A Laser-Induced Graphene-Based Sensor Modified with CeO2 for Determination of Organophosphorus Pesticides with Improved Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
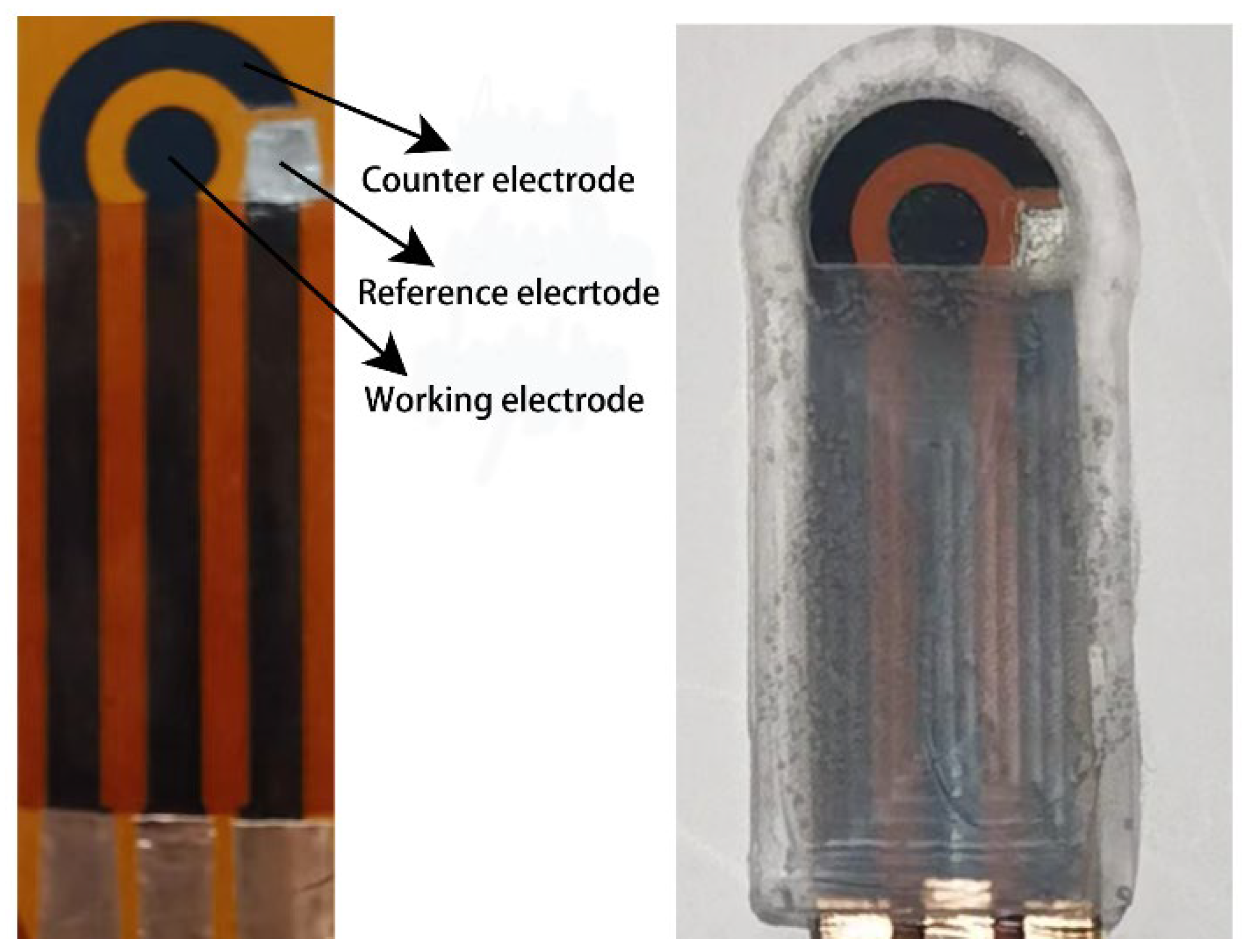
2.2. Preparation of the LIG Electrode
2.3. Preparation of the Modified Electrode
2.4. Analysis Program
3. Results
3.1. Basic Characteristics of the LIG Electrode
3.2. Effects of the Laser Parameters on the Electrochemical Properties of LIG
3.3. Effects of the CeO2 Modification on the Electrochemical Properties of the LIG-Based Sensor
3.4. Analytical Performance of the LIG-Based Sensor for Chlorpyrifos Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lykogianni, M.; Bempelou, E.; Karamaouna, F.; Aliferis, K.A. Do pesticides promote or hinder sustainability in agriculture? The challenge of sustainable use of pesticides in modern agriculture. Sci. Total Environ. 2021, 795, 148625. [Google Scholar] [CrossRef] [PubMed]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Pan, Q. Analysis of Organophosphorus Pesticides by HPLC Using Magnetic SPE with Nitrogen-Doped Reduced Graphene Oxide/Fe3O4 Nanocomposite as the Adsorbent. LCGC Eur. 2020, 33, 438–447. [Google Scholar]
- Yang, Y.; Gao, L.; Sun, H.; Luo, X.; Lu, D. Determination of 47 organophosphorus pesticide residues in drinking water by membrane extraction-gas chromatography-PFPD. J. Hyg. Res. 2012, 41, 287–292. [Google Scholar]
- Azzouz, A.; Colon, L.P.; Hejji, L.; Ballesteros, E. Determination of alkylphenols; phenylphenols; bisphenol A; parabens, organophosphorus pesticides and triclosan in different cereal-based foodstuffs by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 2621–2631. [Google Scholar] [CrossRef]
- Wondimu, K.T.; Geletu, A.K. Residue analysis of selected organophosphorus and organochlorine pesticides in commercial tomato fruits by gas chromatography mass spectrometry. Heliyon 2023, 9, e14121. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Z.; Yang, Q.; Hou, X. Integration of Metallic Nanomaterials and Recognition Elements for the Specifically Monitoring of Pesticides in Electrochemical Sensing. Crit. Rev. Anal. Chem. 2023, 1–22. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, F.; Dubovyk, V.; Guo, M.; Zhu, G.; Ran, Q.; Zhao, H. Electrochemical sensing platform based on graphitized and carboxylated multi-walled carbon nanotubes decorated with cerium oxide nanoparticles for sensitive detection of methyl parathion. J. Mater. Res. Technol. JmrT 2022, 19, 3738–3748. [Google Scholar] [CrossRef]
- Ilager, D.; Shetti, N.P.; Foucaud, Y.; Badawi, M.; Aminabhavi, T.M. Graphene/g-carbon nitride (GO/g-C3N4) nanohybrids as a sensor material for the detection of methyl parathion and carbendazim. Chemosphere 2022, 292, 133450. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Li, M.; Qu, L.; Liu, Z. Acetylcholinesterase-Cu-3(PO4)(2) hybrid nanoflowers for electrochemical detection of dichlorvos using square-wave voltammetry. Anal. Methods 2022, 14, 3911–3920. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 2019, 126, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Peplowski, A.; Janczak, D.; Jakubowska, M. Acetylcholinesterase-based biosensing solutions for the detection of organophosphorus pesticides. In Proceedings of the Photonics Applications in Astronomy, Communications, Industry, and High-Energy Physics Experiments 2019, Wilga, Poland, 25 May–2 June 2019. [Google Scholar]
