2019, 80, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Ferreira, M.; Moraes, A.D.; Carvalho, H. Ordinary Least Products Regression: A Robust Statistical Tool for Assessing Agreement between Measures Attended by High Variability. Res. Sq. 2020; in review. [Google Scholar] [CrossRef]
- Matlab, MathWorks Help Center—Linear Regression. Available online: https://fr.mathworks.com/help/matlab/data_analysis/linear-regression.html (accessed on 24 April 2023).
- Gibbons, J.D.; Chakraborti, S. Nonparametric Statistical Inference, 4th ed.; Statistics, Textbooks and Monographs; Rev.Expanded.; Marcel Dekker: New York, NY, USA, 2003; ISBN 978-0-8247-4052-8. [Google Scholar]
- Matlab, MathWorks Help Center—Linear Regression. Available online: https://fr.mathworks.com/help/stats/fitlm.html (accessed on 9 November 2023).
- Matlab, MathWorks Help Center—Linear Regression. Available online: https://fr.mathworks.com/help/stats/linearmodel.coefci.html (accessed on 9 November 2023).
- Cohen, J. Quantitative Methods in Psychology—A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]



| Gait | Horse | Number of Flexion/Extension Movements |
|---|---|---|
| Walk | Horse 1 | 26 |
| Horse 2 | 42 | |
| Horse 3 | 24 | |
| Trot | Horse 1 | 48 |
| Horse 2 | 88 | |
| Horse 3 | 26 | |
| Horse 4 | 38 | |
| Gallop | Horse 2 | 26 |
| Horse 3 | 2 | |
| Horse 4 | 20 |
| Gait | Motion Capture | IMUs | ||
|---|---|---|---|---|
| Flexion | Extension | Flexion | Extension | |
| Walk | 2.6° (±1.3°) | 2.5° (±1.3°) | 3.7° (±1.1°) | 3.8° (±1.1°) |
| Trot | 3.9° (±1.1°) | 3.9° (±1.1°) | 4.3° (±1.6°) | 4.2° (±1.7°) |
| Gallop | 9.5° (±1.3°) | 9.8° (±1.4°) | 11.3° (±1.6°) | 11.4° (±1.7°) |
| Bias | SD | ULA | LLA | Pearson’s r | |
|---|---|---|---|---|---|
| Flexion | 0.8° | 1.5° | 3.7° | −2.1° | 0.86 |
| Extension | 0.8° | 1.4° | 3.6° | −2.0° | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatrisse, C.; Macaire, C.; Hebert, C.; Hanne-Poujade, S.; De Azevedo, E.; Audigié, F.; Ben Mansour, K.; Marin, F.; Martin, P.; Mezghani, N.; et al. A Method for Quantifying Back Flexion/Extension from Three Inertial Measurement Units Mounted on a Horse’s Withers, Thoracolumbar Region, and Pelvis. Sensors 2023, 23, 9625. https://doi.org/10.3390/s23249625
Hatrisse C, Macaire C, Hebert C, Hanne-Poujade S, De Azevedo E, Audigié F, Ben Mansour K, Marin F, Martin P, Mezghani N, et al. A Method for Quantifying Back Flexion/Extension from Three Inertial Measurement Units Mounted on a Horse’s Withers, Thoracolumbar Region, and Pelvis. Sensors. 2023; 23(24):9625. https://doi.org/10.3390/s23249625
Chicago/Turabian StyleHatrisse, Chloé, Claire Macaire, Camille Hebert, Sandrine Hanne-Poujade, Emeline De Azevedo, Fabrice Audigié, Khalil Ben Mansour, Frederic Marin, Pauline Martin, Neila Mezghani, and et al. 2023. "A Method for Quantifying Back Flexion/Extension from Three Inertial Measurement Units Mounted on a Horse’s Withers, Thoracolumbar Region, and Pelvis" Sensors 23, no. 24: 9625. https://doi.org/10.3390/s23249625
APA StyleHatrisse, C., Macaire, C., Hebert, C., Hanne-Poujade, S., De Azevedo, E., Audigié, F., Ben Mansour, K., Marin, F., Martin, P., Mezghani, N., Chateau, H., & Chèze, L. (2023). A Method for Quantifying Back Flexion/Extension from Three Inertial Measurement Units Mounted on a Horse’s Withers, Thoracolumbar Region, and Pelvis. Sensors, 23(24), 9625. https://doi.org/10.3390/s23249625







