1. Introduction
Hyperspectral imaging has emerged as a versatile and powerful technique that enables capturing spectral information across various applications. This technology allows for the acquisition of images in numerous narrow and contiguous spectral bands, facilitating detailed analysis and characterization of materials, objects, and scenes [
1]. The wealth of spectral data obtained through hyperspectral imaging has applications in remote sensing, agriculture, geology, biology, and industrial inspection.
Various hyperspectral imaging techniques have been developed to cater to different requirements and scenarios. These techniques encompass push-broom [
2] and whisk-broom systems for spatial scanning, snapshot methods utilizing filters or dispersive elements, and Fourier transform-based approaches for spectral decomposition [
3]. Each technique has advantages and limitations, often tailored to specific applications and equipment constraints.
Active illumination hyperspectral imaging is a specialized technique that uses controlled and deliberate illumination sources to enhance the accuracy and quality of spectral information captured in an image [
4,
5,
6,
7]. In traditional passive hyperspectral imaging, the scene is illuminated by ambient light or natural light sources, and the sensor measures the reflected or emitted light across multiple spectral bands. On the other hand, active illumination techniques introduce an element of control over the illumination source, allowing for precise manipulation of its spectral characteristics.
In active illumination hyperspectral imaging, the illumination source is typically chosen to emit light at specific wavelengths. This illumination enables the acquisition of images with more accurate spectral information, which can be crucial in applications where distinguishing between closely related materials is essential. By using controlled illumination, the signal received by the sensor can be optimized for the specific spectral bands of interest [
7], which leads to improved SNR in those bands, enhancing the overall quality of the hyperspectral data. Active illumination also minimizes the impact of lighting variations due to natural lighting conditions by providing consistent and controlled lighting conditions. In addition, active illumination allows the adaptation of illumination to specific applications and compensation for sensor characteristics.
In this article, we present a different variant of active illumination hyperspectral imaging, where a spectral shape variation of broadband illumination is utilized instead of multiple spectral bands at specific wavelengths. From the images recorded with many different illumination spectra, a spectral response consisting of a small number of spectral channels is reconstructed. The light source with variable illumination spectra is an incandescent lamp with adjustable filament temperature. Control of the filament temperature can be achieved by controlling the electric current through the bulb. As the temperature of the filament changes, the emitted spectrum follows Planck’s Law for black body radiation, allowing for controlled spectral variations. In this way, the illumination spectrum is tunable with arbitrary precision, with a small caveat that the maximal filament temperature limits the available spectra variations.
The reflected or transmitted spectrum reconstruction is formulated as an inverse problem. As the illumination spectra are of similar spectral shape due to blackbody emission characteristics, the inverse problem is ill-posed, necessitating robust mathematical algorithms and regularization techniques to mitigate the ill-posedness. We present a mathematical formulation of the reconstruction algorithm and the simulation study assessing the performance of the hyperspectral imaging approach under various circumstances. Through this investigation, we seek to provide a deep understanding of the approach’s capabilities, limitations, and sensitivity to imaging parameters.
The proposed active illumination approach holds promise in scenarios where illumination is already implemented with incandescent lamps, like classical microscopy or macroscopic imaging using RGB sensors. Moreover, the simplicity and affordability of incandescent lamps make this technique accessible and practical for various novel imaging setups.
4. Results
The signals S were simulated using the default parameters as described in the previous section. Values of regularization parameters for the default simulation were set to k1 = 10−6 and k2 = 10−2. The k2 parameter was always an inverse value of SNR, while k1 was for a factor of 104 smaller. Suitable values for regularization parameters depend on the imaging signal’s scale.
