Microspherical Titanium-Phosphorus Double Oxide: Hierarchical Structure Development for Sensing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Electrochemical Measurements
2.3. Electrode Preparation
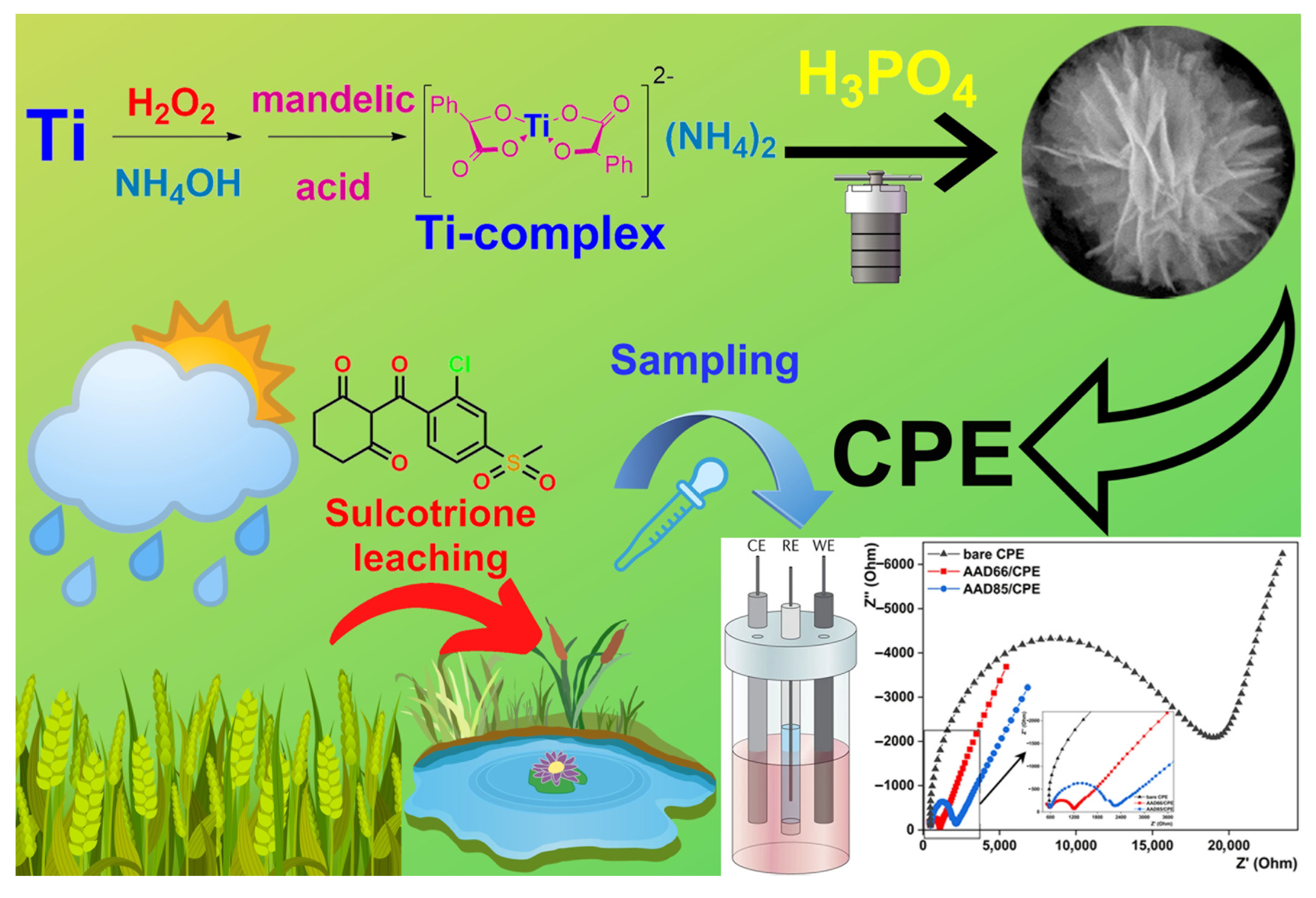
2.4. Synthesis of Ti-Complex with DL-Mandelic Acid
2.5. Synthesis of Microstructured Titanium-Phosphorus Double Oxide by Hydrothermal Method
2.6. Material Characterization
3. Results
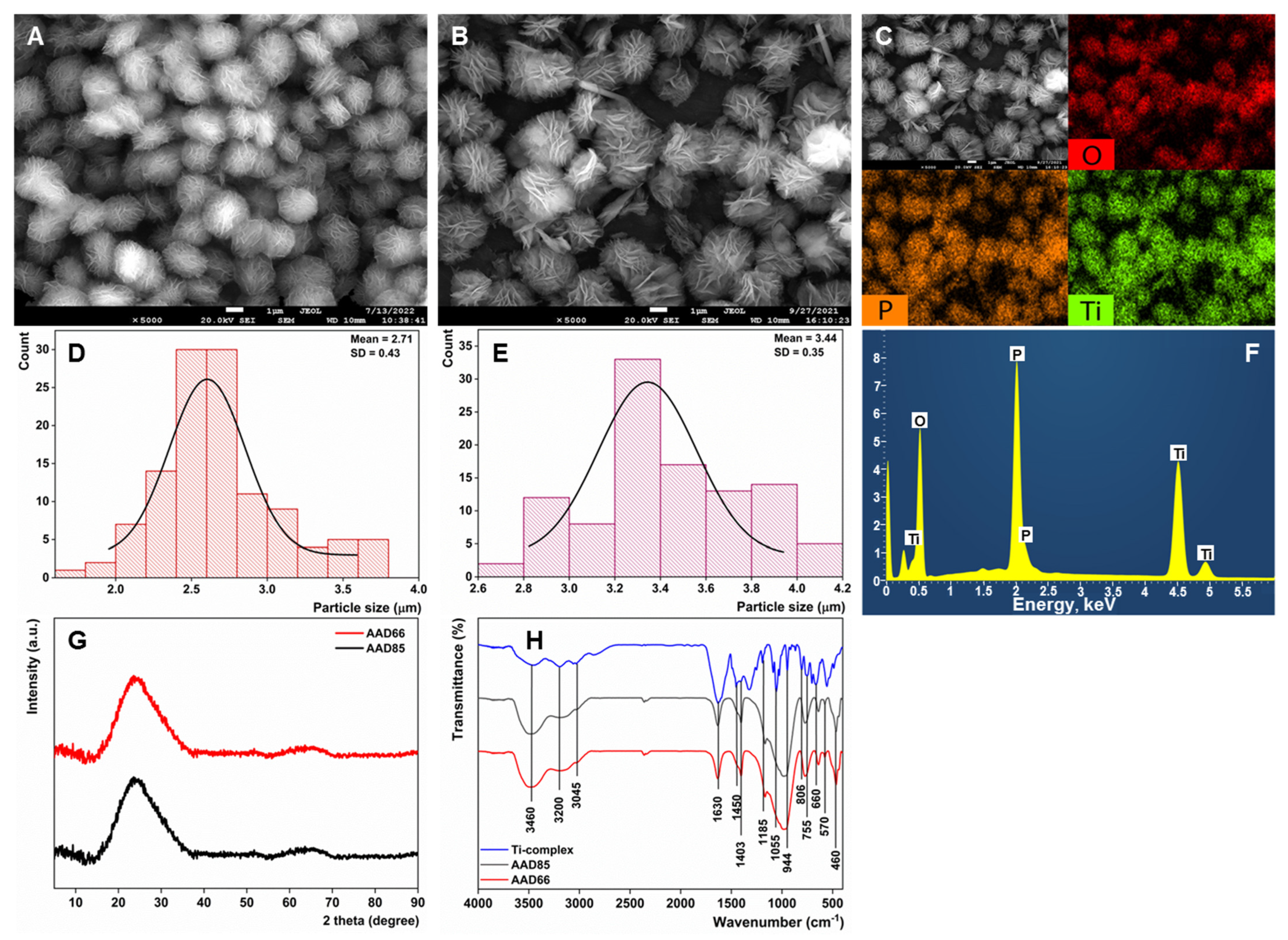
3.1. Material Characterization
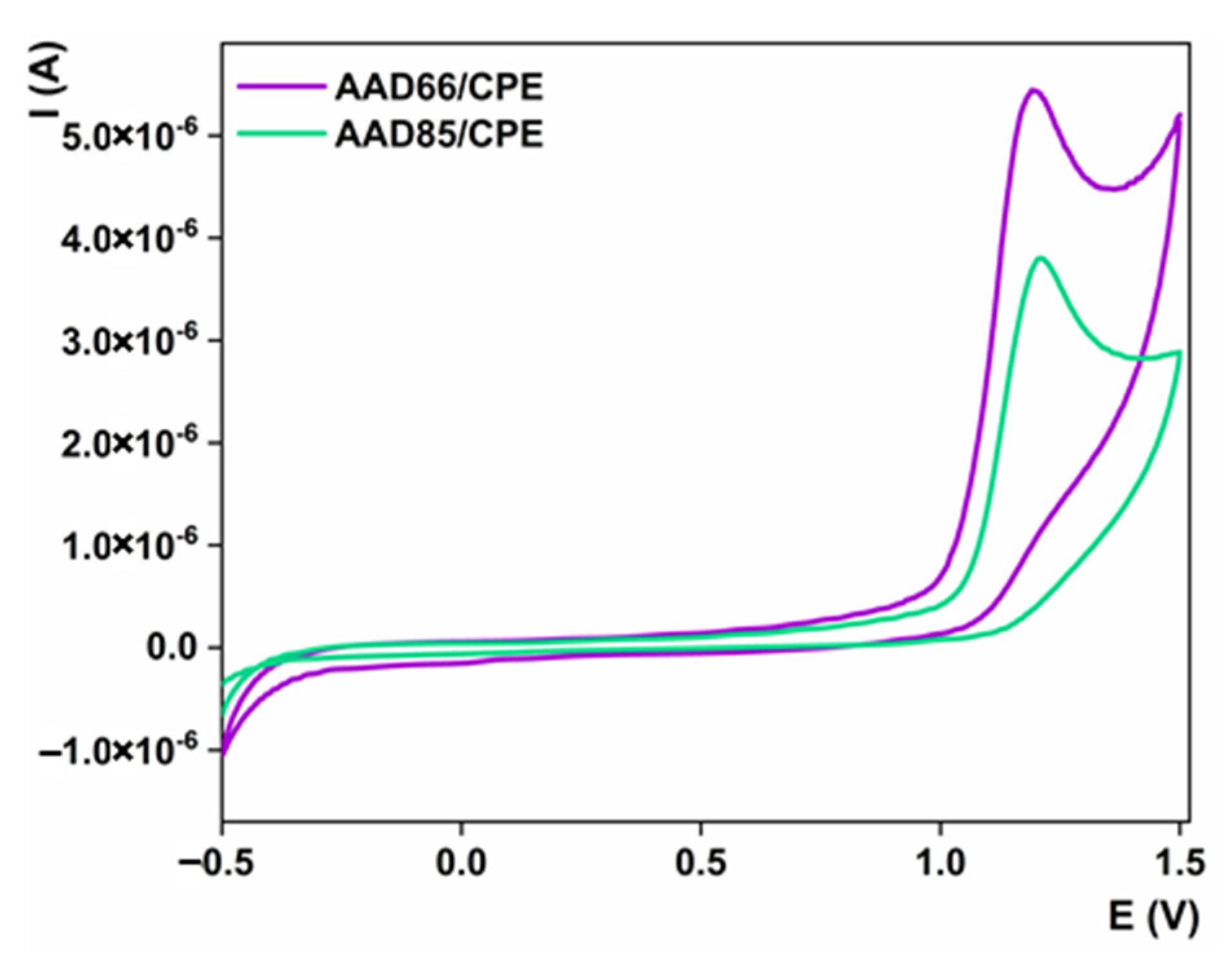
3.2. Electrocatalytic Properties of the Materials
3.3. Electrochemical Performance of Prepared Structures toward Sulcotrione
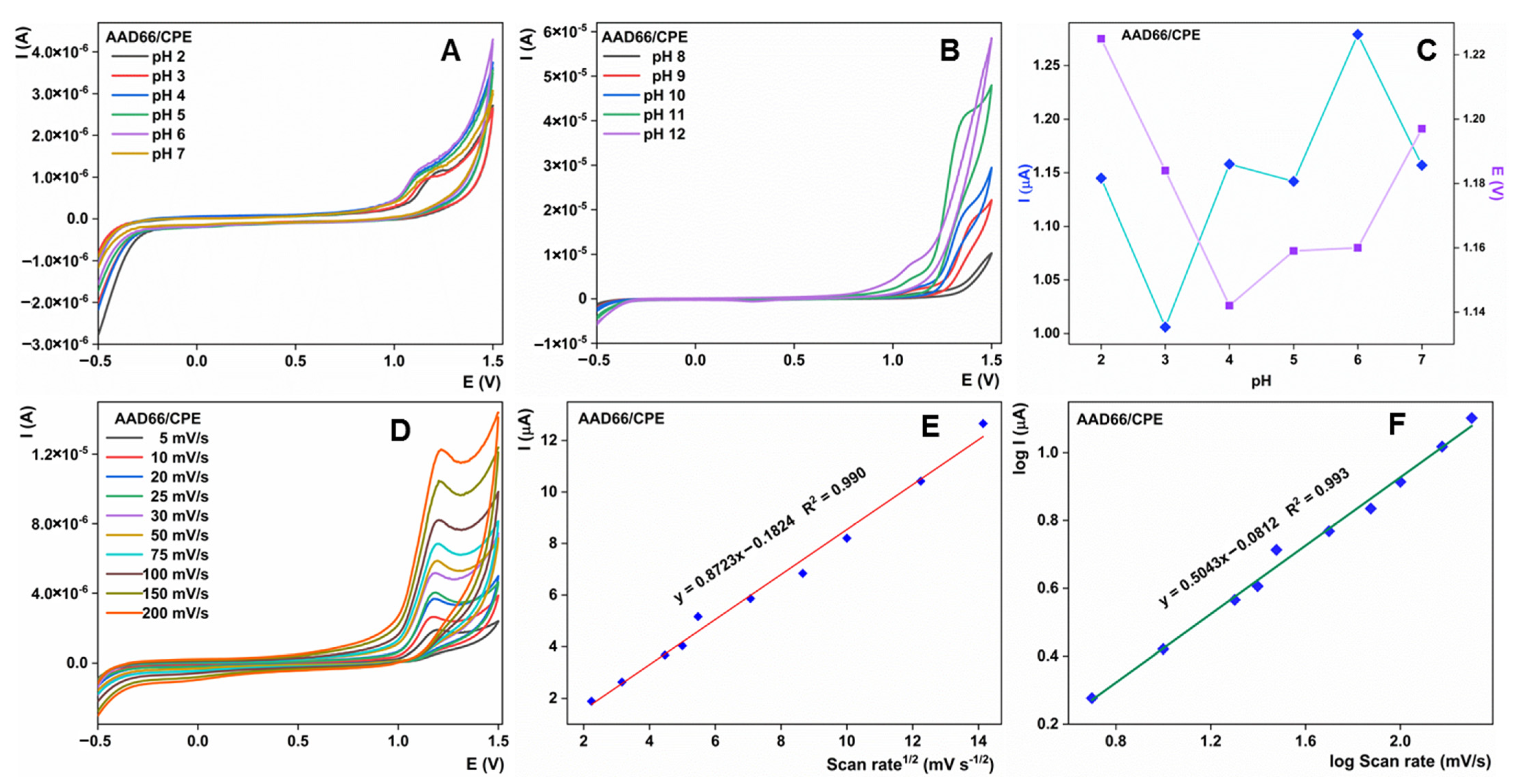
3.4. Effect of Various Parameters toward Sulcotrione Detection Using AAD66/CPE
3.5. Sulcotrione Determination on AAD66/CPE
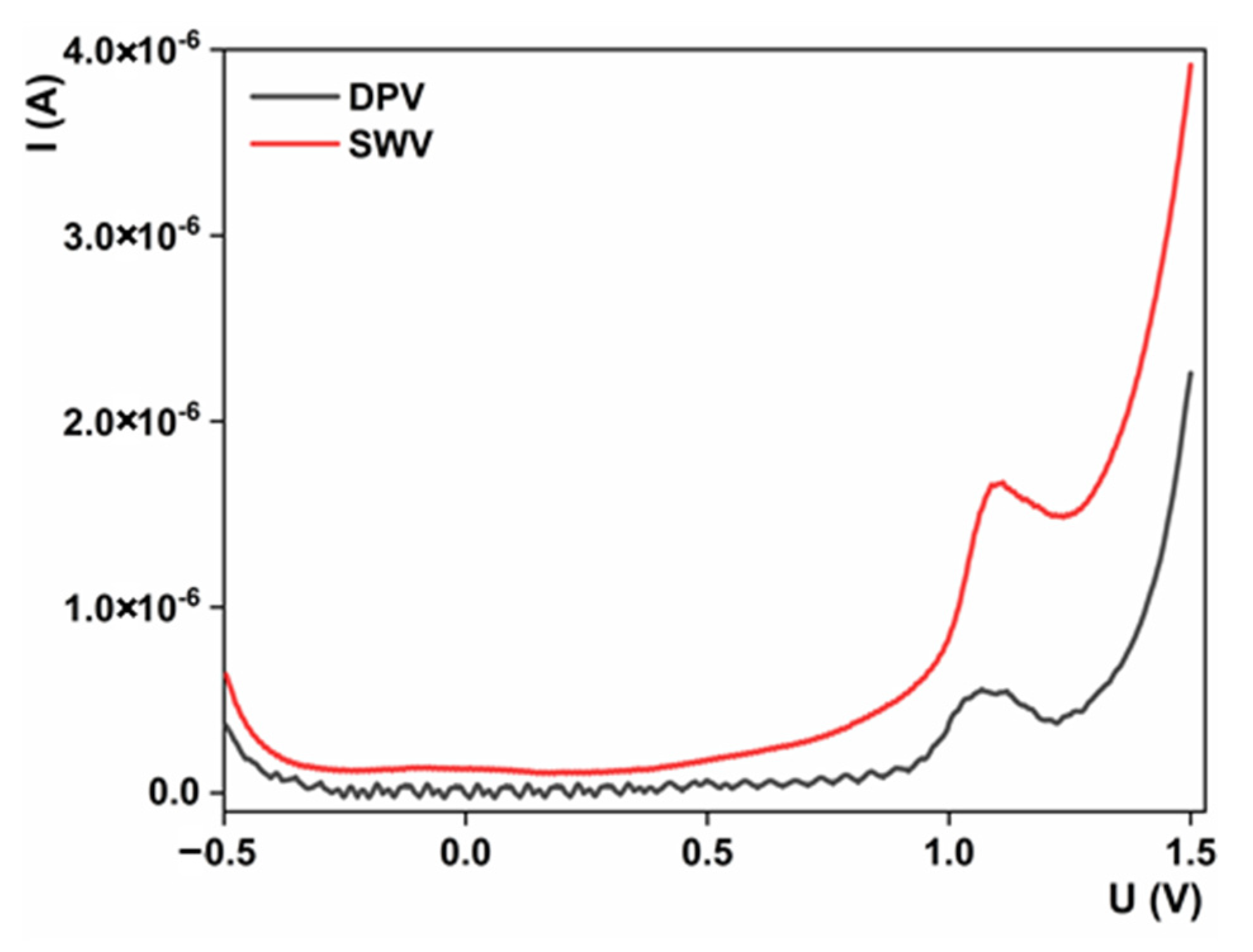
3.5.1. Method Selection and Optimization
3.5.2. Analytical Method Development
3.6. Interference Studies and Practical Applicability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koohsaryan, E.; Anbia, M. Nanosized and hierarchical zeolites: A short review. Chin. J. Catal. 2016, 37, 447–467. [Google Scholar] [CrossRef]
- Trogadas, P.; Ramani, V.; Strasser, P.; Fuller, T.F.; Coppens, M.-O. Hierarchically Structured Nanomaterials for Electrochemical Energy Conversion. Angew. Chem. Int. Ed. 2015, 55, 122–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, Z.-Y.; Su, B.-L. Hierarchically Structured Porous Materials for Energy Conversion and Storage. Adv. Funct. Mater. 2012, 22, 4634–4667. [Google Scholar] [CrossRef]
- Zhang, Q.; Myers, D.; Lan, J.; Jenekhe, S.A.; Cao, G. Applications of light scattering in dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2012, 14, 14982–14998. [Google Scholar] [CrossRef]
- Wu, S. An Overview of Hierarchical Design of Textile-Based Sensor in Wearable Electronics. Crystals 2022, 12, 555. [Google Scholar] [CrossRef]
