Electrochemical Sensors for Controlling Oxygen Content and Corrosion Processes in Lead-Bismuth Eutectic Coolant—State of the Art
Abstract
1. Introduction
2. Discussion
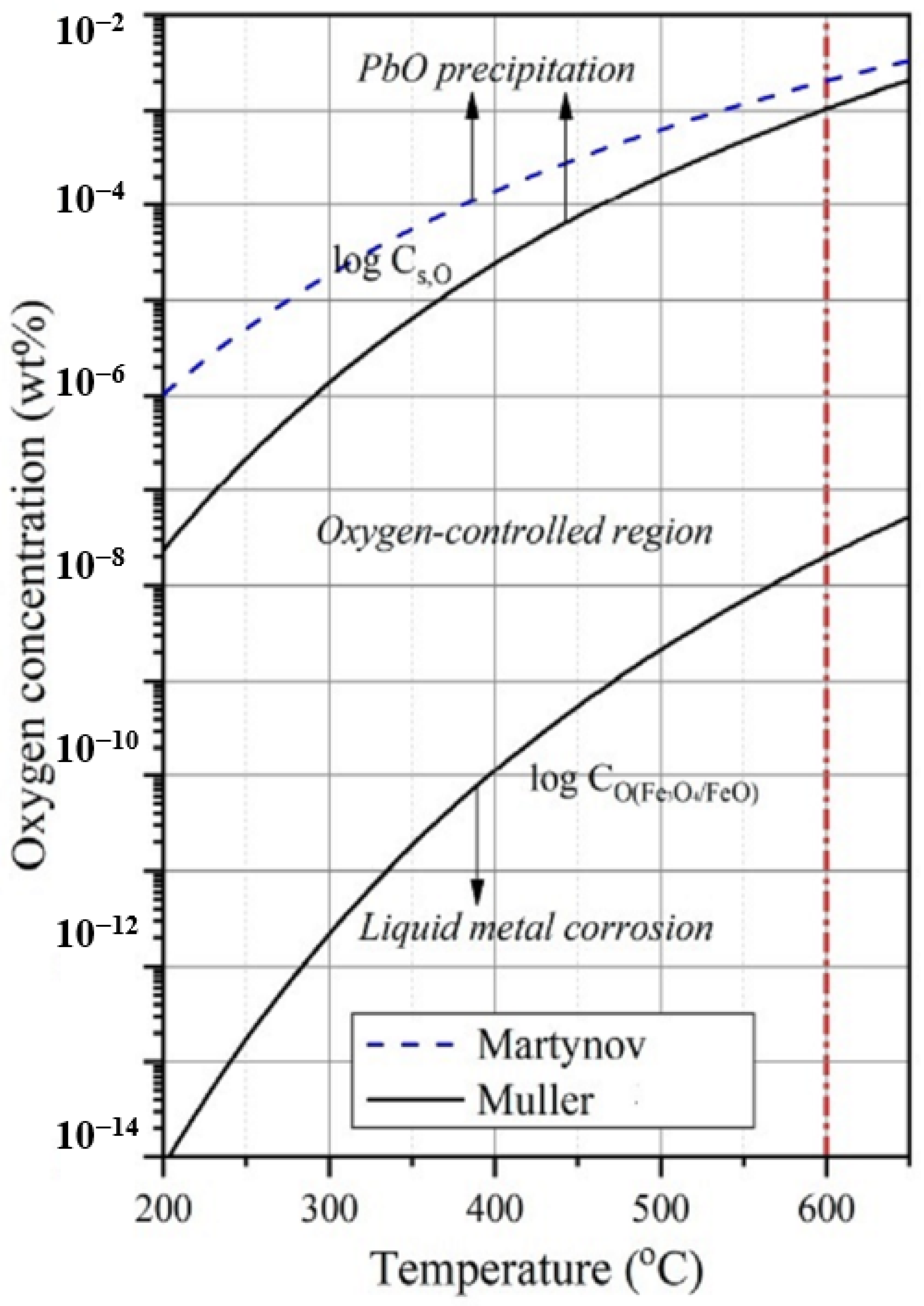
2.1. The Operating Range of Oxygen Concentrations
- (1)
- High oxygen content: from 1 × 10−6% to 1 × 10−3% by weight;
- (2)
- Average oxygen content: from 1 × 10−6%–1 × 10−7% by weight;
- (3)
- Low oxygen content: ≤1 × 10−7%.