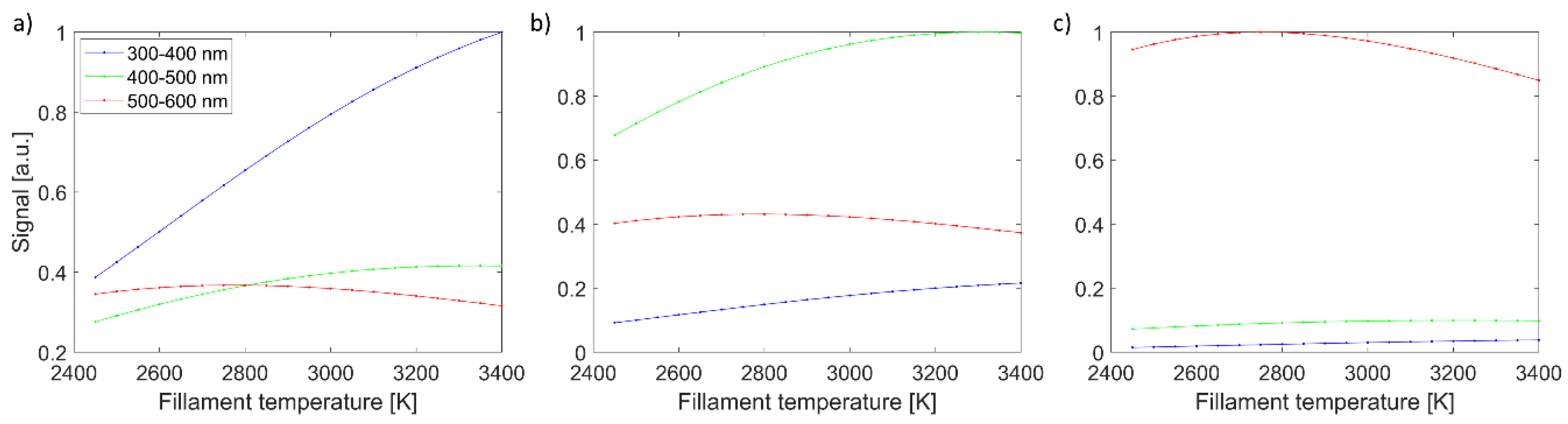
Imaging signals for all three channels, in dependence on filament temperature, are shown in
Figure 2. They are presented for selected three spectra: blue (spectrum #13), green (spectrum#14), and red (spectrum #15). As expected, the blue color (spectrum #13) with the strongest signal in the 300–400 nm channel increases with filament temperature. Similarly, the green color (spectrum #14) has the strongest signal in the 400–500 nm channel and reaches the maximum when the filament temperature is close to 3400 K. The red color (spectrum #15) is the strongest signal in the 500–600 nm channel and reaches its maximum slightly below 2800 K. All three colors have distinctive temperature dependence for three spectral channels, facilitating the reconstruction of spectra shape. It should also be stressed that the shape of imaging signal vs. filament temperature is affected by various factors, namely Planck’s black body radiation law, normalization to
T4 that mimics exposition adaption to higher radiation power at higher temperature, spectral-dependant quantum efficiency of the sensor, and filter spectral dependant transmission.
The corresponding reconstructed spectra are shown in
Figure 3. In general, the reconstructed spectra (dashed lines) follow the original spectra (solid lines), with somewhat higher errors near the edge of the spectral range in case of the spectra with increased dynamic in that spectral region (e.g., spectra #3, #5, #19). The spectra that have a transition from low to high reflectivity (e.g., spectra #16, #17, #18) typically have a well-reconstructed location of the transition. At the same time, the shape does not always fully agree with the original spectra. Some spectra featuring ripples are reconstructed with high accuracy (e.g., spectra #6, #11), while others show some discrepancies (e.g., spectra #4, #13, #16). The original grey-scale spectra are mostly flat (
Figure 3d), except at the low wavelengths, where the reflectivity is significantly lower. The corresponding reconstructed spectra have some ripples and do not capture well the reduced reflectivity at low wavelengths. However, on average, the reconstructed grey-scale spectra are quantitatively close to the original spectra.
