An Odor Trace Visualization System Using a Two-Dimensional Backside Scattering Localized Surface Plasmon Resonance Gas Sensor
Abstract
:1. Introduction
2. Materials and Methods
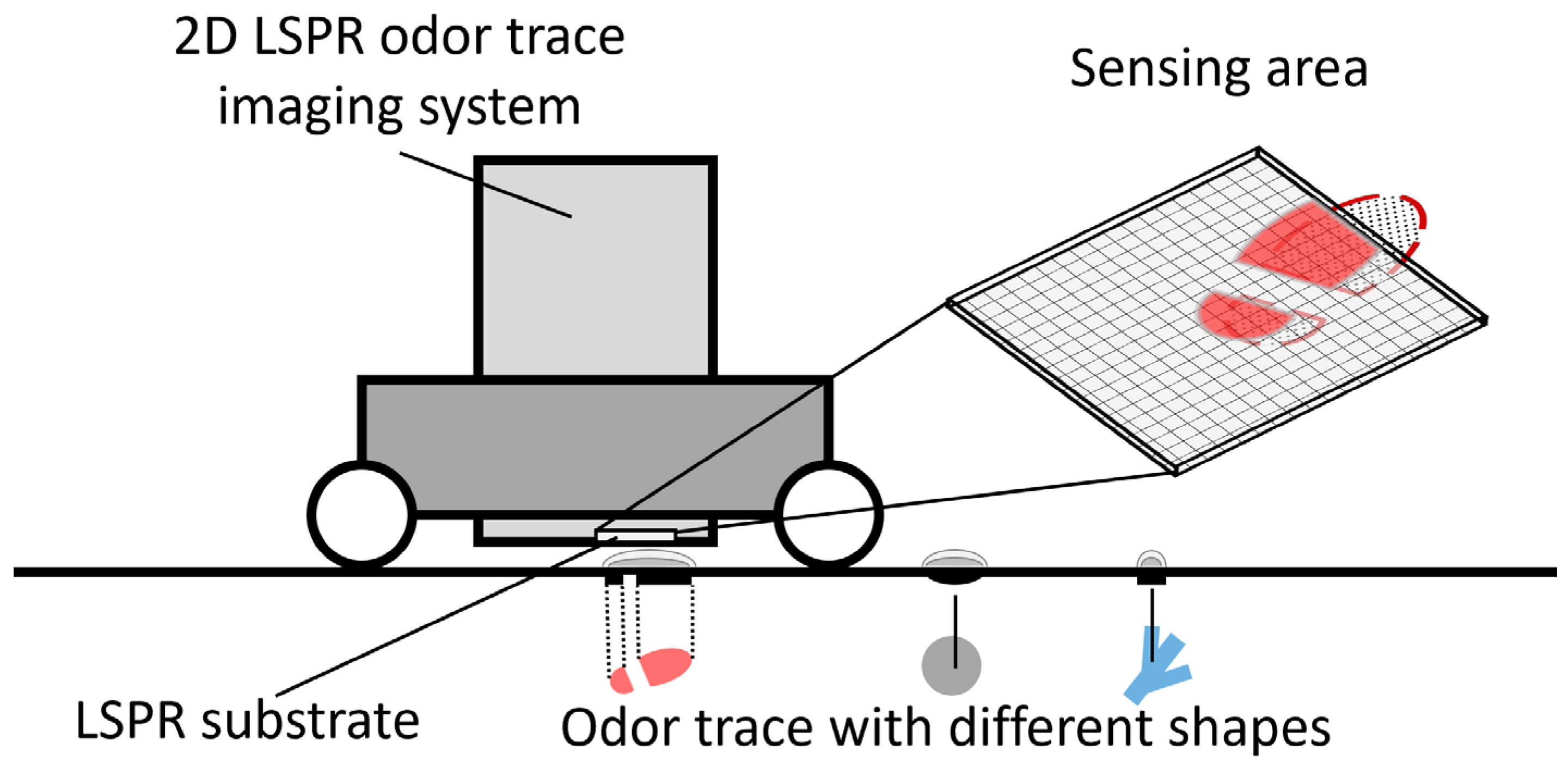
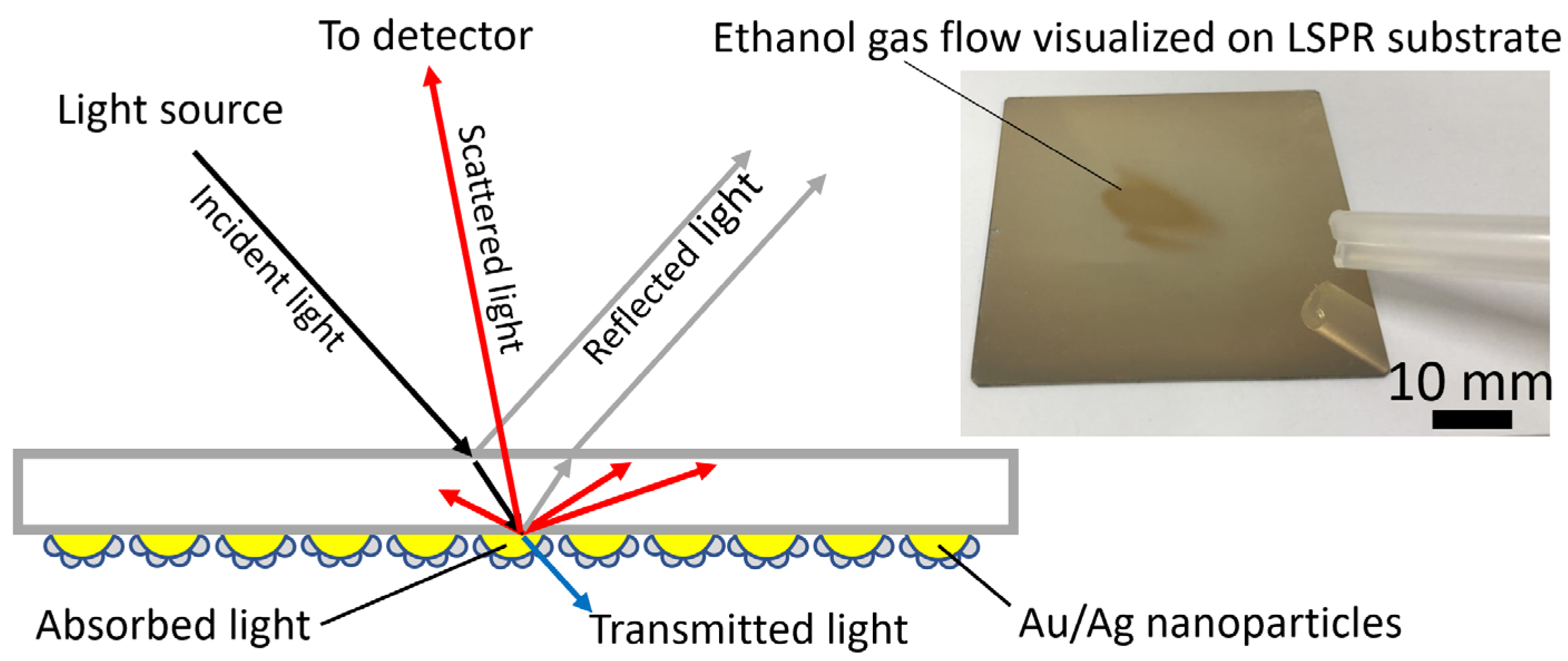
2.1. Visualization Concept and Principle of the On-Ground Odor Trace Imaging System
2.2. Fabrication of an LSPR Sensor Substrate with an Au/Ag Core-Shell Nanostructure
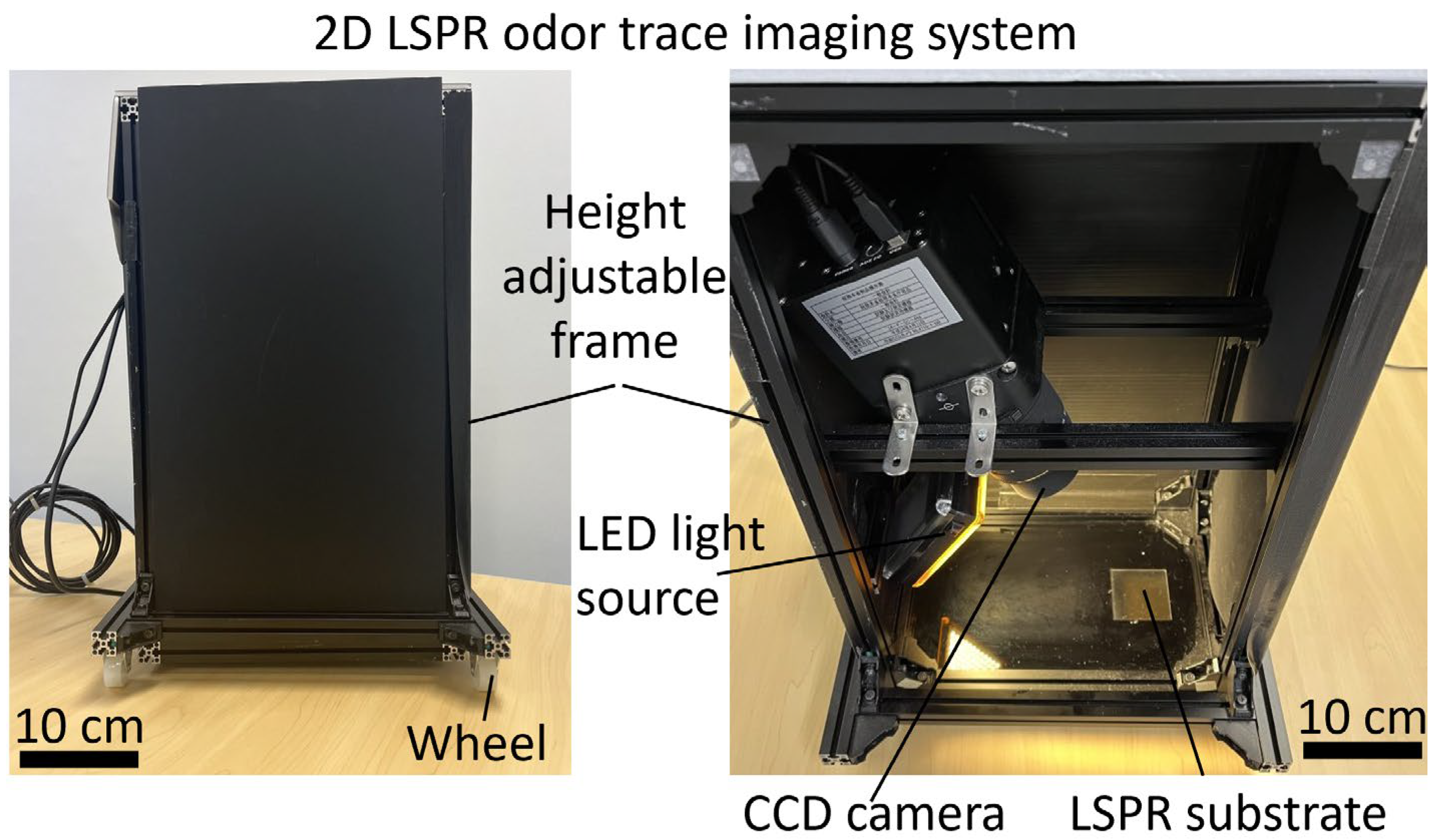
2.3. Fabrication of a 2D LSPR Odor Trace Imaging System
3. Results and Discussion
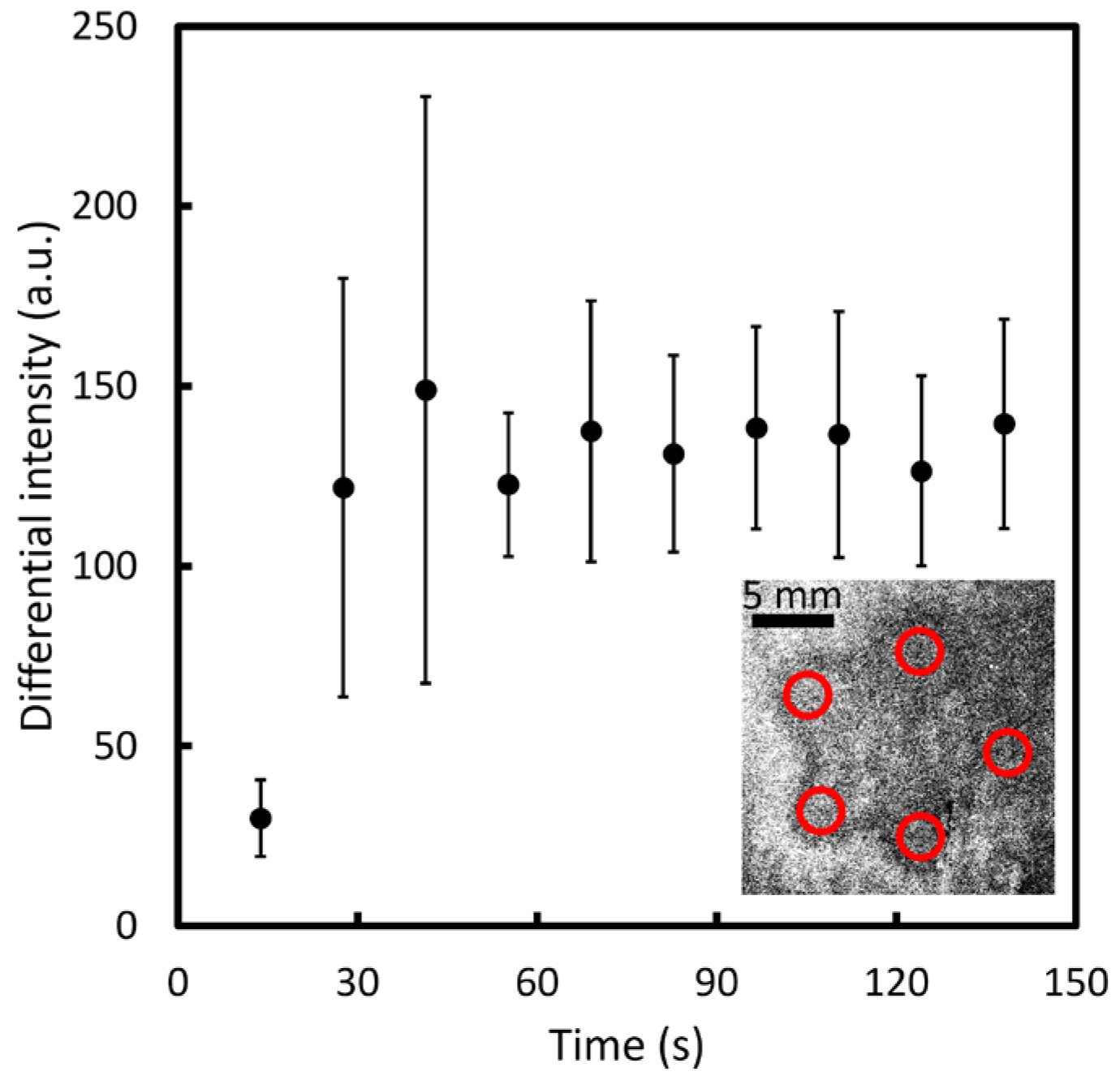
3.1. Characterization of the Au/Ag Core-Shell LSPR Substrate
3.2. Comparative Experiment of the Visualization of an A-Shaped Odor Trace
3.3. Indoor Environmental On-Ground Star-Shaped Odor Trace Visualization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Murlis, J.; Elkinton, J.S.; Carde, R.T. Odor Plumes and How Insects Use Them. Annu. Rev. Entomol. 1992, 37, 505–532. [Google Scholar] [CrossRef]
- Baron, R.; Saffell, J. Amperometric Gas Sensors as a Low Cost Emerging Technology Platform for Air Quality Monitoring Applications: A Review. ACS Sens. 2017, 2, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- He, T.Y.Y.; Wen, F.; Yang, Y.Q.; Le, X.H.; Liu, W.X.; Lee, C.K. Emerging Wearable Chemical Sensors Enabling Advanced Integrated Systems toward Personalized and Preventive Medicine. Anal. Chem. 2023, 95, 490–514. [Google Scholar] [CrossRef]