2.2. Measurement of Oxygen in a Lead-Bismuth Eutectic Coolant
- −
- −
- −
- Oxides deposition on the surface of the solid electrolyte;
- −
- adsorption of metallic impurities on the electrolyte surface;
- −
- formation of microcracks;
- −
- reduction of the resistance of the interelectrode insulator;
- −
- the presence of open micropores on the surface of the electrolyte.
2.3. Control of Corrosion and Slag Formation Processes in LBE Using Oxygen Activity Sensors
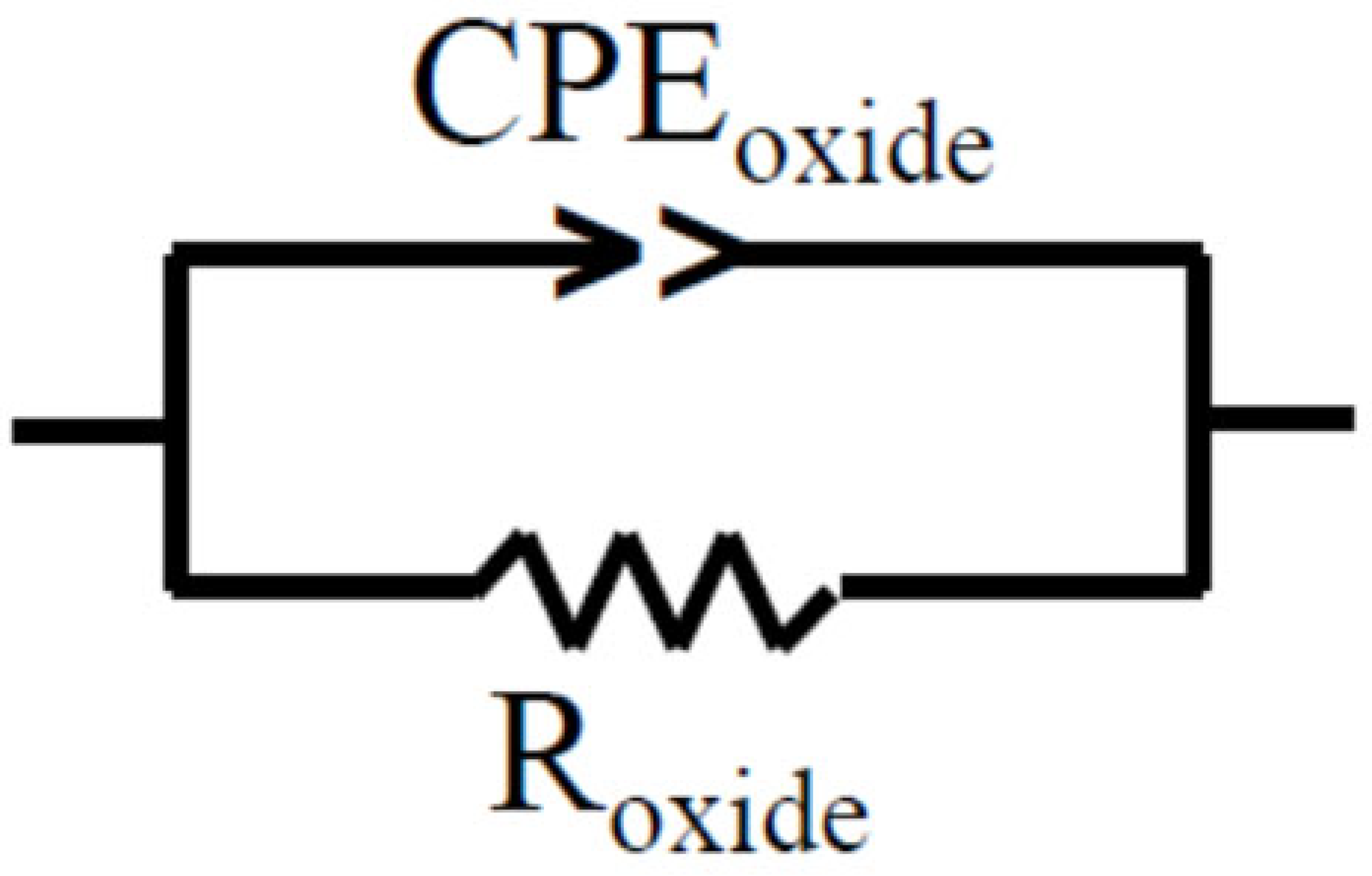
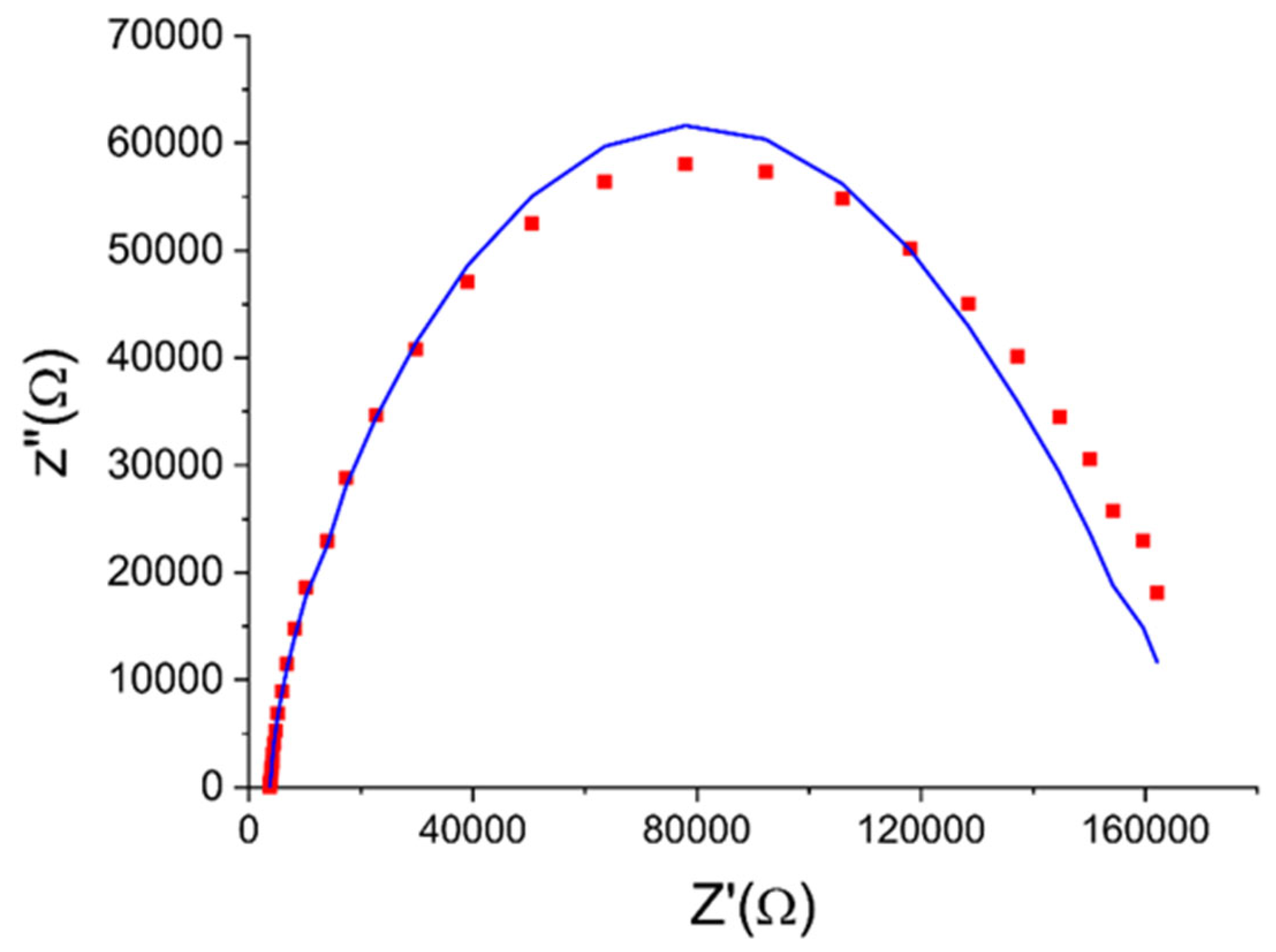
2.4. Impedance Spectroscopy Application to Monitor Corrosion Processes in LBE
3. Conclusions
- The elements that make up austenitic and martensitic steels were effectively dissolved in lead-bismuth coolant. Oxide films protected the structural materials of the primary circuit from destruction.
