Passive Acoustic Sampling Enhances Traditional Herpetofauna Sampling Techniques in Urban Environments
Abstract
:1. Introduction
2. Materials and Methods
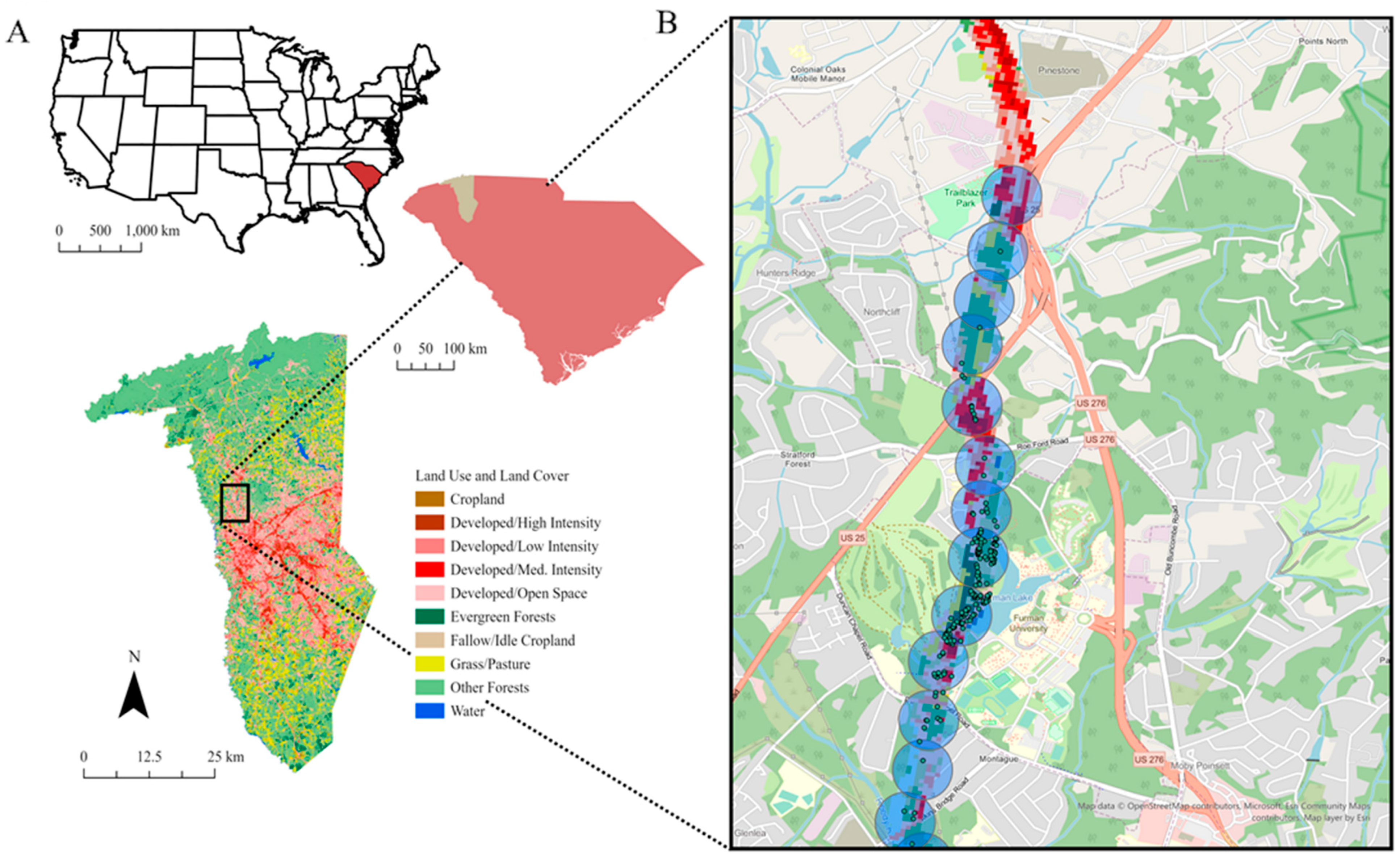
2.1. Study Area
2.2. Data Collection
2.3. Data Processing
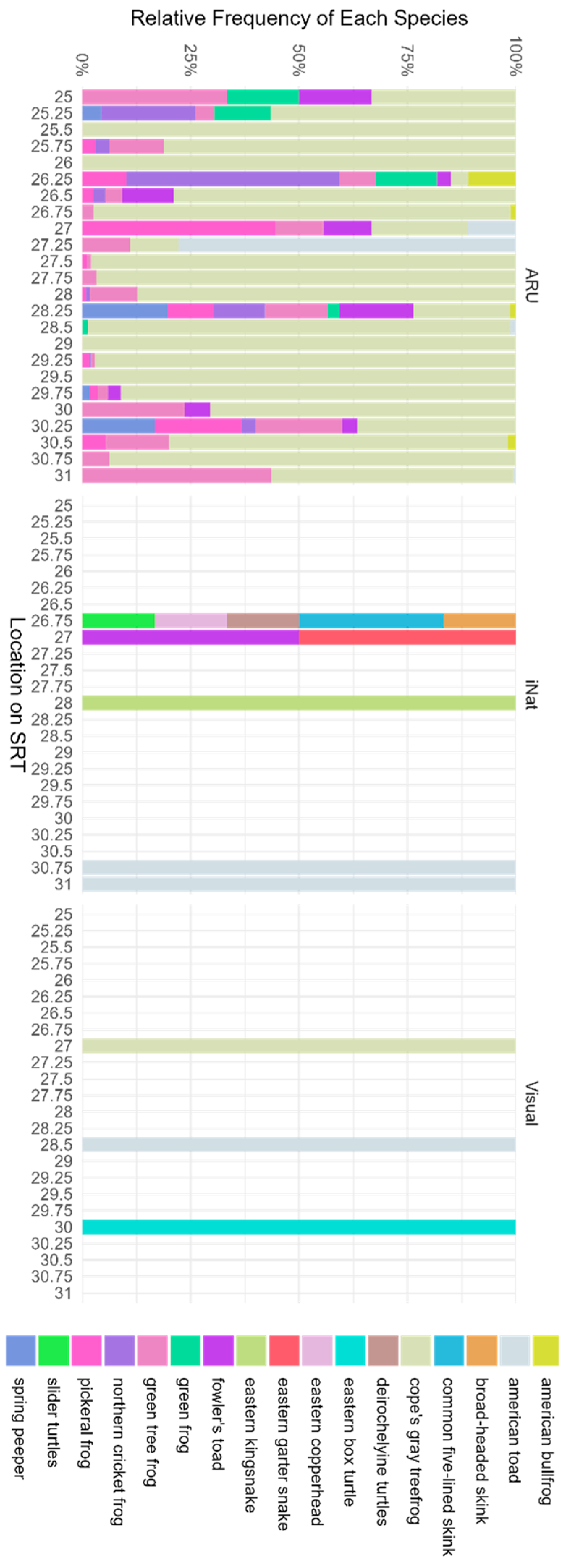
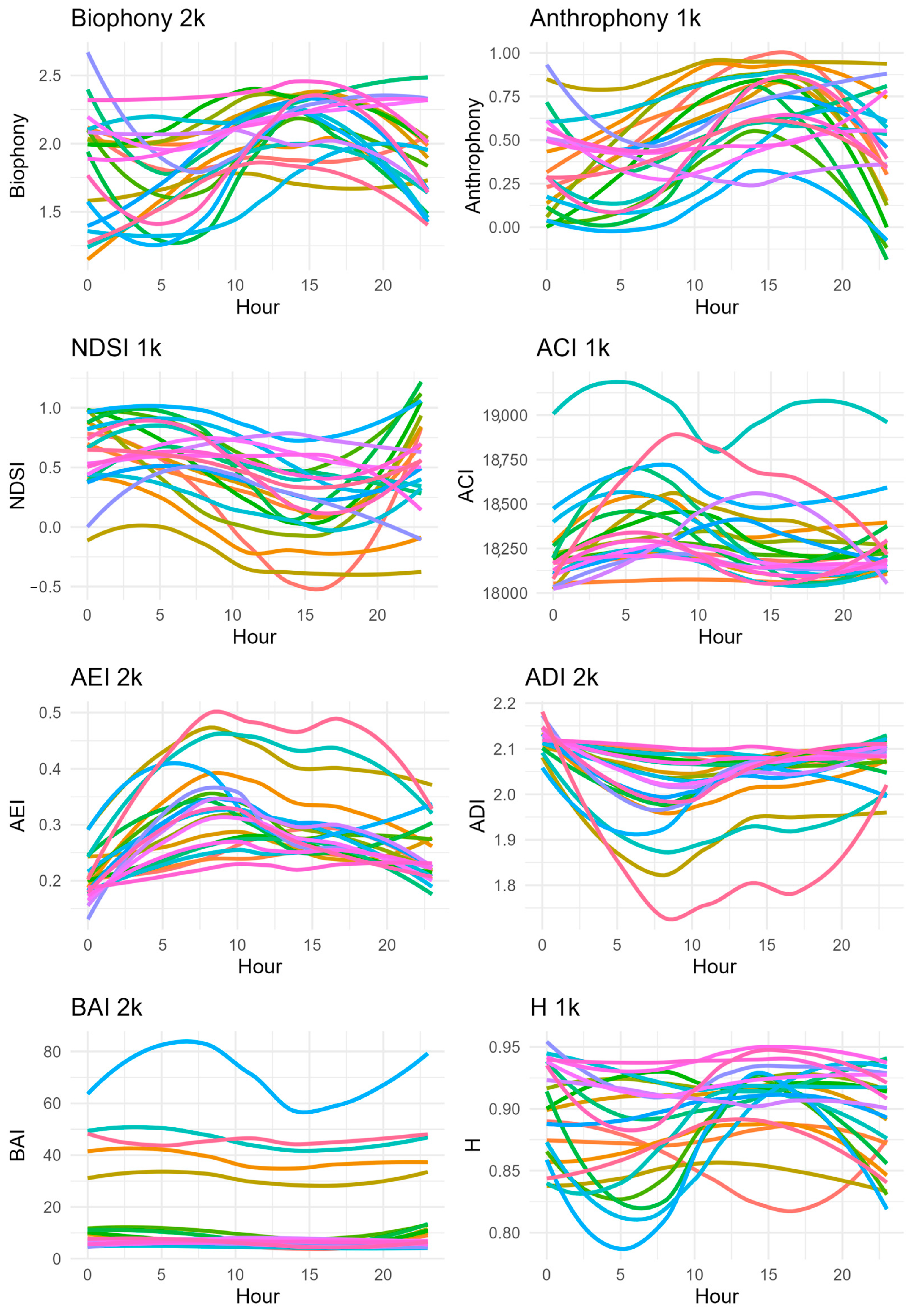
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, J.; Michael, T.; Zhang, J.; Roe, P. Detecting frog calling activity based on acoustic event detection and multi-label learning. Procedia Comput. Sci. 2016, 80, 627–638. [Google Scholar] [CrossRef]
- Eakin, C.; Calhoun, A.J.K.; Hunter Jr., M. L. Indicators of wood frog (Lithobates sylvaticus) condition in a suburbanizing landscape. Ecosphere 2019, 10, e02789. [Google Scholar] [CrossRef]
- Pyles, M.V.; Prado-Junior, J.A.; Magnago, L.F.; de Paula, A.; Meira-Neto, J.A. Loss of biodiversity and shifts in aboveground biomass drivers in tropical rainforests with different disturbance histories. Biodivers. Conserv. 2018, 27, 3215–3231. [Google Scholar] [CrossRef]
- Howell, H.J.; Rothermel, B.B.; White, N.; Searcy, C.A. Gopher tortoise demographic responses to a novel disturbance regime. J. Wildl. Manag. 2020, 84, 56–65. [Google Scholar] [CrossRef]