Analyzing reconstructed spectra under default simulation parameters has provided valuable insights into the algorithm’s performance. The algorithm’s ability to closely follow true spectra is evident, with slight deviations near the edges of the spectral range for some spectra. Spectra exhibiting transitions from low to high reflectivity generally exhibit accurate transition locations, although the precise shape is not always consistently captured. Notably, the spectra with ripples, such as spectra #6 and #11, demonstrate high accuracy in reconstruction. However, discrepancies arise for specific spectra like #4, #13, and #16. Original gray-scale spectra, characterized by mostly flat reflectance, show subtle ripples in the reconstructed versions. Although these reconstructions of gray-scale spectra do not fully capture the reduced reflectivity at low wavelengths, their quantitative proximity to true values is promising.
All
RMSEs are less than 5.1%, on average less than 2.3%. The largest
RMSE is obtained in spectrum #19, which has a reflectivity of about 90% at almost all wavelengths. For spectrum 24, which has a reflectivity of only a few percent over the entire spectrum, the error is only 0.1%. However, the results are different when we inspect the relative error
rRMSE, considering also the amplitude of the spectra. The
rRMSE values are generally larger, on average 9.9%, reaching 25.1% in the case of spectrum #13. Specific spectra, like spectrum #13, exhibit
RMSE that can get up to 25%. The reason for high relative error for some spectra, like spectrum #13, is because the spectrum has ripples and relatively high error in the spectrum where the reflectivity is high, while most parts of the spectrum are low. The reconstruction errors in terms of
RMSE and
rRMSE are presented in
Table 1.
4.1. Sensitivity Analysis
Changing
SNR from 100 to 1000, which can be experimentally realized by increasing integration time or light intensity, consistently improves the
RMSE for all reconstructed spectra. Improvement in
RMSE is higher for some spectra (e.g., spectrum #18, where
RMSE goes from 2.9% to 0.6%), while others have moderate improvement (e.g., spectrum #7, where
RMSE goes from 3% to 2.5%). On average, the
RMSE was reduced to 1.4% compared to 2.3% for
SNR = 100. In general,
rRMSE decreases by increasing
SNR, but surprisingly, it also slightly increases for some spectra (spectra #7, #12. #15, and #15, where the
RMSE does not improve considerably). The reason for such unexpected behavior of
rRMSE is that those spectra have ripples, hence the notable error in the area of very low reflectivity in the case of
SNR = 1000. Therefore, a substantial relative error in this part of the spectrum is present (note that the absolute error is normalized to the true value of reflectivity, which is very low) and, consequently, the increase in
rRMSE. The increased
rRMSE is possible because our spectrum reconstruction uses the means for least squares absolute error, so it tries to find the minimum in
RMSE, not the
rRMSE. The average
rRMSE in the case of
SNR = 1000 is still improved; it is 8.2% compared to 9.9% for
SNR = 100, resulting in a much smaller error reduction than
RMSE. The errors for all spectra are collected in
Table 2 and
Table 3.
A similar improvement as with the increased SNR is achieved by doubling the number of acquisition spectral channels to p = 6. The average RMSE is 1.4%, and the average rRMSE is 7.2%. The reconstruction error reduction is smaller in the case of the decrease in the filament temperature step (ΔT = 10 K). Namely, the average RMSE is 1.8%, and the average rRMSE is 8.3%. Increasing the number of acquisition spectral channels and smaller increments in filament temperature provides twice and five times more measurements, which have the same effect as improved SNR. When combining repeated measurements, the SNR increases with the square root of the number of repeated measurements. We expect similar behavior (i.e., an apparent improvement in SNR) if the number of acquisition spectral channels is doubled or the number of filament temperatures is five times higher. The apparent improvement in SNR should be for the or . A smaller increment in filament temperature provides information that could be obtained by interpolating the data with default simulation parameters. Hence, the improvement is likely due to more data and apparent improvement of the SNR. Increasing the number of acquisition spectral channels provides better spectral resolution in the measured data, so improvement in reconstructed spectra is also expected due to this reason. Worse performance for p = 6 acquisition spectral channels, compared to SNR = 1000 in gray-scale spectra, is because improved SNR facilitates better estimation of relatively low reflectivity at low wavelength range.