- To preserve the protective functions of oxide films, a stable oxygen concentration was maintained in the primary coolant in a narrow range from 1 × 10−6% to 4 × 10−6% or up to 1 × 10−8–1 × 10−7% when using protective coatings made of aluminum oxide.
- Measurement of the oxygen content in the LBE was carried out by the method of potentiometry. At the moment, this method is fully developed, but does not exclude a number of metrological difficulties in interpretation and ensuring the reliability of measurement results.
- The use of oxygen activity sensors to monitor the processes of corrosion and slag formation in LBE was hampered by the lack of a quantitative relationship between the sensor readings and the content of impurities in the coolant.
- To obtain data on the state of protective oxide films (including thickness and mechanical damage) on structural materials in static LBE at temperatures up to 600 °C, impedance spectroscopy sensors can be used.
- The next stage for the introduction of impedance spectroscopy sensors into the corrosion monitoring system of the first circuit of promising nuclear reactors with LBE is to confirm their operability in flowing LBE loops.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fedorovich, E.D.; Kurdyukov, I.I. Analytical review of operational experience and modern developments of medium and low-power nuclear power plants with liquid metal coolant. (Modern developments). Technol. Ensuring Life Cycle Nucl. Power Plants 2020, 21 Pt 2, 9–31. [Google Scholar]
- Toshinskiy, G.I.; Komlev, O.G.; Martynov, P.N.; Rusanov, A.E.; Stepanov, V.S.; Klimov, N.N.; Dedul’, A.V.; Bolvanchikov, S.N. Lead-bismuth reactors for regional energetics. Nucl. Power 2011, 111, 290–293. [Google Scholar]
- Loewen, E.P.; Tokuhiro, A.T. Status of Research and Development of the Lead-Alloy-Cooled Fast Reactor. J. Nucl. Sci. Technol. 2003, 40, 614–627. [Google Scholar] [CrossRef]
- Choi, S.; Cho, J.-H.; Bae, M.-H.; Lim, J.; Puspitarini, D.; Jeun, J.H.; Joo, H.-G.; Hwang, I.S. PASCAR: Long burning small modular reactor based on natural circulation. Nucl. Eng. Des. 2011, 241, 1486–1499. [Google Scholar] [CrossRef]
- Shin, Y.-H.; Choi, S.; Cho, J.; Kim, J.H.; Hwang, I.S. Advanced passive design of small modular reactor cooled by heavy liquid metal natural circulation. Prog. Nucl. Energy 2015, 83, 433–442. [Google Scholar] [CrossRef]
- Takahashi, M.; Igashira, M.; Obara, T.; Sekimoto, H.; Kikuchi, K.; Aoto, K.; Kitano, T. Studies on materials for heavy liquid metal cooled rectors in Japan. In Proceedings of the ICONE10: Tenth International Conference on Nuclear Engineering, Arlington, VA, USA, 14–18 April 2002; pp. 5549–5559. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N. Review of the studies on fundamental issues in LBE corrosion. J. Nucl. Mater. 2008, 373, 351–377. [Google Scholar] [CrossRef]
- Ilincev, G. Research results on the corrosion effects of liquid heavy metals Pb, Bi and Pb–Bi on structural materials with and without corrosion inhibitors. Nucl. Eng. Des. 2002, 217, 167–177. [Google Scholar] [CrossRef]
- Klok, O. Liquid Metal Corrosion Effects in MYRRHA Candidate 316L Austenitic Stainless Steel. Ph.D. Thesis, Vrije Universiteit, Brussel, Belgium, 2018; 253p. [Google Scholar]