- Mengak, M.T.; Guynn, D.C. Pitfalls and Snap Traps for Sampling Small Mammals and Herpetofauna. Am. Midl. Nat. 1987, 118, 284. [Google Scholar] [CrossRef]
- Ribeiro-Júnior, M.A.; Gardner, T.A.; Ávila-Pires, T.C.S. Evaluating the Effectiveness of Herpetofaunal Sampling Techniques across a Gradient of Habitat Change in a Tropical Forest Landscape. J. Herpetol. 2008, 42, 733. [Google Scholar] [CrossRef]
- Trimble, M.J.; van Aarde, R.J. A note on polyvinyl chloride (PVC) pipe traps for sampling vegetation-dwelling frogs in South Africa. Afr. J. Ecol. 2013. [Google Scholar] [CrossRef]
- Field, S.A.; Tyre, A.J.; Thorn, K.H.; O’Connor, P.J.; Possingham, H.P. Improving the efficiency of wildlife monitoring by estimating detectability: A case study of foxes (Vulpes vulpes) on the Eyre Peninsula, South Australia. Wildl. Res. 2005, 32, 253. [Google Scholar] [CrossRef]
- Sugai, L.S.M.; Silva, T.S.F.; Ribeiro, J.W., Jr.; Llusia, D. Terrestrial passive acoustic monitoring: Review and perspectives. BioScience 2019, 69, 15–25. [Google Scholar] [CrossRef]
- Acevado, M.A.; Villanueva-Rivera, L.J. Using automated digital recording systems as effective tools for the monitoring of birds and amphibians. Wildl. Soc. Bull. 2006, 34, 211–214. [Google Scholar] [CrossRef]