In the next step, we impaired these three imaging parameters. By reducing SNR to 10, the reconstruction accuracy also reduces. The average RMSE is 3.8% compared to 2.3% for the default set, and the average rRMSE is 23.7% compared to 9.9% for the default set. Similarly, by increasing ΔT to 100 K, reconstruction accuracy is reduced, resulting in the average RMSE = 2.7% and rRMSE = 11.8%. However, the errors increase substantially less when increasing ΔT than decreasing SNR, thus showing that the reconstruction is very sensitive to low SNR. A more significant increment in filament temperature does not exclude the information that could not be obtained by interpolating the data with default simulation parameters, so slightly worse results are likely due to less data and apparent degradation of the SNR. That explains why reducing SNR tenfold (to SNR = 10) affects the accuracy of reconstructed spectra more severely than the simulated larger filament temperature increments, which would reduce the apparent SNR only for a factor of .
Then, we tested the effect of minimal filament temperature on the reconstructed spectra accuracy and found that by decreasing the minimum filament temperature, a slight improvement in the reconstruction accuracy was achieved. Still, the increase in the minimum filament temperature results in a significant reduction in the reconstruction accuracy. Specifically, if the minimum temperature is decreased to 1950 K, the average RMSE and rRMSE are 1.9% and 8.9%, respectively. If the minimum temperature increases to 2950 K, the average RMSE and rRMSE are 3.1% and 18.2%, respectively. These results can be explained by noting that lower and higher minimal filament temperatures determine how different illumination spectra are used in the inverse problem. Going to a very low filament temperature cannot have much effect because, in that case, the spectrum is heavily shifted towards infrared. Even at the maximal achievable temperature T = 3400 K, the black body radiation spectrum has maximum in infrared (λmax = 853 nm). Still, this spectrum has to be weighted with the CMOS quantum efficiency, which rapidly decreases at 700 nm and above.
On the other hand, if the lowest temperature is set at a higher value, we omit spectra at lower temperatures. Consequentially, the inverse problem is formed with similar spectra, and the reconstruction problem is more ill-posed. In addition, increasing the lowest temperature also reduces the number of available temperatures and, therefore, the number of acquired signals and the apparent SNR. All of that explains the lower accuracy of the reconstructed spectra when the minimal temperature was raised to Tmin = 2950 K.
The next test was introducing the illumination spectra errors by assuming the wrong estimation of the filament temperature (δT). While RMSE is still below 10%, on average 4.8%, the spectra are visually inaccurate. That is especially true for the gray-scale spectra (spectra #19 to #24), where pronounced ripples appear. Poor quality of the reconstructed spectra is also revealed by the rRMSE, which reaches 45% and is, on average, 25.7%. These results demonstrate that the filament temperature error is the most prominent source of error in the reconstructed spectra and should not be surprising. Our hyperspectral algorithm relies on inverse reconstruction of the spectra, using linearly independent illumination spectra, but they are of similar spectral shape. As the spectra are similar, it is not surprising that the algorithm is sensitive to the error of the illumination spectra. Even with a small spectral error (δT = 10 K), differences among the different temperature illumination spectra are lost due to the spectral error. Therefore, this issue must be adequately addressed.
The final sensitivity test was changing the wavelength step size of the reconstructed spectra Δλ. It has some effect, but it depends on the actual spectrum. In some cases, smaller wavelength step size improves accuracy, and larger reduces it (e.g., #6, #14, or #18), while in other cases, the smaller wavelength step even reduces the rRMSE (e.g., #3 or #19). Specifically, when Δλ is reduced to 5 nm, the average RMSE and rRMSE are 2.2% and 11.6%, respectively. When Δλ increases to 20 nm, the average RMSE and rRMSE are 2.9% and 16.0%, respectively. The reasons for such behavior are not clear. However, the spectral resolution of the reconstructed spectra is significantly better than the resolution of the detected spectra (p = 3 or 6), implying that the subtle differences could arise from the regularization techniques or other sources.