- Fedorovich, E.D.; Kurdyukov, I.I. Analytical review of operational experience and modern developments of medium and low-power nuclear power plants with liquid metal coolant. (Operational experience). Technol. Ensuring Life Cycle Nucl. Power Plants 2020, 20 Pt 1, 19–26. [Google Scholar]
- Tan, T. Modeling of the Protective Oxide Layer Growth in Non-Isothermal Lead-Alloys Coolant Systems. Ph.D. Thesis, University of Nevada, Reno, NV, USA, 2007; 193p. [Google Scholar]
- Lambrinou, K.; Koch, V.; Coen, G.; Van den Bosch, J.; Schroer, C. Corrosion scales on various steels after exposure to liquid lead–bismuth eutectic. J. Nucl. Mater. 2014, 450, 244–255. [Google Scholar] [CrossRef]
- Lee, S.G.; Shin, Y.-H.; Park, J.; Hwang, I.S. High-Temperature Corrosion Behaviors of Structural Materials for Lead-Alloy-Cooled Fast Reactor Application. Appl. Sci. 2021, 11, 2349. [Google Scholar] [CrossRef]
- Klok, O. The Effect of Corrosion of Liquid Metals on Austenitic Stainless Steel of the MYRRHA-316L Brand. Ph.D. Thesis, Vrige of the University of Brussels, Brussels, Belgium, 2018; 253p. [Google Scholar]
- Weisenburg, A.; Schroer, S.; Gianu, A.; Heinzel, A.; Konis, J.; Steiner, H.; Muller, G.; Fazio, S.; Gessi, A.; Babayan, S.; et al. Long-term corrosion on steel T91 and AISI1 316L in a fluid lead alloy and the development of a barrier for corrosion protection: Experiments and Models. J. Nucl. Mater. 2011, 415, 260–269. [Google Scholar] [CrossRef]
- Martin-Munoz, F.J.; Soler-Crespo, L.; Gomez-Briceno, D. Corrosion behaviour of martensitic and austenitic steels in flowing lead–bismuth eutectic. J. Nucl. Mater. 2011, 416, 87–93. [Google Scholar] [CrossRef]
- Martynov, P.N.; Ivanov, K.D. Properties of Lead-Bismuth Coolant and Perspectives of Non-Electric Applications of Lead-Bismuth Reactor: Technical Report; IAEA: Vienna, Austria, 1997. [Google Scholar]
- Müller, G.; Heinzel, A.; Schumacher, G.; Weisenburger, A. Control of oxygen concentration in liquid lead and lead–bismuth. J. Nucl. Mater. 2003, 321, 256–262. [Google Scholar] [CrossRef]
- Askhadullin, R.S.; Storozhenko, A.N.; Melnikov, V.P.; Legkikh, A.Y.; Uliyanov, V.V. Provision of heavy liquid metal coolant technology in new generation reactor installations. In Proceedings of the Fifth Conference: Heavy Liquid Metal Heat Carriers in Nuclear Technologies, Obninsk, Russia, 8–10 October 2018; pp. 136–137. [Google Scholar]
- Ding, W.; Jiang, Z.; Xin, J.; Zhang, M.; Zheng, M. Interactions between alloy elements and oxygen at the steel–liquid LBE interface determined from first-principles molecular dynamics simulations. Phys. Chem. Chem. Phys. 2019, 21, 25735–25742. [Google Scholar] [CrossRef]
- Deloffre, P.; Balbaud-Célérier, F.; Terlain, A. Corrosion behaviour of aluminized martensitic and austenitic steels in liquid Pb–Bi. J. Nucl. Mater. 2004, 335, 180–184. [Google Scholar] [CrossRef]
- García Ferré, F.; Bertarelli, E.; Chiodoni, A.; Carnelli, D.; Gastaldi, D.; Vena, P.; Beghi, M.G.; Di Fonzo, F. The mechanical properties of a nanocrystalline Al2O3/α-Al2O3 composite coating measured by nanoindentation and Brillouin spectroscopy. Acta Mater. 2013, 61, 2662–2670. [Google Scholar] [CrossRef]