- Tai, H.L.; Duan, Z.H.; Wang, Y.; Wang, S.; Jiang, Y.D. Paper-Based Sensors for Gas, Humidity, and Strain Detections: A Review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Liu, J.W.; Wang, J.N.; Yan, W. Gas Sensing Technology for the Detection and Early Warning of Battery Thermal Runaway: A Review. Energy Fuels 2022, 36, 6038–6057. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.L.; Huang, X.J.; Ren, J.H.; Feng, H.; Zhang, Y.; Yang, X.X. High-sensitivity gas leak detection sensor based on a compact microphone array. Measurement 2021, 174, 109017. [Google Scholar] [CrossRef]
- Schoofs, G.R. Operation of Adsorbers for Purifying Ammonia-Synthesis Make-Up Gas. Ind. Eng. Chem. Res. 1993, 32, 613–619. [Google Scholar] [CrossRef]
- Reyes, S.J.; Durocher, Y.; Pham, P.L.; Henry, O. Modern Sensor Tools and Techniques for Monitoring, Controlling, and Improving Cell Culture Processes. Processes 2022, 10, 189. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Y.; Gopalan, S.; Luo, F.; Amreen, K.; Singh, R.K.; Goel, S.; Lin, Z.Y.; Naidu, R. Review and Perspective: Gas Separation and Discrimination Technologies for Current Gas Sensors in Environmental Applications. ACS Sens. 2023, 8, 1373–1390. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y. Metal-organic frameworks for QCM-based gas sensors: A review. Sens. Actuators A-Phys. 2020, 307, 111984. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.Y.; Wu, G.; Cao, J.X.; Zhang, Z.Y.; Zhang, Y. Carbon Nanotube-Based Field-Effect Transistor-Type Sensor with a Sensing Gate for Ppb-Level Formaldehyde Detection. ACS Appl. Mater. Interfaces 2021, 13, 56309–56319. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, S.; Chiyomaru, Y.; Sassa, F.; Liu, C.J.; Hayashi, K. Molecularly Imprinted Filtering Adsorbents for Odor Sensing. Sensors 2016, 16, 1974. [Google Scholar] [CrossRef]