The complete results of the sensitivity analysis are shown in
Table 2 and
Table 3. The reconstructed reflectance spectra, together with the original spectra for all parameter variations within the sensitivity analysis, are included in
Supplementary Materials.
4.2. Spectra from Real-Life Examples
RGB images from real-life examples are shown in
Figure 4. Three points where the spectra were taken for the simulation tests are marked on those images.
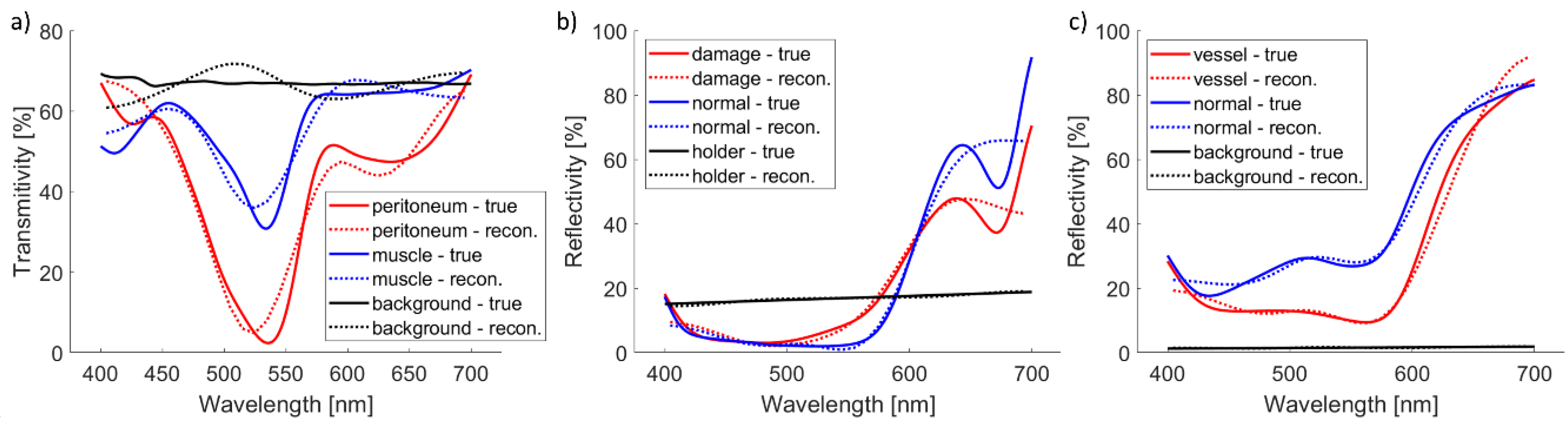
Simulated and reconstructed spectra are shown in
Figure 5. Background or apple holder have flat spectra, which are correctly reconstructed. Other spectra are more complex and do not have all details properly reconstructed. However, the basic shape is reconstructed correctly.
Accuracy in terms of RMSE and rRMSE is reported in
Table 4. While RMSE is always within 5%, rRMSE can be high. That is the case when part of the true spectrum is very low, and the relative spectrum error is significant even though the absolute error is not that large (e.g., peritoneum around 550 nm).
5. Discussion
This study presents a novel hyperspectral imaging approach that harnesses the potential of active illumination and a small number of detection channels. Our choice to explore three- and six-channel scenarios reflects the practical feasibility of integrating this approach into existing imaging setups. The three-channel configuration is particularly advantageous due to its compatibility with commonly used filter cubes in microscopes and RGB cameras. In contrast, the six-channel configuration aligns with the prevalent design of filter wheels. The findings of this study are relevant to both reflectance imaging, which was the selected imaging method in this study, and transmittance imaging since the imaging approach and spectral reconstruction algorithm are the same.