- Thompson, B.R.; Horozov, T.S.; Stoyanov, S.D.; Paunov, V.N. Hierarchically structured composites and porous materials from soft templates: Fabrication and applications. J. Mater. Chem. A 2019, 7, 8030–8049. [Google Scholar] [CrossRef]
- Herrera-Beurnio, M.C.; Hidalgo-Carrillo, J.; López-Tenllado, F.J.; Martin-Gómez, J.; Estévez, R.C.; Urbano, F.J.; Marinas, A. Bio-Templating: An Emerging Synthetic Technique for Catalysts. A Review. Catalysts 2021, 11, 1364. [Google Scholar] [CrossRef]
- Berglund, L.A.; Burgert, I. Bioinspired Wood Nanotechnology for Functional Materials. Adv. Mater. 2018, 30, 1704285. [Google Scholar] [CrossRef]
- Jeffryes, C.; Campbell, J.; Li, H.; Jiao, J.; Rorrer, G. The potential of diatom nanobiotechnology for applications in solar cells, batteries, and electroluminescent devices. Energy Environ. Sci. 2011, 4, 3930–3941. [Google Scholar] [CrossRef]
- Vogel, N.; Retsch, M.; Fustin, C.-A.; del Campo, A.; Jonas, U. Advances in Colloidal Assembly: The Design of Structure and Hierarchy in Two and Three Dimensions. Chem. Rev. 2015, 115, 6265–6311. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Y.; Jin, S.; Zhao, C. Development of Core-Shell Nanoparticle Drug Delivery Systems Based on Biomimetic Mineralization. Chembiochem 2020, 21, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Matson, J.; Zha, R.; Stupp, S.I. Peptide self-assembly for crafting functional biological materials. Curr. Opin. Solid State Mater. Sci. 2011, 15, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Goujon, E.; Richard, C.; Goupil, P.; Ledoigt, G. Cytotoxicity on Allium cepa of the two main sulcotrione photoproducts, xanthene-1,9-dione-3,4-dihydro-6-methylsulphonyl and 2-chloro-4-mesylbenzoic acid. Pestic. Biochem. Physiol. 2015, 124, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Goujon, E.; Maruel, S.; Richard, C.; Goupil, P.; Ledoigt, G. Transformation of the Herbicide Sulcotrione into a Root Growth Enhancer Compound by Sequential Photolysis and Hydrolysis. J. Agric. Food Chem. 2016, 64, 563–569. [Google Scholar] [CrossRef]
