Wrist-Worn Sensor Validation for Heart Rate Variability and Electrodermal Activity Detection in a Stressful Driving Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
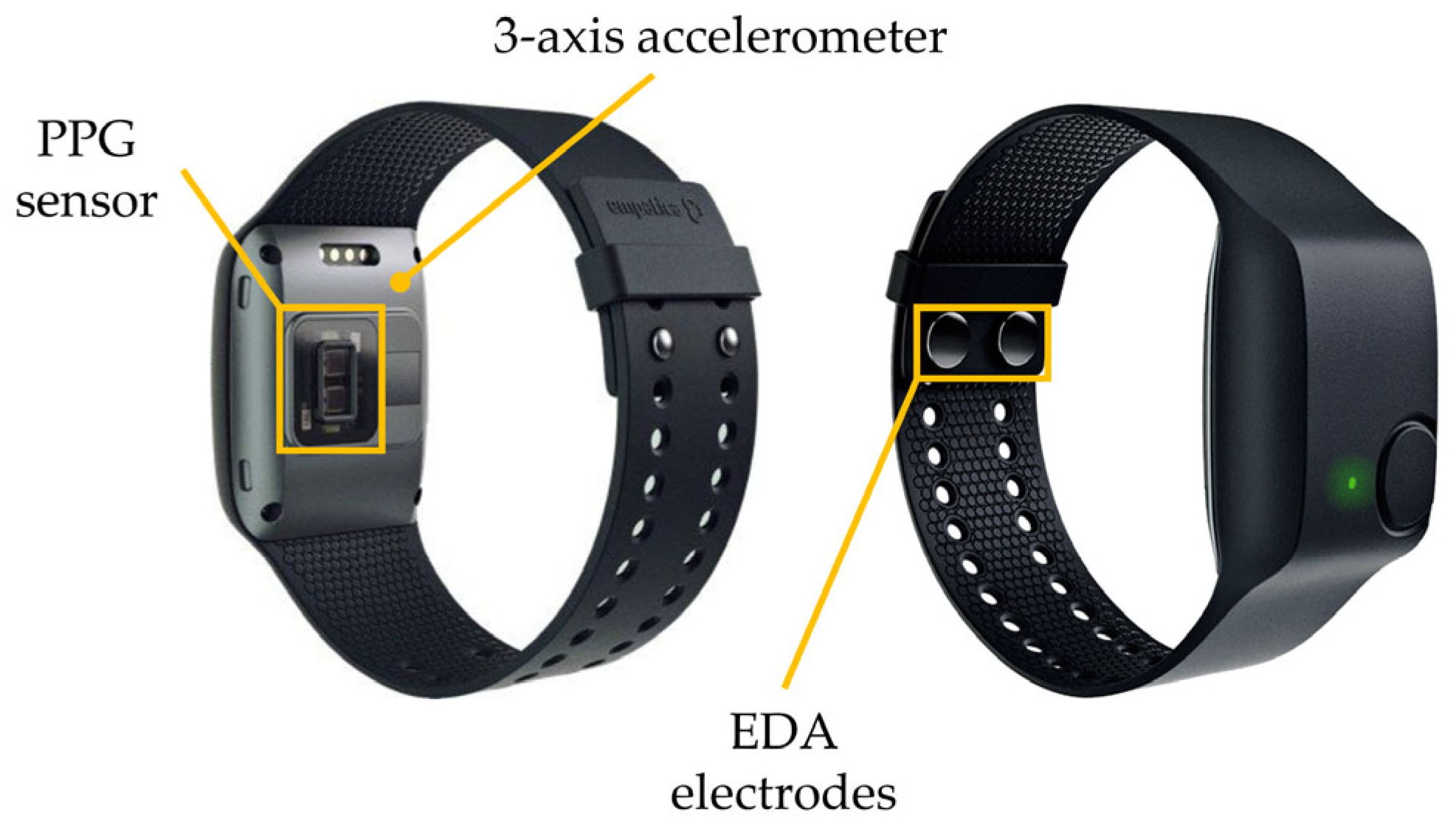
2.2. Recording Devices
2.3. Procedure
2.4. Experimental Conditions
- Baseline: Participants were asked to sit and relax for 3 min in front of a black computer screen, keeping their hands on their legs or the armrests and their eyes closed. The researcher also recommended to the participant not to speak and to stay still as much as possible.