A critical result of our simulation-based investigation is the approach’s sensitivity to the accuracy of the illumination spectrum. Introducing illumination spectrum errors by assuming incorrect filament temperatures is a significant source of the reconstruction error. While RMSE remains generally below 10% and often below 5%, visual discrepancies are prominent, especially in the gray-scale spectra with notable ripples. The elevated rRMSE values, reaching up to 45%, underscore the algorithm’s sensitivity to the accuracy of illumination spectra. Therefore, we must always remember the necessity of precisely knowing the emission spectrum of the illumination source, which can be achieved through spectrometer-based measurements. For example, a fiber probe can collect the emitted spectrum, which a spectrometer samples in real-time. The accurate illumination spectrum information is a foundation for successful spectral reconstruction, ensuring that the algorithm can effectively separate and recover the unique spectral characteristics of the imaged samples.
An alternative avenue for the calibration would involve imaging spectral standards of well-defined spectra at different filament temperatures and subsequently calibrating the system using these standards. However, the effectiveness of this approach hinges on the repeatability of the incandescent lamp’s emission spectrum at these temperatures. If the spectrum remains consistent across different imaging sessions, it could provide a practical calibration route, particularly when spectrometer-based calibration is challenging.
Other parameters that were systematically varied to assess their impact on reconstruction accuracy also affect the algorithm’s accuracy. The first two parameters are the SNR and the number of acquisition spectral channels p. Elevated SNR from 100 to 1000 consistently improves RMSE across all reconstructed spectra. Prominent RMSE reduction is observed for some spectra (e.g., #18) and only moderate improvements for others (e.g., #7). Interestingly, the rRMSE findings differ slightly, with improved SNR generally leading to reduced rRMSE, though occasional increases occur (e.g., #7 and #12). Investigation of the reconstructed spectra shows that the Orange for the SNR = 1000 has a higher error in the part with very low reflectivity, which results in substantial relative error for that part of the spectrum. The effectiveness of improving reconstruction accuracy through an increased number of acquisition spectral channels (up to p = 6) or a smaller increment in filament temperature (ΔT = 10 K) is evident. While both strategies enhance accuracy, the impact of increased spectral channels is more pronounced, underscoring the importance of data quantity and quality in hyperspectral reconstruction. Decreasing SNR to 10 consistently degrades reconstructed spectra accuracy. However, the magnitude of reduction varies among different colors and gray-scale spectra. Particularly, rRMSE values are more adversely affected than RMSE, emphasizing the algorithm’s sensitivity to relative errors under low SNR conditions. Similar trends emerge with larger filament temperature increments (ΔT = 100 K), albeit with less pronounced effects. Notably, specific spectra, like #6, perform relatively well with larger increments in filament temperature but exhibit poorer results with lower SNR.
The influence of filament temperature range selection on reconstruction accuracy is also explored. Lower minimal filament temperature has a marginal effect on accuracy, while higher minimal temperature leads to reduced accuracy. This outcome highlights the role of illumination spectra diversity in the inverse problem, particularly in scenarios where similar spectra exacerbate ill-posedness. The analysis of varying wavelength step sizes in reconstructed spectra demonstrates mixed outcomes. Smaller step sizes enhance accuracy in some cases but worsen it in others, a pattern also observed with larger step sizes.
Our simulations involved spectral channels with sharp passbands commercially available at optical component sellers like Thorlabs. While these filters introduce small ripples within the passband, it is reasonable to assume that they are unlikely to significantly impact the algorithm performance because they are often on a wavelength scale lower than the spectral resolution of this hyperspectral approach. However, it is essential to validate this aspect during the actual implementation.
The simulation study leaned on the regularization techniques for reflectivity spectra reconstruction. Acknowledging that these regularization techniques may not be universally optimal across all scenarios is pertinent. The potential for enhancing results by optimizing these techniques underscores the iterative nature of algorithm refinement and the scope for further improvements in accuracy and applicability. Like the regularization optimization, optimizing the illumination protocol is also essential to implementing the method. Higher filament temperature increases the radiation power (proportional to T4) and spectrum. Increased radiation power should be compensated by decreased exposure time to prevent under- or overexposure. We simulated that by T−4 normalization of the illumination spectra from Planck’s law (Equation (1)). However, different normalizations might improve spectrum identifiability as the illumination power in pass wavelength band is not proportional to T4.