- Lonergan, M.C.; Severin, E.J.; Doleman, B.J.; Beaber, S.A.; Grubb, R.H.; Lewis, N.S. Array-based vapor sensing using chemically sensitive, carbon black-polymer resistors. Chem. Mater. 1996, 8, 2298–2312. [Google Scholar] [CrossRef]
- Ge, L.P.; Ye, X.; Yu, Z.P.; Chen, B.; Liu, C.J.; Guo, H.; Zhang, S.Y.; Sassa, F.; Hayashi, K. A fully inkjet-printed disposable gas sensor matrix with molecularly imprinted gas-selective materials. npj Flex. Electron. 2022, 6, 40. [Google Scholar] [CrossRef]
- Nakamoto, T.; Ishida, H. Chemical sensing in spatial/temporal domains. Chem. Rev. 2008, 108, 680–704. [Google Scholar] [CrossRef]
- Noziere, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgic, I.; Hamilton, J.F.; et al. The Molecular Identification of Organic Compounds in the Atmosphere: State of the Art and Challenges. Chem. Rev. 2015, 115, 3919–3983. [Google Scholar] [CrossRef]
- Marin, A.C.; Schaefer, A.T.; Ackels, T. Spatial information from the odour environment in mammalian olfaction. Cell Tissue Res. 2021, 383, 473–483. [Google Scholar] [CrossRef]
- Reddy, G.; Murthy, V.N.; Vergassola, M. Olfactory Sensing and Navigation in Turbulent Environments. Annu. Rev. Condens. Matter Phys. 2022, 13, 191–213. [Google Scholar] [CrossRef]
- Kikas, T.; Ishida, H.; Webster, D.R.; Janata, J. Chemical plume tracking. 1. Chemical information encoding. Anal. Chem. 2001, 73, 3662–3668. [Google Scholar] [CrossRef]