- Emotional stimuli, divided into Video Clip and Scream:
- ○
- Video Clip: Ad hoc videos eliciting strong emotions (i.e., the fear) and based on a validated database [23] were selected and administered to the participants. Specifically, three scenes, each belonging to a different movie and with a length of 4 min, were edited into a single video clip with a total length of about 12 min.
- ○
- Scream: A sudden emotional event composed of a scream accompanied by the image of a frightening face was added at the end of the video clip. Specifically, this condition started a few seconds before the occurrence of the stimulus and lasted about 2 to 3 min. A short break was scheduled after this section to allow the researcher to change the position of the EDA sensors according to the participant’s randomization group.
- Driving videos: The last stage of the experimental protocol consisted of three driving simulations of increasing arousal and potential movement artifacts. The participant was given a steering wheel, a pedalboard, and a gear shifter and was asked to watch the driving videos and follow the main road while simulating the typical gestures of driving (i.e., steering, accelerating, braking, and shifting gears when an acoustic signal was heard). The driving videos had an overall duration of 10 min and 30 s (i.e., 3 min and 30 s per driving video), which were divided as follows:
- ○
- No-Risk Driving: The driving scenario depicted a mountain landscape with no potential hazards or risks;
- ○
- Low-Risk Driving: It was composed of an accurate selection of several shorter clips extracted from the hazard perception video clips, a British state-of-the-art test for assessing drivers’ skills in risk detection and driving behavior [24];
- ○
- High-Risk Driving: This last scenario reproduced potentially dangerous driving simulations, where the drivers’ lack of attention could be harmful.
2.5. Data Processing
2.6. Acceleration Magnitude Signal
2.7. HRV Analysis
2.8. EDA Analysis
2.9. Statistical Analyses
3. Results
3.1. Signals Quality
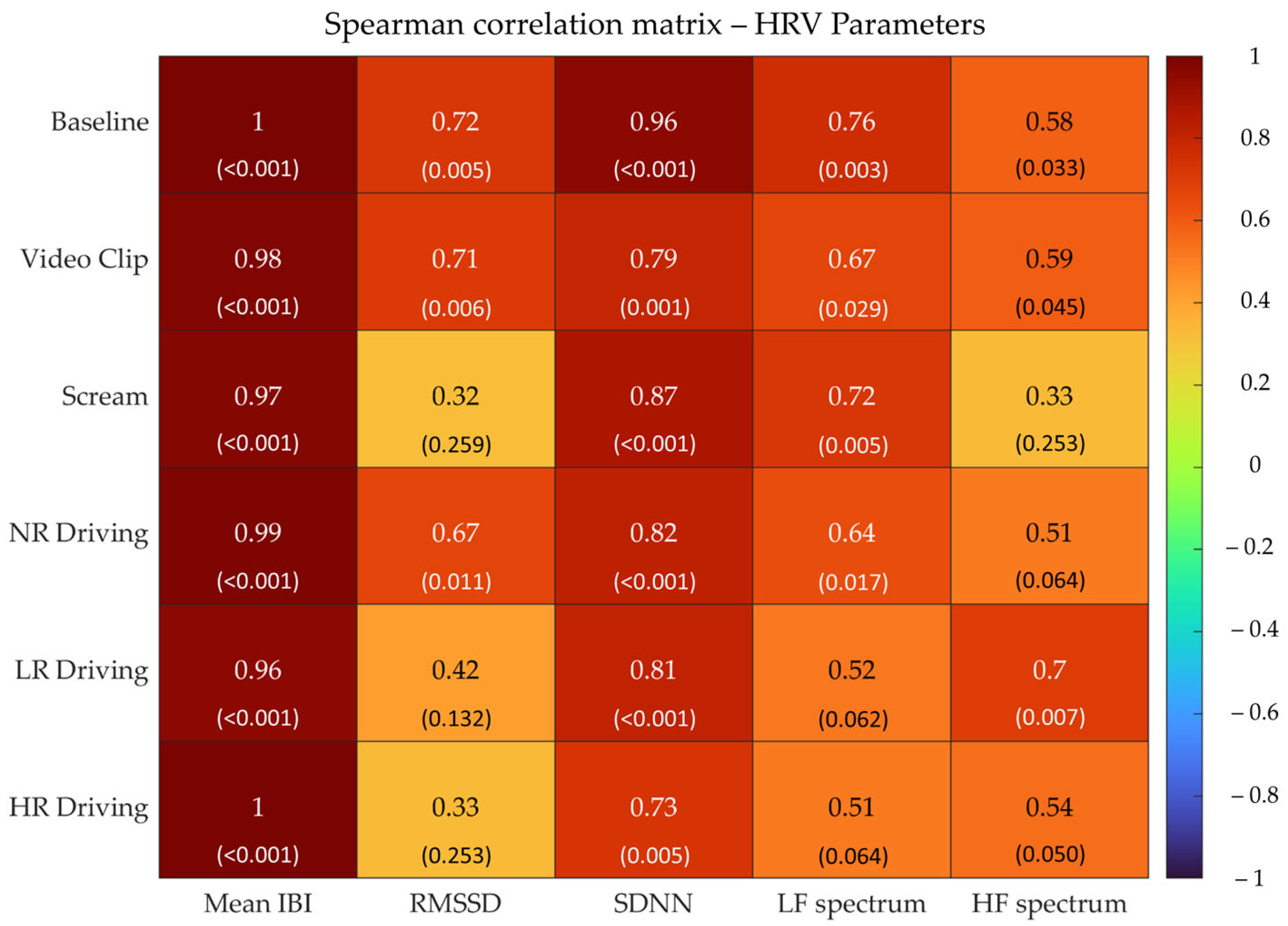
3.2. Correlation Analyses
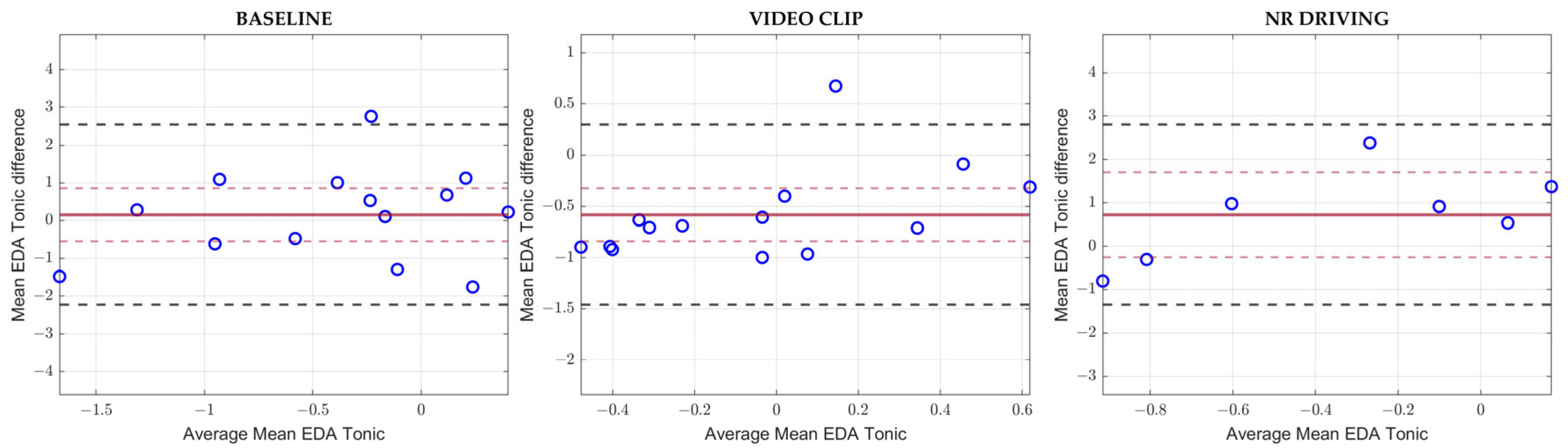
3.3. Bland–Altman Analyses
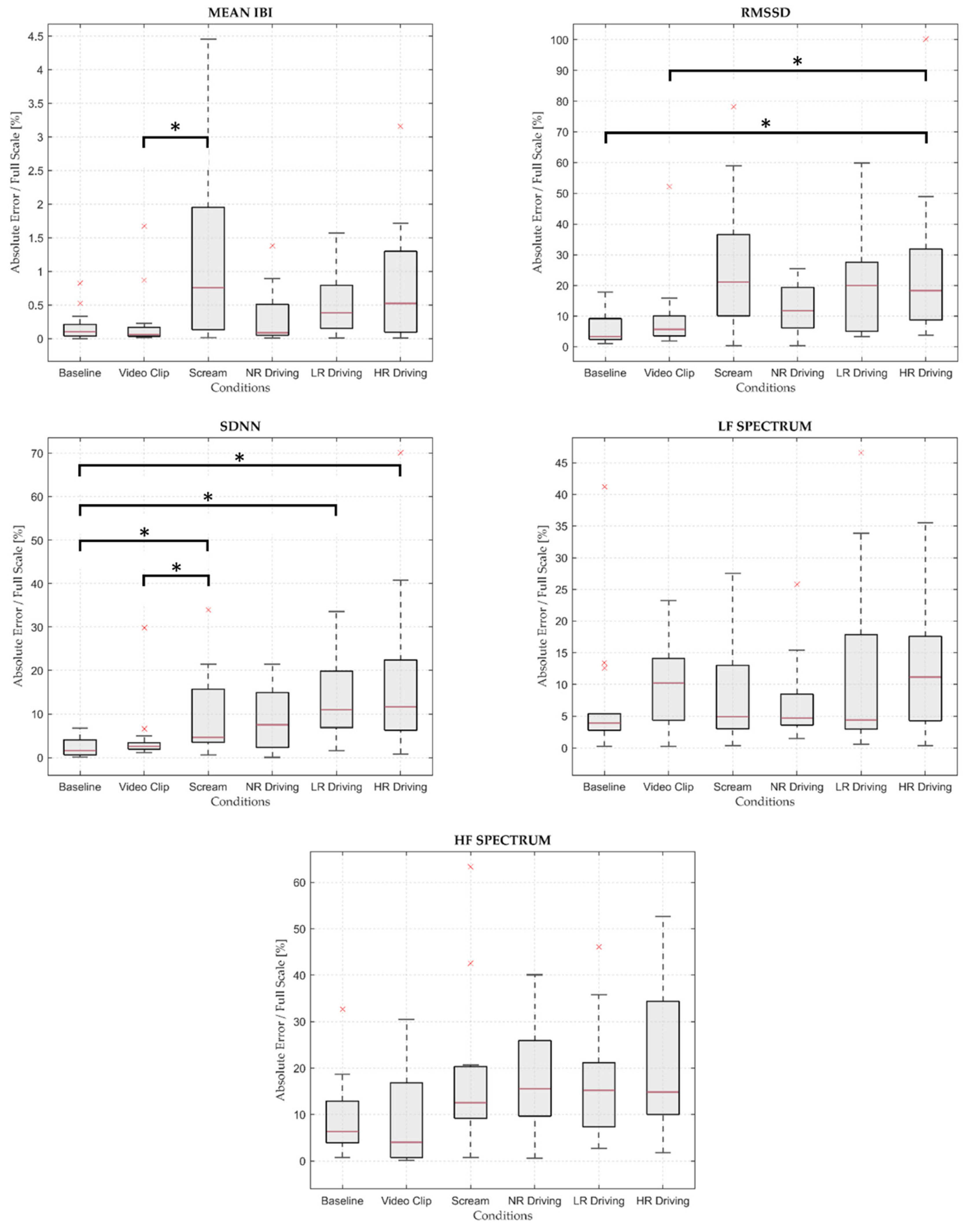
3.4. Friedman Test
4. Discussion
4.1. Heart Rate Variability
4.2. Electrodermal Activity