- He, J.; Zheng, Y.; Chen, X.; Li, N.; Tang, F.; Huo, N.; Wang, J.; Gu, S. Development of an enzyme-linked immunosorbent assay for the detection of mebendazole in chicken and mutton. Anal. Methods 2021, 13, 1740–1746. [Google Scholar] [CrossRef]
- Liu, D.-L.; Li, Y.; Sun, R.; Xu, J.-Y.; Chen, Y.; Sun, C.-Y. Colorimetric Detection of Organophosphorus Pesticides Based on the Broad-Spectrum Aptamer. J. Nanosci. Nanotechnol. 2020, 20, 2114–2121. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Shin, H. Hierarchical Porous Carbon Electrodes with Sponge-Like Edge Structures for the Sensitive Electrochemical Detection of Heavy Metals. Sensors 2021, 21, 1346. [Google Scholar] [CrossRef]
- Hu, P.; Sun, Z.; Shen, Y.; Pan, Y. A Long-Term Stable Sensor Based on Fe@PCN-224 for Rapid and Quantitative Detection of H2O2 in Fishery Products. Foods 2021, 10, 419. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.; Qin, C.; Wang, Z.; Zhao, W.; Li, Y. Flexible Free-Standing CuxO/Ag2O (x = 1, 2) Nanowires Integrated with Nanoporous Cu-Ag Network Composite for Glucose Sensing. Nanomaterials 2020, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, S.; Wang, Y.; Zhang, J.; Yu, X.; Zhou, Y.; Zhang, J. Synthesis of Ni-Co Hydroxide Nanosheets Constructed Hollow Cubes for Electrochemical Glucose Determination. Sensors 2019, 19, 2938. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Wang, W.; Wang, Y.; Hu, X.; Liu, J.; Gong, X.; Miao, W.; Ding, L.; Li, X.; et al. Synthesis, modification and application of titanium dioxide nanoparticles: A review. Nanoscale 2022, 14, 6709–6734. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Nakayama, H.; Tsuhako, M. Intercalation of 2-Aminoethanethiol into Layered Titanium Phosphate and Its Adsorption of Heavy Metal Ions. Bull. Chem. Soc. Jpn. 2002, 75, 1991–1996. [Google Scholar] [CrossRef]