- Dou, P.; Kasada, R. Preliminary study on nano- and micro-composite sol-gel based alumina coatings on structural components of lead–bismuth eutectic cooled fast breeder reactors. J. Nucl. Mater. 2011, 409, 177–182. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, L.; Qiu, C.; Huang, Q. Characterization of multiphase ceramic coatings fabricated via laser in situ reaction technology. Surf. Eng. 2018, 34, 301–308. [Google Scholar] [CrossRef]
- Rivai, A.K.; Takahashi, M. Corrosion investigations of Al–Fe-coated steels, high Cr steels, refractory metals and ceramics in lead alloys at 700 °C. J. Nucl. Mater. 2010, 398, 146–152. [Google Scholar] [CrossRef]
- Shi, Q.; Yan, W.; Sha, W.; Wang, W.; Shan, Y.-Y.; Yang, K. Corrosion resistance of self-growing TiC coating on SIMP steel in LBE at 600 °C. Mater. Corros. 2016, 67, 1204–1212. [Google Scholar] [CrossRef]
- Vogt, J.-B.; Proriol Serre, I. A Review of the Surface Modifications for Corrosion Mitigation of Steels in Lead and LBE. Coatings 2021, 11, 53. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, J.; Wang, H.; Chen, Y.; Yin, X.; Guo, N. Corrosion Behavior and Surface Treatment of Cladding Materials Used in High-Temperature Lead-Bismuth Eutectic Alloy: A Review. Coatings 2021, 11, 364. [Google Scholar] [CrossRef]
- Weisenburger, A.; Heinzel, A.; Müller, G.; Muscher, H.; Rousanov, A. T91 cladding tubes with and without modified FeCrAlY-coatings exposed in LBE at different flow, stress and temperature conditions. J. Nucl. Mater. 2008, 376, 274–281. [Google Scholar] [CrossRef]
- Weisenburger, A.; Heinzel, A.; Fazio, C.; Müller, G.; Markow, V.G.; Kastanov, A.D. Low cycle fatigue tests of surface modified T91 steel in 10−6 wt.% oxygen containing Pb45Bi55 at 550 °C. J. Nucl. Mater. 2008, 377, 261–267. [Google Scholar] [CrossRef]
- Weisenburger, A.; Jianu, A.; An, W.; Fetzer, R.; Del Giacco, M.; Heinzel, A. Creep, creep-rupture tests of Al-surface-alloyed T91 steel in liquid lead bismuth at 500 and 550 °C. J. Nucl. Mater. 2012, 431, 77–84. [Google Scholar] [CrossRef]
- Kashtanov, A.D. Development of Scientific and Technological Principles for the Selection of Materials, Taking into Account the Peculiarities of Their Damage during the Operation of Various Elements of Reactor Equipment with Heavy Liquid Metal Heat Carriers. Ph.D. Thesis, Saint Petersburg, Russia, 2020; 35p. [Google Scholar]
- Wei, Y.; Jiao, Y.; An, D.; Li, D.; Li, V.; Wei, K. Overview of dissolved oxygen detection technology: From laboratory analysis to intelligent online detection. Sensors 2019, 19, 3995. [Google Scholar] [CrossRef]
- Martynov, P.N.; Chernov, M.E.; Gulevsky, V.A.; Provorov, A.A. Development of a capsule-type electrochemical sensor for monitoring oxygen in a heavy coolant. Nucl. Power 2005, 98, 360. [Google Scholar] [CrossRef]
- Merkens, W.; Shmitz, R. A sensor for determining the activity of oxygen in metal melts and the method of its production. Saint Petersburg State Polytechnic University. Patent RU 2325633 C2, 20 October 2003. [Google Scholar]
- Schroer, C.; Konys, J.; Verdaguer, A.; Abella, J.; Gessi, A.; Kobzova, A.; Babayan, S.; Courouau, J.-L. Design and testing of electrochemical oxygen sensors for service in liquid lead alloys. J. Nucl. Mater. 2011, 415, 338–347. [Google Scholar] [CrossRef]
- Brissonneau, L.; Beauchamp, F.; Morier, O.; Schroer, C.; Konys, J.; Kobzova, A.; Gabriele, F.D.; Courouau, J.-L. Oxygen control systems and impurity purification in LBE: Learning from DEMETRA project. J. Nucl. Mater. 2011, 415, 348–360. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, T.Y. Kinetics of Gas Phase Oxygen Control System and Oxygen Concentration Measurement in Liquid Pb and LBE. J. Ind. Eng. Chem. 2007, 13, 602–607. [Google Scholar]