- Moore, P.; Crimaldi, J. Odor landscapes and animal behavior: Tracking odor plumes in different physical worlds. J. Mar. Syst. 2004, 49, 55–64. [Google Scholar] [CrossRef]
- Pearce, T.C.; Gu, J.; Chanie, E. Chemical source classification in naturally turbulent plumes. Anal. Chem. 2007, 79, 8511–8519. [Google Scholar] [CrossRef] [PubMed]
- Thesen, A.; Steen, J.B.; Doving, K.B. Behavior of Dogs During Olfactory Tracking. J. Exp. Biol. 1993, 180, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Furton, K.G.; Myers, L.J. The scientific foundation and efficacy of the use of canines as chemical detectors for explosives. Talanta 2001, 54, 487–500. [Google Scholar] [CrossRef]
- Ansari, A.A.A.; Aldajani, K.M.M.; AlHazaa, A.N.N.; Albrithen, H.A.A. Recent progress of fluorescent materials for fingermarks detection in forensic science and anti-counterfeiting. Coord. Chem. Rev. 2022, 462, 214523. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Ishida, H.; Nakayama, G.; Nakamoto, T.; Moriizumi, T. Controlling a gas/odor plume-tracking robot based on transient responses of gas sensors. IEEE Sens. J. 2005, 5, 537–545. [Google Scholar] [CrossRef]
- Yoshioka, H.T.; Liu, C.J.; Hayashi, K. Multispectral fluorescence imaging for odorant discrimination and visualization. Sens. Actuators B-Chem. 2015, 220, 1297–1304. [Google Scholar] [CrossRef]
- Iitani, K.; Sato, T.; Naisierding, M.; Hayakawa, Y.; Toma, K.; Arakawa, T.; Mitsubayashi, K. Fluorometric Sniff-Cam (Gas-Imaging System) Utilizing Alcohol Dehydrogenase for Imaging Concentration Distribution of Acetaldehyde in Breath and Transdermal Vapor after Drinking. Anal. Chem. 2018, 90, 2678–2685. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.H.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Badilescu, S.; Raju, D.; Bathini, S.; Packirisamy, M. Gold Nano-Island Platforms for Localized Surface Plasmon Resonance Sensing: A Short Review. Molecules 2020, 25, 4661. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Borges, J.; Lopes, C.; Pereira, R.M.S.; Vasilevskiy, M.I.; Vaz, F. Gas Sensors Based on Localized Surface Plasmon Resonances: Synthesis of Oxide Films with Embedded Metal Nanoparticles, Theory and Simulation, and Sensitivity Enhancement Strategies. Appl. Sci. 2021, 11, 5388. [Google Scholar] [CrossRef]
- Karakouz, T.; Tesler, A.B.; Bendikov, T.A.; Vaskevich, A.; Rubinstein, I. Highly Stable Localized Plasmon Transducers Obtained by Thermal Embedding of Gold Island Films on Glass. Adv. Mater. 2008, 20, 3893–3899. [Google Scholar] [CrossRef]
- Tesler, A.B.; Chuntonov, L.; Karakouz, T.; Bendikov, T.A.; Haran, G.; Vaskevich, A.; Rubinstein, I. Tunable Localized Plasmon Transducers Prepared by Thermal Dewetting of Percolated Evaporated Gold Films. J. Phys. Chem. C 2011, 115, 24642–24652. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Hironou, A.; Shen, Z.J.; Tamiya, E. Versatile Micropatterning of Plasmonic Nanostructures by Visible Light Induced Electroless Silver Plating on Gold Nanoseeds. ACS Appl. Mater. Interfaces 2016, 8, 23932–23940. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wang, Y.; Song, C.Q.; Zheng, L.H.; Ma, N.; Liu, X.; Li, S.W.; Lin, L.L.; Li, M.Z.; Xu, Y.; et al. Hybrid Au-Ag Nanostructures for Enhanced Plasmon-Driven Catalytic Selective Hydrogenation through Visible Light Irradiation and Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2018, 140, 864–867. [Google Scholar] [CrossRef]
- Chen, B.; Guo, H.; Liu, C.J.; Shang, L.; Ye, X.; Chen, L.; Feng, C.H.; Hayashi, K. Molecularly imprinted sol-gel/Au@Ag core-shell nano-urchin localized surface plasmon resonance sensor designed in reflection mode for detection of organic acid vapors. Biosens. Bioelectron. 2020, 169, 112639. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Sassa, F.; Hayashi, K. A Robot Equipped with a High-Speed LSPR Gas Sensor Module for Collecting Spatial Odor Information from On-Ground Invisible Odor Sources. ACS Sens. 2018, 3, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Soeda, T.; Sassa, F.; Hayashi, K. Visualization of Spatial Distribution of On Ground Gas Sources with LSPR based 1D/2D High Speed Gas Sensor Robot System. In Proceedings of the 2018 IEEE Sensors, New Delhi, India, 28–31 October 2018; pp. 539–542. [Google Scholar]
- Yang, Z.Y.; Soeda, T.; Sassa, F.; Hayashi, K. Odor Trace Visualization by Mobile Robot Equipped with Two-Dimensional Lspr Gas Sensor. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (Transducers & Eurosensors XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1203–1206. [Google Scholar] [CrossRef]
- Blakey, I.; Merican, Z.; Thurecht, K.J. A Method for Controlling the Aggregation of Gold Nanoparticles: Tuning of Optical and Spectroscopic Properties. Langmuir 2013, 29, 8266–8274. [Google Scholar] [CrossRef] [PubMed]