- Dong, J.; Hou, J.; Jiang, J.; Ai, S. Innovative approach for the electrochemical detection of non-electroactive organophosphorus pesticides using oxime as electroactive probe. Anal. Chim. Acta 2015, 885, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, L.; Mikaelsson, T.; Hakonen, A.; Schmidt, M.S.; Rindzevicius, T.; Boisen, A.; Kall, M.; Andersson, P.O. Selective surface-enhanced Raman scattering detection of Tabun, VX and Cyclosarin nerve agents using 4-pyridine amide oxime functionalized gold nanopillars. Talanta 2020, 211, 120721. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Chen, Y.; Fan, K.; Wu, J.; Ying, Y. Exploring pralidoxime chloride as a universal electrochemical probe for organophosphorus pesticides detection. Anal. Chim. Acta 2017, 982, 78–83. [Google Scholar] [CrossRef]
- Altundal, M.; Üğe, A.; Gök, Ö.; Zeybek, B. Determination of Cadmium(II) by Differential Pulse Voltammetry (DPV) Using a Cerium(IV) Oxide: Polyaniline Composite Modified Glassy Carbon Electrode (GCE). Anal. Lett. 2021, 54, 2431–2451. [Google Scholar] [CrossRef]
- Sun, Q.; Du, J.; Tian, L.; Wu, J.; Zhang, X. Detection of organophosphorus pesticides: Exploring oxime as a probe with improved sensitivity by CeO2-modified electrode. Anal. Methods 2021, 13, 4634–4641. [Google Scholar] [CrossRef]
- Lu, L.; Wang, W.; Xie, Y.; Tang, Y. Recent Advances in the Fabrication of Graphene-based Flexible Electronic Devices by Laser Direct Writing. J. Mech. Eng. 2021, 57, 234–247. [Google Scholar]
- Yang, D.; Nam, H.; LeTruong-Son, D.; Lee, Y.; Kim, B.; Kim, Y.; Kim, S.; Kim, Y. Laser-induced-graphene formation on fabric based on femtosecond laser direct writing for flexible strain sensors. In Proceedings of the Conference on Lasers and Electro-Optics Pacific Rim, Sapporo, Japan, 31 August–5 September 2022. [Google Scholar]
- Liao, J.; Guo, W.; Peng, P. Direct laser writing of copper-graphene composites for flexible electronics. Opt. Lasers Eng. 2021, 147, 106605. [Google Scholar] [CrossRef]
- Wang, Y.; Han, C.; Zhou, Y.; Ke, C.; Wang, Y. Direct laser writing of polyimide for flexible graphene photodetectors. In Proceedings of the AOPC 2021: Advanced Laser Technology and Applications, Beijing, China, 20–22 June 2021; p. 120601P. [Google Scholar]
- Lucchetta, D.; Di Donato, A.; Francescangeli, O.; Singh, G.; Castagna, R. Light-Controlled Direction of Distributed Feedback Laser Emission by Photo-Mobile Polymer Films. Nanomaterials 2022, 12, 2890. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31, e1803621. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; Peng, Z.; Li, Y.; Gao, C.; Ji, Y.; Ye, R.; Kim, N.D.; Zhong, Q.; Yang, Y.; et al. High-Performance Pseudocapacitive Microsupercapacitors from Laser-Induced Graphene. Adv. Mater. 2016, 28, 838–845. [Google Scholar] [CrossRef]