- Takahashi, H.; Oi, T.; Hosoe, M. Characterization of semicrystalline titanium(iv) phosphates and their selectivity of cations and lithium isotopes. J. Mater. Chem. 2002, 12, 2513–2518. [Google Scholar] [CrossRef]
- Liu, J.; Wei, X.; Yu, Y.; Song, J.; Wang, X.; Li, A.; Liu, X.-W.; Deng, W.-Q. Uniform core–shell titanium phosphate nanospheres with orderly open nanopores: A highly active Brønsted acid catalyst. Chem. Commun. 2010, 46, 1670–1672. [Google Scholar] [CrossRef]
- Sarkar, K.; Nandi, M.; Bhaumik, A. Enhancement in microporosity and catalytic activity on grafting silica and organosilica moieties in lamellar titanium phosphate framework. Appl. Catal. A Gen. 2008, 343, 55–61. [Google Scholar] [CrossRef]
- Santos-Peña, J.; Soudan, P.; Cruz-Yusta, M.; Franger, S. Increasing the electrochemical activity of transition metal phosphates in lithium cells by treatment with intimate carbon: The case of titanium phosphate. Electrochim. Acta 2006, 51, 4841–4849. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, R.; Wang, J.; Yu, J.; Lan, T.; Wang, W.; Yang, H.; Wu, H.; Deng, C. Promoting Effect of Layered Titanium Phosphate on the Electrochemical and Photovoltaic Performance of Dye-Sensitized Solar Cells. Nanoscale Res. Lett. 2010, 5, 1313–1319. [Google Scholar] [CrossRef]
- Knežević, S.; Ognjanović, M.; Stanković, V.; Zlatanova, M.; Nešić, A.; Gavrović-Jankulović, M.; Stanković, D. La(OH)3 Multi-Walled Carbon Nanotube/Carbon Paste-Based Sensing Approach for the Detection of Uric Acid—A Product of Environmentally Stressed Cells. Biosensors 2022, 12, 705. [Google Scholar] [CrossRef]
- Ognjanović, M.; Nikolić, K.; Bošković, M.; Pastor, F.; Popov, N.; Marciuš, M.; Krehula, S.; Antić, B.; Stanković, D.M. Electrochemical Determination of Morphine in Urine Samples by Tailoring FeWO4/CPE Sensor. Biosensors 2022, 12, 932. [Google Scholar] [CrossRef]
- Jia, K.; Pan, B.; Lv, L.; Zhang, Q.; Wang, X.; Pan, B.; Zhang, W. Impregnating titanium phosphate nanoparticles onto a porous cation exchanger for enhanced lead removal from waters. J. Colloid Interface Sci. 2009, 331, 453–457. [Google Scholar] [CrossRef]