This study partially relates to existing active illumination spectral imaging [
4,
5,
6,
7]. However, it fundamentally differs from these studies by introducing continuously variable spectrum illumination, possibly enabling the reconstruction of far more spectral points than active illumination using a few distinct spectra. Specifically, Kaariainen and Donsberg combined a supercontinuum laser (SC) and a Fabry–Perot interferometer (FPI) to build a tunable light source enabling active illumination at selected spectral bands. Since the spectral transmittance of FPI is tuned by voltage, custom spectral bands can be selected, resulting in multiple spectral images (100 in this study). In contrast to our approach, a set of monochromatic images is recorded in [
4], like the filtered hyperspectral imaging method [
11]. However, the SC–FPI active illumination hardware is expensive and bulky compared to our approach and cannot be easily implemented in existing imaging setups. A similar method of active illumination for HIS was implemented in [
5]. Here, 27 LEDs with different and disjunct spectral emissions covering the 300–1050 nm spectral region were combined in a light-pipe module carefully designed to provide a homogenous illumination field. The same illumination idea, but in a ring illumination geometry, was demonstrated also in [
7]. In this study, 19 LEDs covering the spectral range 365–1050 nm were distributed within a ring. These two approaches are as expensive as the SC-FPI illumination. The imaging is limited to only a small number of preselected spectral bands, while the illumination modules are still bulky and mostly incompatible with existing imaging setups. The authors of these studies showed some HSI results, namely spectra and spatial images, but they neither provided a comparison to reference imaging modalities nor assessed the effect of different imaging parameters on the imaging performance. However, it is necessary to point out that our proposed active illumination differs entirely from the above methods since multiple images integrated over the whole detection spectral range are acquired, and hypercubes are obtained by performing reconstruction.
Traditionally, the approach with active illumination is to perform imaging of multiple narrow spectral bands [
4,
5,
6,
7]. There are also methods for spectra reconstruction from an RGB image, which is an underdetermined problem and cannot be solved without a-priori information [
12]. Moreover, it is argued that the same RGB can map to different spectra depending on the context [
13]. The problem is typically solved by deep neural networks (DNN). One DNN approach primarily focuses on training a “pixel-centric” mapping, wherein each pixel’s RGB values are mapped to its spectral estimate without considering neighboring pixels [
12,
13,
14,
15]. More recently, DNN has shifted towards “patch-centric” mappings. In this approach, substantial image content information is anticipated to be extracted from extensive image patches and integrated into the super-resolution process [
16,
17]. However, our proposed approach does not need a-priori information since multiple spectral images are recorded, which are used to reconstruct hyperspectral cubes.
Like the spectrum reconstruction from RGB images, our approach to hyperspectral imaging could produce better results if the regularized inversion of the mathematically formulated problem was inverted with machine learning techniques such as Random Forest, Artificial Neural Networks (ANN), and Convolutional Neural Networks (CNN). Improved results are anticipated due to the inherent nonlinearity of machine learning methods, which should yield superior results.
The inherent problem of our approach is the spectrum of the incandescent lamp, which has substantial power in red and NIR ranges for all achievable filament temperatures. This problem could be solved with a custom-designed filter that has high transmission in the blue region and gradually decreased transmission for longer wavelengths. However, an appropriate filter may not be available as a standard component, so it should be custom-designed, which likely defeats the idea of having a simple and cheap hyperspectral imaging system. On the other hand, that might not be a problem if the presented concept of hyperspectral imaging is employed in a device that is produced in a large quantity.
Given their widespread availability and simplified hardware requirements, the allure of employing RGB cameras instead of dedicated spectral filters is evident. However, this transition poses notable challenges. Spectral bands in RGB cameras tend to overlap, which could affect the separability of the spectral information. Furthermore, some RGB cameras exhibit outstanding quantum efficiency in the red and near-infrared (NIR) regions for all three spectral channels. Therefore, successfully adapting this methodology to RGB cameras necessitates meticulous camera selection, focusing on cameras with low quantum efficiency for the G and B channels within the red and NIR wavelength ranges or using a suitable cut-on optical filter to eliminate the NIR light contribution to the signal.