- Liu, J.; Ji, H.; Lv, X.; Zeng, C.; Li, H.; Li, F.; Qu, B.; Cui, F.; Zhou, Q. Laser-induced graphene (LIG)-driven medical sensors for health monitoring and diseases diagnosis. Microchim. Acta 2022, 189, 54. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Chen, Z.D.; Mao, J.W.; Han, D.D.; Sun, X. Laser Fabrication of Graphene-Based Electronic Skin. Front. Chem. 2019, 7, 461. [Google Scholar] [CrossRef] [PubMed]
- Zamarchi, F.; Silva, T.; Winiarski, J.; Santana, E.; Vieira, I. Polyethylenimine-Based Electrochemical Sensor for the Determination of Caffeic Acid in Aromatic Herbs. Chemosensors 2022, 10, 357. [Google Scholar] [CrossRef]
- You, R.; Liu, Y.Q.; Hao, Y.L.; Han, D.D.; Zhang, Y.L.; You, Z. Laser Fabrication of Graphene-Based Flexible Electronics. Adv. Mater. 2020, 32, e1901981. [Google Scholar] [CrossRef]
- Gonzalez, J.P.A.; Aguilar, R.G.; López, J.O. Synthesis and Characterization of Laser Induced Graphene (LIG) and laser Reduced Graphene Oxide (lrGO) by using a pulsed CO2 laser. MRS Adv. 2019, 4, 3327–3335. [Google Scholar] [CrossRef]
- Yoon, H.; Nah, J.; Kim, H.; Ko, S.; Sharifuzzaman, M.; Barman, S.C.; Xuan, X.; Kim, J.; Park, J.Y. A chemically modified laser-induced porous graphene based flexible and ultrasensitive electrochemical biosensor for sweat glucose detection. Sens. Actuators B Chem. 2020, 311, 127866. [Google Scholar] [CrossRef]
- Clerici, F.; Fontana, M.; Bianco, S.; Serrapede, M.; Perrucci, F.; Ferrero, S.; Tresso, E.; Lamberti, A. In situ MoS2 Decoration of Laser-Induced Graphene as Flexible Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 10459–10465. [Google Scholar] [CrossRef]
- Duy, L.X.; Peng, Z.; Li, Y.; Zhang, J.; Ji, Y.; Tour, J.M. Laser-induced graphene fibers. Carbon 2018, 126, 472–479. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Z.; Gao, L.; Wang, S.; Zhao, J. Effective design of MnO2 nanoparticles embedded in laser-induced graphene as shape-controllable electrodes for flexible planar microsupercapacitors. Appl. Surf. Sci. 2022, 571, 151385. [Google Scholar] [CrossRef]
- Nasraoui, S.; Al-Hamry, A.; Ameur, S.; Ali, M.B.; Kanoun, O. Electrochemical Sensor for 4-Aminophenol Based on Flexible Laser Induced Graphene. In Proceedings of the 2021 International Workshop on Impedance Spectroscopy (IWIS), Chemnitz, Germany, 29 September–1 October 2021; pp. 94–96. [Google Scholar]
- Samoson, K.; Soleh, A.; Saisahas, K.; Promsuwan, K.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Chang, K.H.; Abdullah, A.F.L.; Tayayuth, K.; et al. Facile fabrication of a flexible laser induced gold nanoparticle/chitosan/ porous graphene electrode for uric acid detection. Talanta 2022, 243, 123319. [Google Scholar] [CrossRef] [PubMed]
- Caetano, K.D.S.; da Rosa, D.S.; Pizzolato, T.M.; Santos, P.A.M.D.; Hinrichs, R.; Benvenutti, E.V.; Dias, S.L.P.; Arenas, L.T.; Costa, T.M.H. MWCNT/zirconia porous composite applied as electrochemical sensor for determination of methyl parathion. Microporous Mesoporous Mater. 2020, 309, 110583. [Google Scholar] [CrossRef]