- Jorge, F.C.; Machado, C.G.; Siqueira da Cunha Nogueira, S.; Nogueira-Filho, S.L.G. The effectiveness of acoustic indices for forest monitoring in Atlantic rainforest fragments. Ecol. Indic. 2018, 91, 71–76. [Google Scholar] [CrossRef]
- Schindler, A.R.; Gerber, J.E.; Quinn, J.E. An ecoacoustic approach to understand the effects of human sound on soundscapes and avian communication. Biodiversity 2020, 21, 15–27. [Google Scholar] [CrossRef]
- Caldwell, K.L.; Carter, T.C.; Doll, J.C. A comparison of bat activsity in a managed central hardwood forest. Am. Midl. Nat. 2019, 181, 225–244. [Google Scholar] [CrossRef]
- MacLaren, A.R.; Crump, P.S.; Forstner, M.R. Optimizing the power of human performed audio surveys for monitoring the endangered Houston toad using automated recording devices. PeerJ 2021, 9, 119–135. [Google Scholar] [CrossRef]
- Quinn, J.E.; Brandle, J.R.; Johnson, R.J.; Tyre, A.J. Application of detectability in the use of indicator species: A case study with birds. Ecol. Indic. 2011, 11, 1413–1418. [Google Scholar] [CrossRef]
- Quinn, J.E.; Schindler, A.E.; Blake, L.; Schaffer, S.K.; Hyland, E. Loss of winter wonderland: Proximity to different road types has variable effects on winter soundscapes. Landsc. Ecol. 2021, 37, 381–391. [Google Scholar] [CrossRef]
- Allen-Ankins, S.; McKnight, D.T.; Nordberg, E.J.; Hoefer, S.; Roe, P.; Watson, D.M.; McDonald, P.G.; Fuller, R.A.; Schwarzkopf, L. Effectiveness of acoustic indices as indicators of vertebrate biodiversity. Ecol. Indic. 2023, 147, 109937. [Google Scholar] [CrossRef]