- Nam, H.O.; Lim, J.; Han, D.Y.; Hwang, I.S. Dissolved oxygen control and monitoring implementation in the liquid lead–bismuth eutectic loop: HELIOS. J. Nucl. Mater. 2008, 376, 381–385. [Google Scholar] [CrossRef]
- Courouau, J.-L.; Deloffre, P.; Adriano, R. Oxygen control in lead-bismuth eutectic: First validation of electrochemical oxygen sensors in static conditions. J. Phys. IV 2002, 12, 141–153. [Google Scholar] [CrossRef]
- Courouau, J.-L.; Trabuc, P.; Laplanche, G.; Deloffre, P.; Taraud, P.; Olliver, M.; Adriano, R.; Trambaud, S. Impurities and oxygen control in lead alloys. J. Nucl. Mater. 2002, 301, 53–59. [Google Scholar] [CrossRef]
- Konys, J.; Schroer, C.; Wedemeyer, O. Electrochemical Oxygen Sensors for Corrosion Control in Lead-Cooled Nuclear Reactors. Corrosion 2009, 65, 798–808. [Google Scholar] [CrossRef]
- Lim, J.; Manfredi, G.; Gavrilov, S.; Rosseel, K.; Aerts, A.; Van den Bosch, J. Control of dissolved oxygen in liquid LBE by electrochemical oxygen pumping. Sens. Actuators B 2014, 204, 388–392. [Google Scholar] [CrossRef]
- Manfredi, G. Oxygen Sensors and Electrochemical Oxygen Pumps for Lead Alloy Cooled Nuclear Systems. Ph.D. Thesis, Belgian Nuclear Research Centre, Wallonia, Belgium, 2019; 124p. [Google Scholar]
- Adhi, P.M.; Kamal, D.M.; Vilcu, A.; Kondo, M.; Takahashi, M. Measurement of Oxygen Concentration in Static and Flowing Liquid Pb-Bi by Using Zirconia Based Sensor. Indones. J. Phys. 2020, 5, 31. [Google Scholar] [CrossRef]
- Martynov, P.N.; Askhadullin, R.S.; Ivanov, K.D.; Chernov, M.E.; Ulyanov, V.V.; Shelemetev, V.M.; Sadovnicchiy, R.P.; Cheporov, R.Y.; Niyazov, S.-A.S. Features of the use of iron oxide reference electrodes in solid-electrolyte sensors for monitoring the thermodynamic activity of oxygen. Izv. Vuzov. Yad. Energ. 2012, 3, 62–67. [Google Scholar] [CrossRef]
- Tarantino, M.; Angiolini, M.; Bassini, S.; Cataldo, S.; Ciantelli, C.; Cristalli, C.; Del Nevo, A.; Di Piazza, I.; Diamanti, D.; Eboli, M. Overview on Lead-Cooled Fast Reactor Design and Related Technologies Development in ENEA. Energies 2021, 14, 5157. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Q.; Meng, X.; Chen, Z.; Zhang, Y.; Yan, Q.; Wei, Y. Oxygen concentration measurement and control of lead-bismuth eutectic in a small, static experimental facility. J. Nucl. Sci. Technol. 2019, 57, 590–598. [Google Scholar] [CrossRef]
- Askhadullin, R.S.; Mzartynov, P.N.; Rachkov, V.I.; Legkikh, A.Y.; Storozhenko, A.N.; Ul’yanov, V.V.; Gurlevskiy, V.A. Control and regulation of oxygen content in heavy liquid metal heat carriers for anticorrosive protection of steel. High Temp. 2016, 54, 564–572. [Google Scholar] [CrossRef]
- Musikhin, Y.A. On Electrode Polarization of Electrochemical Oxygen Sensor in Liquid Metal Coolants. Izv. Vuzov. Yad. Energetica 2012, 4, 75–84. [Google Scholar] [CrossRef]
- Adhi, P.M.; Kondo, M.; Takahashi, M. Performance of solid electrolyte oxygen sensor with solid and liquid reference electrode for liquid metal. Sens. Actuators B Chem. 2017, 241, 1261–1269. [Google Scholar] [CrossRef]