- Kapnisti, M.; Noli, F.; Misaelides, P.; Vourlias, G.; Karfaridis, D.; Hatzidimitriou, A. Enhanced sorption capacities for lead and uranium using titanium phosphates; sorption, kinetics, equilibrium studies and mechanism implication. Chem. Eng. J. 2018, 342, 184–195. [Google Scholar] [CrossRef]
- Ortiz-Oliveros, H.B.; Flores-Espinosa, R.; Ordoñez-Regil, E.; Fernández-Valverde, S. Synthesis of α-Ti(HPO4)2 H2O and sorption of Eu (III). Chem. Eng. J. 2014, 236, 398–405. [Google Scholar] [CrossRef]
- Peng, W.; Du, S.; Shaoning, Z.; Xieyi, H.; Qingyuan, B.; Meng, Q.; Wei, Z.; Fuqiang, H. Constructing mesoporous phosphated titanium oxide for efficient Cr(III) removal. J. Hazard. Mater. 2020, 384, 121278. [Google Scholar] [CrossRef]
- Sahu, B.B.; Parida, K. Cation Exchange and Sorption Properties of Crystalline α-Titanium(IV) Phosphate. J. Colloid Interface Sci. 2002, 248, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Ban, T.; Asano, K.; Takai-Yamashita, C.; Ohya, Y. Bottom-up synthesis of titanophosphate nanosheets by the aqueous solution process. Nanoscale Adv. 2020, 2, 3542–3549. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, M.E.; Brito, J.L.; Bastardo-González, E.; Méndez, F.J. Adsorption properties of novel layered titanium phosphate prepared from mesoporous titania by sol–gel processing. J. Sol Gel Sci. Technol. 2020, 97, 431–440. [Google Scholar] [CrossRef]
- Jia, K.; Pan, B.; Zhang, Q.; Zhang, W.; Jiang, P.; Hong, C.; Pan, B.; Zhang, Q. Adsorption of Pb2+, Zn2+, and Cd2+ from waters by amorphous titanium phosphate. J. Colloid Interface Sci. 2008, 318, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, X.; Cai, J.; Miao, T.; Li, L.; Li, G.; Deng, D.; Jiang, L.; Wang, C. Novel flower-like titanium phosphate microstructures and their application in lead ion removal from drinking water. J. Mater. Chem. A 2014, 2, 6718–6722. [Google Scholar] [CrossRef]