- Brown, M.G.; Quinn, J.E. Zoning does not improve the availability of ecosystem services in urban watersheds. A case study from Upstate South Carolina, USA. Ecosyst. Serv. 2018, 34, 254–265. [Google Scholar] [CrossRef]
- Boughton, R.G.; Staiger, J.; Franz, R. Use of PVC Pipe Refugia as a Sampling Technique for Hylid Treefrogs. Am. Midl. Nat. 2000, 144, 168–177. [Google Scholar] [CrossRef]
- Dorcas, M.E.; Gibbons, W. Frogs & Toads of the Southeast; University of Georgia Press: Athens, GA, USA, 2008; Volume 264. [Google Scholar]
- Sidie-Slettedahl, A.M.; Jensen, K.C.; Johnson, R.R.; Arnold, T.W.; Austin, J.E.; Stafford, J.D. Evaluation of autonomous recording units for detecting 3 species of secretive marsh birds. Wildl. Soc. Bull. 2015, 39, 626–634. [Google Scholar] [CrossRef]
- Campos-Cerqueira, M.; Aide, T.M. Improving distribution data of threatened species by combining acoustic monitoring and occupancy modeling. Methods Ecol. Evol. 2016, 7, 1340–1348. [Google Scholar] [CrossRef]
- Ligges, U.; Krey, S.; Mersmann, O.; Schnackenberg, S. tuneR: Analysis of Music and Speech. 2018. Available online: https://CRAN.R-project.org/package=tuneR (accessed on 6 October 2023).