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bitkina, O.V.; Kim, J.; Park, J.; Park, J.; Kim, H.K. Identifying traffic context using driving stress: A longitudinal preliminary case study. Sensors 2019, 19, 2152. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.; Dorn, L.; Hoyes, T.W.; Davies, D.R.; Glendon, A.I.; Taylor, R.G. Driver Stress and Performance on a Driving Simulator. Hum. Factors 1998, 40, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.D.; Boyle, L.N. Driver stress as influenced by driving maneuvers and roadway conditions. Transp. Res. Part F Traffic Psychol. Behav. 2007, 10, 177–186. [Google Scholar] [CrossRef]
- Paschalidis, E.; Choudhury, C.F.; Hess, S. Combining driving simulator and physiological sensor data in a latent variable model to incorporate the effect of stress in car-following behaviour. Anal. Methods Accid. Res. 2019, 22, 100089. [Google Scholar] [CrossRef]
- Zontone, P.; Affanni, A.; Bernardini, R.; Piras, A.; Rinaldo, R. Stress Detection Through Electrodermal Activity(EDA) and Electrocardiogram (ECG) Analysis inCar Drivers. In Proceedings of the 27th European Signal Processing Conference (EUSIPCO), A Coruña, Spain, 2–6 September 2017. [Google Scholar]
- Deng, Y.; Wu, Z.; Chu, C.H.; Zhang, Q.; Hsu, D.F. Sensor feature selection and combination for stress identification using combinatorial fusion. Int. J. Adv. Robot. Syst. 2013, 10, 306. [Google Scholar] [CrossRef]
- Healey, J.A.; Picard, R.W. Detecting stress during real-world driving tasks using physiological sensors. IEEE Trans. Intell. Transp. Syst. 2005, 6, 156–166. [Google Scholar] [CrossRef]
- Tagliabue, M.; Sarlo, M. Affective components in training to ride safely using a moped simulator. Transp. Res. Part F Traffic Psychol. Behav. 2015, 35, 132–138. [Google Scholar] [CrossRef]
- Gianfranchi, E. A Tool for Training Hazard Perception and for Assessing Driving Behaviors in Adolescents and Inexperienced Drivers: A Simulation Study. Ph.D. Thesis, Università degli Studi di Padova, Padova, Italy, 2019. [Google Scholar]
- Crundall, D.; Underwood, G. Visual Attention while Driving: Measures of Eye Movements Used in Driving Research. In Handbook of Traffic Psychology; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 137–148. [Google Scholar] [CrossRef]
- Kinnear, N.; Kelly, S.W.; Stradling, S.; Thomson, J. Understanding how drivers learn to anticipate risk on the road: A laboratory experiment of affective anticipation of road hazards. Accid. Anal. Prev. 2013, 50, 1025–1033. [Google Scholar] [CrossRef]