- Guo, S.-Y.; Han, S. Constructing a novel hierarchical 3D flower-like nano/micro titanium phosphate with efficient hydrogen evolution from water splitting. J. Power Sources 2014, 267, 9–13. [Google Scholar] [CrossRef]
- Guo, S.-Y.; Han, S.; Chi, B.; Pu, J.; Li, J. Synthesis of shape-controlled mesoporous titanium phosphate nanocrystals: The hexagonal titanium phosphate with enhanced hydrogen generation from water splitting. Int. J. Hydrog. Energy 2014, 39, 2446–2453. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kato, H.; Kakihana, M. Synthesis of Titanium Dioxide Nanocrystals with Controlled Crystal- and Micro-Structures from Titanium Complexes. Nanomater. Nanotechnol. 2013, 3, 23. [Google Scholar] [CrossRef]
- Katsumata, K.-I.; Ohno, Y.; Tomita, K.; Sakai, M.; Nakajima, A.; Kakihana, M.; Fujishima, A.; Matsushita, N.; Okada, K. Preparation of TiO2 Thin Films Using Water-soluble Titanium Complexes and Their Photoinduced Properties. Photochem. Photobiol. 2011, 87, 988–994. [Google Scholar] [CrossRef]
- Truong, Q.D.; Dien, L.X.; Vo, D.-V.N.; Le, T.S. Controlled synthesis of titania using water-soluble titanium complexes: A review. J. Solid State Chem. 2017, 251, 143–163. [Google Scholar] [CrossRef]
- Yada, M.; Inoue, Y.; Sakamoto, A.; Torikai, T.; Watari, T. Synthesis and Controllable Wettability of Micro- and Nanostructured Titanium Phosphate Thin Films Formed on Titanium Plates. ACS Appl. Mater. Interfaces 2014, 6, 7695–7704. [Google Scholar] [CrossRef]
- Kalaimani, N.; Ramya, K.; Aarthi, R.; Raja, C.R. Growth and Characterization of Solution Grown Nonlinear Optical Ammonium Tartrate Crystal. Rasayan J. Chem. 2018, 11, 1263–1269. [Google Scholar] [CrossRef]
- Badawi, H.M.; Förner, W. Analysis of the infrared and Raman spectra of phenylacetic acid and mandelic (2-hydroxy-2-phenylacetic) acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1162–1167. [Google Scholar] [CrossRef]