- Villanueva-Rivera, L.J.; Pijanowski, B.C. Soundecology: Soundscape Ecology. R Package Version 1.3.3. 2018. Available online: https://CRAN.R-project.org/package=soundecology (accessed on 6 October 2023).
- Villanueva-Rivera, L.J.; Pijanowski, B.C.; Doucette, J.; Pekin, B. A primer of acoustic analysis for landscape ecologists. Landsc. Ecol. 2011, 26, 1233. [Google Scholar] [CrossRef]
- Sueur, J.; Aubin, T.; Simonis, C. Seewave: A free modular tool for sound analysis and synthesis. Bioacoustics 2008, 18, 213–226. [Google Scholar] [CrossRef]
- Kasten, E.P.; Gage, S.H.; Fox, J.; Joo, W. The remote environmental assessment laboratory’s acoustic library: An archive for studying soundscape ecology. Ecol. Inform. 2012, 12, 50–67. [Google Scholar] [CrossRef]
- Pieretti, N.; Farina, A.; Morri, D. A new methodology to infer the singing activity of an avian community: The Acoustic Complexity Index (ACI). Ecol. Indic. 2011, 11, 868–873. [Google Scholar] [CrossRef]
- Boelman, N.T.; Asner, G.P.; Hart, P.J.; Martin, R.E. Multitrophic invasion resistance in Hawaii: Bioacoustics, field surveys, and airborne remote sensing. Ecol. Appl. 2007, 17, 2137–2144. [Google Scholar] [CrossRef]
- Hyland, E.B.; Schulz, A.; Quinn, J.E. Quantifying the Soundscape: How filters change acoustic indices. Ecol. Indic. 2023, 148, 110061. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; Francois, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Beninde, J.; Delaney, T.W.; Gonzalez, G.H.; Shaffer, B. Harnessing iNaturalist to quantify hotspots of urban biodiversity: The Los Angeles case study. Front. Ecol. Evol. 2023, 11, 983371. [Google Scholar] [CrossRef]
- Martin, F. PVC Pipe Samplers for Hylid Frogs: A Cautionary Note; Herpetological Natural History; US Department of Energy Environmental Services and Technology Department: Oak Ridge, TN, USA, 2004. [Google Scholar]

| Cope’s Gray Treefrog | Green Frog | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | p Value | Estimate | Std. Error | p Value | Estimate | Std. Error | p Value | |
| Intercept | 0.243 | 0.120 | 2.599 | 0.139 | 0.054 | 0.035 | |||
| Water (%) | −0.029 | 0.024 | 0.250 | −0.001 | 0.001 | 0.800 | 0.000 | 0.001 | 0.800 |
| Forest (%) | 0.000 | 0.002 | 0.920 | −0.026 | 0.001 | 0.0003 | −0.001 | 0.001 | 0.320 |
| Developed (%) | 0.001 | 0.003 | 0.810 | −0.026 | 0.001 | 0.0003 | 0.001 | 0.001 | 0.255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnes, I.L.; Quinn, J.E. Passive Acoustic Sampling Enhances Traditional Herpetofauna Sampling Techniques in Urban Environments. Sensors 2023, 23, 9322. https://doi.org/10.3390/s23239322
Barnes IL, Quinn JE. Passive Acoustic Sampling Enhances Traditional Herpetofauna Sampling Techniques in Urban Environments. Sensors. 2023; 23(23):9322. https://doi.org/10.3390/s23239322
Chicago/Turabian StyleBarnes, Isabelle L., and John E. Quinn. 2023. "Passive Acoustic Sampling Enhances Traditional Herpetofauna Sampling Techniques in Urban Environments" Sensors 23, no. 23: 9322. https://doi.org/10.3390/s23239322
APA StyleBarnes, I. L., & Quinn, J. E. (2023). Passive Acoustic Sampling Enhances Traditional Herpetofauna Sampling Techniques in Urban Environments. Sensors, 23(23), 9322. https://doi.org/10.3390/s23239322