- Horswill, M.S.; Mckenna, F.P. Drivers’ hazard perception ability: Situation awareness on the road. In A Cognitive Approach to Situation Awareness; Ashgate: Aldershot, UK, 2004; pp. 155–175. [Google Scholar]
- Minen, M.; Gregoret, L.; Seernani, D.; Wilson, J. Systematic Evaluation of Driver’s Behavior: A Multimodal Biometric Study. In Communications in Computer and Information Science; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2023; pp. 57–64. [Google Scholar] [CrossRef]
- Affanni, A. Wireless sensors system for stress detection by means of ECG and EDA acquisition. Sensors 2020, 20, 2026. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. NeuroEng. Rehabil. 2012, 9, 1–17. [Google Scholar] [CrossRef]
- Mccarthy, C.; Pradhan, N.; Redpath, C.; Adler, A. Validation of the Empatica E4 Wristband. In Proceedings of the IEEE EMBS International Student Conference (ISC), Ottawa, ON, Canada, 29–31 May 2016. [Google Scholar]
- Borrego, A.; Latorre, J.; Alcaniz, M.; Llorens, R. Reliability of the Empatica E4 wristband to Measure Electrodermal Activity to Emotional Stimuli. In Proceedings of the 2019 International Conference on Virtual Rehabilitation (ICVR), Magdeburg, Germany, 22–24 November 2019. [Google Scholar] [CrossRef]
- Milstein, N.; Gordon, I. Validating Measures of Electrodermal Activity and Heart Rate Variability Derived from the Empatica E4 Utilized in Research Settings That Involve Interactive Dyadic States. Front. Behav. Neurosci. 2020, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Gianfranchi, E.; Cellini, N.; Patron, E.; Tagliabue, M.; Sarlo, M. Stressing the accuracy: Wrist-worn wearable sensor validation over different conditions. Psychophysiology 2019, 56, e13441. [Google Scholar] [CrossRef] [PubMed]
- Stuyck, H.; Costa, L.D.; Cleeremans, A.; Van den Bussche, E. Validity of the Empatica E4 wristband to estimate resting-state heart rate variability in a lab-based context. Int. J. Psychophysiol. 2022, 182, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Gruden, T.; Stojmenova, K.; Sodnik, J.; Jakus, G. Assessing drivers’ physiological responses using consumer grade devices. Appl. Sci. 2019, 9, 5353. [Google Scholar] [CrossRef]
- Van Dooren, M.; de Vries, J.J.G.G.J.; Janssen, J.H. Emotional sweating across the body: Comparing 16 different skin conductance measurement locations. Physiol. Behav. 2012, 106, 298–304. [Google Scholar] [CrossRef]