- Attia, A.; Wang, Q.; Huang, X.; Yang, Y. Titanium phosphates as positive electrode in lithium-ion batteries: Composition, phase purity and electrochemical performance. J. Solid State Electrochem. 2011, 16, 1461–1471. [Google Scholar] [CrossRef]
- Janusz, W.; Khalameida, S.; Skwarek, E.; Skubiszewska-Zięba, J.; Sydorchuk, V.; Charmas, B. Modification of titanium phosphate precipitated from titanylsulfate. J. Therm. Anal. Calorim. 2018, 135, 2925–2934. [Google Scholar] [CrossRef]
- Li, Y.H.; Ling, Y.H.; Bai, X. De Preparation and Characterization of Anisotropic Ammonium Titanium Phosphate Crystals via Hydrothermal Route. In High-Performance Ceramics III., Key Engineering Materials, Vol. 280–283; Trans Tech Publications, Ltd.: Bäch, Switzerland, 2007; pp. 597–600. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Lu, H.; Yuan, B.; Hu, Y. Facile Synthesis of Poly(vinyl alcohol)/α-Titanium Phosphate Nanocomposite with Markedly Enhanced Properties. Ind. Eng. Chem. Res. 2011, 50, 11109–11116. [Google Scholar] [CrossRef]
- Güler, H.; Kurtuluş, F. A rapid synthesis of sodium titanium phosphate, NaTi2(PO4)3 by using microwave energy. Mater. Chem. Phys. 2006, 99, 394–397. [Google Scholar] [CrossRef]
- Maslova, M.V.; Rusanova, D.; Naydenov, V.; Antzutkin, O.N.; Gerasimova, L.G. Formation of titanium phosphate composites during phosphoric acid decomposition of natural sphene. J. Solid State Chem. 2008, 181, 3357–3365. [Google Scholar] [CrossRef]
- Stanković, D.M.; Mehmeti, E.; Svorc, L.; Kalcher, K. Simple, Rapid and Sensitive Electrochemical Method for the Determination of the Triketone Herbicide Sulcotrione in River Water Using a Glassy Carbon Electrode. Electroanalysis 2015, 27, 1587–1593. [Google Scholar] [CrossRef]
- Rajaji, U.; Kumar, K.Y.; Arumugam, R.; Alothman, A.A.; Ouladsmane, M.; Chung, R.-J.; Liu, T.-Y. Sonochemical construction of hierarchical strontium doped lanthanum trisulfide electrocatalyst: An efficient electrode for highly sensitive detection of ecological pollutant in food and water. Ultrason. Sonochemistry 2023, 92, 106251. [Google Scholar] [CrossRef] [PubMed]