The simulation study primarily explored hyperspectral imaging within the optical 400–700 nm range, leveraging the standard ColorChecker spectra. However, we posit that the technique’s potential extends to wider spectral ranges. The CMOS cameras can operate within the 300–1000 nm range, which presents new opportunities for hyperspectral imaging in this wavelength range, but they have lower quantum efficiency in the UV and NIR range. However, we must note the interplay between the camera’s quantum efficiency and the incandescent lamp’s emission spectrum within the NIR range. This compensatory effect should contribute to the algorithm’s viability in this extended range.
We have discussed some of the study’s limitations earlier (e.g., ignoring the actual transmissivity of the bandpass filters that may have ripples). Another limitation was using a direct method to solve linear systems and obtain reflectance spectra from the simulated signals. Iterative methods may yield better results, such as preconditioned conjugate gradients or least squares. We used a simple T4 normalization factor to normalize the signals at different temperatures. However, this may not be the best approach, and we should explore other normalization strategies. We used the same regularization weights, k1 and k2, for the same SNR values. Yet, the optimal weights may vary depending on the signal shapes and noise levels. We could develop a method to select the optimal weights based on these factors. We used ColorChecker as a sample to evaluate our approach. ColorChecker is a standard reference object in visible light imaging, RGB, and spectral imaging, but we could also simulate other reference standards, such as skin colors or minerals, to mimic specific applications. We expect that using more suitable reference standards would improve the reconstructed spectra for these narrower sample sets. Finally, the main limitation of this study is that we only report numerical simulation results without experimental validation. However, this study aims to suggest the possibility of using active illumination for hyperspectral imaging, which needs to be tested experimentally in the future.
For implementation of this technique in real-world applications, some challenges remain to be solved. One of them is the accurate determination of illumination spectra, which could be solved by including a spectrometer in the system to measure the spectrum of the emitted light in real-time. Proper calibration makes it possible to provide the spectrum of adequate accuracy as the input parameter of the reconstruction technique. The information about the spectral sensitivity of the detector, including the filters’ transmissivity, can be obtained either from the manufacturer or experimentally measured. Another possible solution to get an accurate transformation matrix
A is to thoroughly calibrate different measured illumination spectra on different reflectance standards. This information is used to estimate the
A’s for different imaging conditions by the Wiener estimation method [
18]. In this case, it is unnecessary to know the detector sensitivity and filter transmissivity since they would already be part of the estimated Wiener transformation matrices. Another challenge is the time needed to change the incandescent bulb filament temperature. The filament temperature is approximately proportional to the electric current; thus, it is possible to change the filament temperature rapidly by rapidly changing the current. It was shown that under normal operating conditions, starting at 300 K after turning on an incandescent lamp, it reaches its final temperature of approx. 3000 K in less than 0.5 s [
19]. Based on that, a quick estimate would be that to change filament temperature to 50 K, it would be necessary to wait 10 ms. A complete imaging involving 20 temperature steps would take less than 1 s. In real imaging conditions, this time would be longer due to the image recording, acquisition of spectra, and other steps involved in the imaging procedure, but the imaging would be relatively fast. A challenge might also be integrating the new imaging components, including the programmable current source and spectrometer, in an existing system. However, in most imaging systems, this should not be a problem since both components are relatively small, compact, and non-expensive.
The versatility of this approach is underscored by its applicability to both hyperspectral microscopy and macroscopic hyperspectral imaging. However, the design of illumination schemes and calibration procedures must be tailored to the specifics of each technique. Integrating this approach into hyperspectral microscopy could revolutionize the spatial analysis of biological samples, while its application to macroscopic imaging holds promise in diverse fields such as medicine, material characterization, and industrial inspections.