- Grayson, G.B.; Sexton, B.F. The Development of Hazard Perception Testing Prepared for Road Safety Division, Department for Transport; TRL: Villenave-d’Ornon, France, 2002. [Google Scholar]
- McKenna, F.P.; Crick, J.L. Hazard Perception in Drivers: A Methodology for Testing and Training; Transport and Road Research Laboratory (TRRL): London, UK, 1994. [Google Scholar]
- Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar]
- Schuster, A. On The Investigation of Hidden Periodicities with Application to a Supposed 26 Day Period of Metheorological Phenomena. Terr. Magn. 1898, 3, 13–41. [Google Scholar]
- Berntson, G.G.; Quigley, K.S.; Jang, J.F.; Boysen, S.T. An Approach to Artifact Identification: Application to Heart Period Data. Psychophysiology 1990, 27, 586–598. [Google Scholar] [CrossRef]
- Lomb, N.R. Least-Squares Frequency Analysis of Unequally Spaced Data. Astrophys. Space Sci. 1976, 39, 447–462. [Google Scholar] [CrossRef]
- Scargle, J.D. Studies in astronomical time series analysis. II. statistical aspects of spectral analysis of unevenly spaced data. Astrophys. J. 1982, 263, 835–853. [Google Scholar] [CrossRef]
- De Geus, E.J.C.; Gianaros, P.J.; Brindle, R.C.; Jennings, J.R.; Berntson, G.G. Should heart rate variability be ‘corrected’ for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology 2019, 56, e13287. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, e13287. [Google Scholar] [CrossRef] [PubMed]
- Aqajari, S.A.H.; Labbaf, S.; Naeini, E.K.; Rahmani, A.M.; Mehrabadi, M.A.; Dutt, N. GSR Analysis for Stress: Development and Validation of an Open Source Tool for Noisy Naturalistic GSR Data. arXiv 2020, arXiv:2005.01834. [Google Scholar]
- Greco, A.; Valenza, G.; Lanata, A.; Scilingo, E.P.; Citi, L. CvxEDA: A convex optimization approach to electrodermal activity processing. IEEE Trans. Biomed. Eng. 2016, 63, 797–804. [Google Scholar] [CrossRef]
- Greco, A.; Valenza, G.; Citi, L.; Scilingo, E.P. Arousal and valence recognition of affective sounds based on electrodermal activity. IEEE Sens. J. 2017, 17, 716–725. [Google Scholar] [CrossRef]
- Chen, W.; Jaques, N.; Taylor, S.; Sano, A.; Fedor, S.; Picard, R.W. Wavelet-Based Motion Artifact Removal for Electrodermal Activity. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015. [Google Scholar] [CrossRef]
- Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures; Boucsein, W.; Fowles, D.C.; Grimnes, S.; Ben-Shakhar, G.; Roth, W.T.; Dawson, M.E.; Filion, D.L. Publication recommendations for electrodermal measurements. Psychophysiology 2012, 49, 1017–1034. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Florian, J.P.; Orjuela-Cañón, A.D.; Aljama-Corrales, T.; Charleston-Villalobos, S.; Chon, K.H. Power Spectral Density Analysis of Electrodermal Activity for Sympathetic Function Assessment. Ann. Biomed. Eng. 2016, 44, 3124–3135. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 1988. [Google Scholar]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Euser, A.M.; Dekker, F.W.; le Cessie, S. A practical approach to Bland-Altman plots and variation coefficients for log transformed variables. J. Clin. Epidemiol. 2008, 61, 978–982. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. Trust An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Costantini, S.; Storm, F. Wrist-worn sensor validation for heart rate variability and electrodermal activity detection in a stressful driving environment. Zenodo 2023. [Google Scholar] [CrossRef]
- Reali, P.; Lolatto, R.; Coelli, S.; Tartaglia, G.; Bianchi, A.M. Information Retrieval from Photoplethysmographic Sensors: A Comprehensive Comparison of Practical Interpolation and Breath-Extraction Techniques at Different Sampling Rates. Sensors 2022, 22, 1428. [Google Scholar] [CrossRef] [PubMed]


| Condition | Mean Number of Detected IBIs | Mean Detection Rate (%) (SD) | Mean Artifact Rate (%) (SD) | ||
|---|---|---|---|---|---|
| Standard | E4 | Standard | E4 | ||
| Baseline | 166 | 165 | 99.80% (0.34%) | 0.84% (2.38%) | 0.64% (0.88%) |
| Video Clip | 378 | 378 | 99.58% (0.60%) | 0.31% (1.07%) | 0.85% (1.58%) |
| Scream | 135 | 132 | 98.03% (1.89%) | 0.09% (0.22%) | 1.07% (2.64%) |
| NR Driving | 203 | 201 | 99.18% (2.00%) | 0.15% (0.56%) | 1.38% (1.85%) |
| LR Driving | 192 | 192 | 99.32% (0.83%) | 0.42% (1.31%) | 3.16% (2.28%) |
| HR Driving | 174 | 174 | 98.51% (1.53%) | 0.54% (1.08%) | 2.9% (2.82%) |
| System | Baseline | Video Clip | Scream | NR Driving | LR Driving | HR Driving |
|---|---|---|---|---|---|---|
| Shoulder GS | 50% | 43% | 21% | 43% | 43% | 57% |
| Fingers GS | 29% | 0% | 7% | 43% | 43% | 29% |
| E4 | 36% | 14% | 7% | 43% | 14% | 14% |