- Rocaboy-Faquet, E.; Barthelmebs, L.; Calas-Blanchard, C.; Noguer, T. A novel amperometric biosensor for ß-triketone herbicides based on hydroxyphenylpyruvate dioxygenase inhibition: A case study for sulcotrione. Talanta 2016, 146, 510–516. [Google Scholar] [CrossRef] [PubMed]



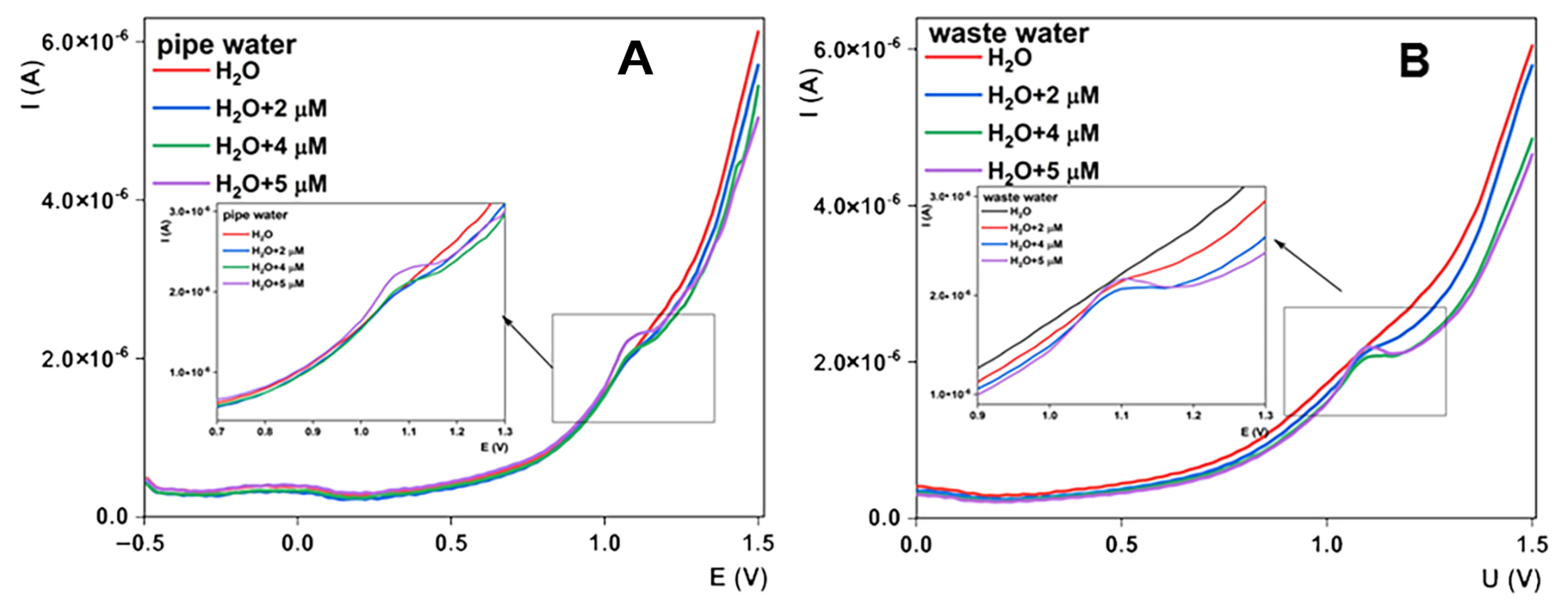
| Found (µM) | Added (µM) | Found (µM)/Recovery (%) | Added (µM) | Found (µM)/Recovery (%) | Added (µM) | Found (µM)/Recovery (%) | |
|---|---|---|---|---|---|---|---|
| Pipe water | 0.00 | 2.00 | 1.94/97 | 2.00 | 4.04/101 | 1.00 | 5.13/103 |
| Wastewater | 0.00 | 2.00 | 1.98/98 | 2.00 | 4.09/102 | 1.00 | 5.08/102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korina, E.; Abramyan, A.; Bol’shakov, O.; Avdin, V.V.; Savić, S.; Manojlović, D.; Stanković, V.; Stanković, D.M. Microspherical Titanium-Phosphorus Double Oxide: Hierarchical Structure Development for Sensing Applications. Sensors 2023, 23, 933. https://doi.org/10.3390/s23020933
Korina E, Abramyan A, Bol’shakov O, Avdin VV, Savić S, Manojlović D, Stanković V, Stanković DM. Microspherical Titanium-Phosphorus Double Oxide: Hierarchical Structure Development for Sensing Applications. Sensors. 2023; 23(2):933. https://doi.org/10.3390/s23020933
Chicago/Turabian StyleKorina, Elena, Anton Abramyan, Oleg Bol’shakov, Vyacheslav V. Avdin, Sladjana Savić, Dragan Manojlović, Vesna Stanković, and Dalibor M. Stanković. 2023. "Microspherical Titanium-Phosphorus Double Oxide: Hierarchical Structure Development for Sensing Applications" Sensors 23, no. 2: 933. https://doi.org/10.3390/s23020933
APA StyleKorina, E., Abramyan, A., Bol’shakov, O., Avdin, V. V., Savić, S., Manojlović, D., Stanković, V., & Stanković, D. M. (2023). Microspherical Titanium-Phosphorus Double Oxide: Hierarchical Structure Development for Sensing Applications. Sensors, 23(2), 933. https://doi.org/10.3390/s23020933