- Liu, C.; Fu, H.; Pei, Y.; Wu, J.; Pisharodi, V.; Hu, Y.; Gao, G.; Yang, R.J.; Yang, J.; Cao, G. Understanding the Electrochemical Potential and Diffusivity of MnO/C Nanocomposites at Various Charge/discharge States. J. Mater. Chem. A 2019, 7, 7831–7842. [Google Scholar] [CrossRef]
- Jos, T.; Issac, S.; Joseph, R.; Rajith, L.; Kumar, K.G. Electrocatalysis and determination of pyridine-2-aldoxime methochloride using carbon nanotube-modified gold electrode. Micro Nano Lett. 2012, 7, 854–858. [Google Scholar] [CrossRef]
- Tun, W.S.T.; Saenchoopa, A.; Daduang, S.; Daduang, J.; Kulchat, S.; Patramanon, R. Electrochemical biosensor based on cellulose nanofibers/graphene oxide and acetylcholinesterase for the detection of chlorpyrifos pesticide in water and fruit juice. RSC Adv. 2023, 13, 9603–9614. [Google Scholar]
- Tunesi, M.M.; Kalwar, N.; Abbas, M.W.; Karakus, S.; Soomro, R.A.; Kilislioglu, A.; Abro, M.I.; Hallam, K.R. Functionalised CuO nanostructures for the detection of organophosphorus pesticides: A non-enzymatic inhibition approach coupled with nano-scale electrode engineering to improve electrode sensitivity. Sens. Actuators B Chem. 2018, 260, 480–489. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Q.; Huang, W.; Yang, W. Construction of a novel electrochemical sensor based on molecularly imprinted polymers for the selective determination of chlorpyrifos in real samples. J. Sep. Sci. 2017, 40, 4839–4846. [Google Scholar] [CrossRef]













| Electrode Material | Linear Range | LOD | Reference |
|---|---|---|---|
| GO/g-C3N4/GCE | 0.8 nM−0.01 μM | 0.82 nM | [10] |
| AChE/CS-GO/GO/CNFs | 25–1000 nM | 2.2 nM | [41] |
| CeO2/MWCNTs/GCE | 0.05 μM−1 μM | 2.5 nM | [19] |
| CuO/ITO (PAM) | 0.01–0.16 μM | 1.6 nM | [42] |
| MIPs/GCE | 0.0001–10 μM | 4.1 nM | [17] |
| GCE (PAMCl) | 25 nM−13.5 μM | 18 nM | [43] |
| CeO2/LIG (PAMCl) | 0.014–0.112 μM | 0.7 nM | This work |
| Sample | Taken (nM) | Found (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Spinach | 30 | 31.26 ± 1.00 | 104.2 ± 3.33 | 3.20 |
| 100 | 96.97 ± 3.2 | 96.97 ± 3.19 | 3.30 | |
| Lettuce | 30 | 28.79 ± 0.58 | 95.97 ± 1.94 | 2.01 |
| 100 | 103.67 ± 2.39 | 103.67 ± 2.39 | 2.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Sun, Q.; Zhang, X.; Yuan, W.; Wu, J. A Laser-Induced Graphene-Based Sensor Modified with CeO2 for Determination of Organophosphorus Pesticides with Improved Performance. Sensors 2023, 23, 9605. https://doi.org/10.3390/s23239605
Zhang W, Sun Q, Zhang X, Yuan W, Wu J. A Laser-Induced Graphene-Based Sensor Modified with CeO2 for Determination of Organophosphorus Pesticides with Improved Performance. Sensors. 2023; 23(23):9605. https://doi.org/10.3390/s23239605
Chicago/Turabian StyleZhang, Wenna, Qiu Sun, Xuelin Zhang, Weijian Yuan, and Jianfeng Wu. 2023. "A Laser-Induced Graphene-Based Sensor Modified with CeO2 for Determination of Organophosphorus Pesticides with Improved Performance" Sensors 23, no. 23: 9605. https://doi.org/10.3390/s23239605
APA StyleZhang, W., Sun, Q., Zhang, X., Yuan, W., & Wu, J. (2023). A Laser-Induced Graphene-Based Sensor Modified with CeO2 for Determination of Organophosphorus Pesticides with Improved Performance. Sensors, 23(23), 9605. https://doi.org/10.3390/s23239605