- Soeda, T.; Yang, Z.Y.; Sassa, F.; Hayashi, K. Gas Visualization with Photo-Induced 2d Pixel Patterned Au/Ag Core-Shell Lspr Imaging Device by Mask-Less Exposure System. In Proceedings of the 2019 20th International Conference on Solid-State Sens. Actuators Microsyst. Eurosensors XXXIII (Transducers Eurosensors XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1274–1276. [Google Scholar] [CrossRef]
- Matsuoka, M.; Lingpu, G.; Sassa, F.; Hayashi, K. Spatiotemporal Visualization of Gases Using 2-D LSPR Gas Sensor. IEEE Sens. Lett. 2023, 7, 5000704. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Sassa, F.; Hayashi, K. An Odor Trace Visualization System Using a Two-Dimensional Backside Scattering Localized Surface Plasmon Resonance Gas Sensor. Sensors 2023, 23, 9525. https://doi.org/10.3390/s23239525
Yang Z, Sassa F, Hayashi K. An Odor Trace Visualization System Using a Two-Dimensional Backside Scattering Localized Surface Plasmon Resonance Gas Sensor. Sensors. 2023; 23(23):9525. https://doi.org/10.3390/s23239525
Chicago/Turabian StyleYang, Zhongyuan, Fumihiro Sassa, and Kenshi Hayashi. 2023. "An Odor Trace Visualization System Using a Two-Dimensional Backside Scattering Localized Surface Plasmon Resonance Gas Sensor" Sensors 23, no. 23: 9525. https://doi.org/10.3390/s23239525
APA StyleYang, Z., Sassa, F., & Hayashi, K. (2023). An Odor Trace Visualization System Using a Two-Dimensional Backside Scattering Localized Surface Plasmon Resonance Gas Sensor. Sensors, 23(23), 9525. https://doi.org/10.3390/s23239525