| Condition | Bias | Bias CI 95% | p-Value (Bias) | Upper LOA | Lower LOA |
|---|---|---|---|---|---|
| Mean IBI | |||||
| Baseline | 1.70 | 1.53 | 0.03 | 6.89 | −3.49 |
| Video Clip | 1.07 | 3.16 | 0.48 | 11.80 | −9.66 |
| NR Driving | 0.52 | 3.15 | 0.73 | 11.23 | −10.19 |
| RMSSD | |||||
| Baseline | −1.07 | 4.19 | 0.59 | 13.14 | −15.29 |
| Video Clip | 3.18 | 8.88 | 0.45 | 33.34 | −26.97 |
| NR Driving | 8.19 | 6.92 | 0.02 | 31.67 | −15.28 |
| HRV LF Spectrum | |||||
| Baseline | 5.42 | 5.03 | 0.04 | 22.49 | −11.65 |
| Video Clip | 1.29 | 5.85 | 0.64 | 21.15 | −18.58 |
| NR Driving | −0.79 | 4.56 | 0.71 | 14.68 | −16.25 |
| Mean EDA Tonic | |||||
| Baseline | 0.15 | 0.70 | 0.64 | 2.54 | −2.23 |
| Video Clip | −0.58 | 0.26 | <0.001 | 0.30 | −1.46 |
| NR Driving | 0.72 | 0.98 | 0.12 | 2.80 | −1.35 |
| HRV Parameter | Chi-Squared | Significance | Decision |
|---|---|---|---|
| Mean IBI | 18.163 | 0.003 | Reject H0 |
| RMSSD | 21.306 | 0.001 | Reject H0 |
| SDNN | 25.918 | <0.001 | Reject H0 |
| LF Spectrum | 4.898 | 0.428 | Mantain H0 |
| HF Spectrum | 10.449 | 0.063 | Mantain H0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, S.; Chiappini, M.; Malerba, G.; Dei, C.; Falivene, A.; Arlati, S.; Colombo, V.; Biffi, E.; Storm, F.A. Wrist-Worn Sensor Validation for Heart Rate Variability and Electrodermal Activity Detection in a Stressful Driving Environment. Sensors 2023, 23, 8423. https://doi.org/10.3390/s23208423
Costantini S, Chiappini M, Malerba G, Dei C, Falivene A, Arlati S, Colombo V, Biffi E, Storm FA. Wrist-Worn Sensor Validation for Heart Rate Variability and Electrodermal Activity Detection in a Stressful Driving Environment. Sensors. 2023; 23(20):8423. https://doi.org/10.3390/s23208423
Chicago/Turabian StyleCostantini, Simone, Mattia Chiappini, Giorgia Malerba, Carla Dei, Anna Falivene, Sara Arlati, Vera Colombo, Emilia Biffi, and Fabio Alexander Storm. 2023. "Wrist-Worn Sensor Validation for Heart Rate Variability and Electrodermal Activity Detection in a Stressful Driving Environment" Sensors 23, no. 20: 8423. https://doi.org/10.3390/s23208423
APA StyleCostantini, S., Chiappini, M., Malerba, G., Dei, C., Falivene, A., Arlati, S., Colombo, V., Biffi, E., & Storm, F. A. (2023). Wrist-Worn Sensor Validation for Heart Rate Variability and Electrodermal Activity Detection in a Stressful Driving Environment. Sensors, 23(20), 8423. https://doi.org/10.3390/s23208423