- Bassini, S.; Antonelli, A.; Di Piazza, I.; Tarantino, M. Oxygen sensors for heavy liquid metal coolants: Calibration and evaluation of the minimum reading temperature. J. Nucl. Mater. 2017, 486, 197–205. [Google Scholar] [CrossRef]
- Bassini, S.; Di Piazza, I.; Antonelli, A.; Angelucci, M.; Sermengi, V.; Palazzi, G.; Tarantino, M. In-loop oxygen reduction in HLM thermal-hydraulic facility NACIE-UP. Prog. Nucl. Energy 2018, 105, 137–145. [Google Scholar] [CrossRef]
- Hinojo, A.; Soriano, I.; Abellà, J.; Colominas, S. Evaluation of High-Temperature Hydrogen Sensors Based on BaCe0.6Zr0.3Y0.1O3-α and Sr(Ce0.9Zr0.1)0.95Yb0.05O3-α Perovskites for Industrial Applications. Sensors 2020, 20, 7258. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Wan, T.; Okubo, N.; Obayashi, H.; Watanabe, N.; Odaira, N.; Kinoshita, H.; Yamaki, K.; Kita, S.; Yoshimoto, H.; et al. Status of LBE study and experimental plan at JAEA. In Proceedings of the 3rd J-PARC Symposium (J-PARC2019), Tsukuba, Japan, 23–26 September 2019. [Google Scholar] [CrossRef]
- Pityk, A.V.; Arnoldov, M.N. Redox processes as the basis of potentiometric methods for control coolants state of nuclear power systems. Probl. At. Sci. Technol. Nucl. React. Constants 2014, 2, 5–18. [Google Scholar]
- Chang, Y.A.; Fitzner, K.; Zhang, M.-X. The Solubility of Gases in Liquid Metals and Alloys. Prog. Mater. Sci. 1988, 32, 97. [Google Scholar] [CrossRef]
- Blokhin, V.A.; Shimkedvich, A.L.; Musikhin, Y.A. Experience in the creation and operation of solid-electrolyte oxygen activometers in a lead-bismuth coolant. In Proceedings of the Heavy Liquid Metal Heat Carriers in Nuclear Technologies, Obnins, Russia, 3–9 October 1998; Tochinsky, G.I., Ed.; Volume 2, pp. 631–635. [Google Scholar]
- Entler, S.; Soban, Z.; Duran, I.; Kovarik, K.; Vyborny, K.; Sebek, J.; Tazlaru, S.; Strelecek, J.; Sladek, P. Ceramic-Chromium Hall Sensors for Environments with High Temperatures and Neutron Radiation. Sensors 2021, 21, 721. [Google Scholar] [CrossRef]
- Okubo, N.; Okuno, Y.; Kitamura, A.; Taguchi, T. Influence of gamma-ray irradiation on mechanical property of YSZ for oxygen sensors in ADS. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 435, 198–202. [Google Scholar] [CrossRef]
- Okuno, Y.; Okubo, N. Phase Transformation by 100 keV Electron Irradiation in Partially Stabilized Zirconia. Quantum Beam Sci. 2021, 5, 20. [Google Scholar] [CrossRef]
- Courouau, J.-L.; Sellier, S.; Chabert, C.; Pignoly, L. Electrochemical oxygen sensor for on-line measurement in liquid lead alloy systems at relatively low temperature. Afinidad 2005, 62, 519. [Google Scholar]
- Martynov, P.N.; Askhadullin, R.S.; Storozhenko, A.N.; Shelemet’ev, V.M.; Sadovnichiy, R.P.; Skomorokhov, A.N. Method for Measuring the Thermodynamic Activity of Oxygen in Molten Liquid Metals. Patent RU 2584378 C1, 19 December 2014. [Google Scholar]
- Sarkisov, A.A. The Role of Russian Science in the Creation of a Domestic Submarine Fleet; Nauka Publishing House: Moscow, Russia, 2008; p. 654. [Google Scholar]
- Martynov, P.N.; Askhadullin, R.S.; Orlov, Y.P.; Storozhenko, A.N. Modern issues and problems of technology of heavy liquid metal heat carriers (lead, lead-bismuth). In Proceedings of the Forth Conference: Heavy Liquid Metal Heat Carriers in Nuclear Technologies, Obninsk, Russia, 23–28 September 2013; p. 27. [Google Scholar]
- Ivanov, K.D.; Niyazov, S.-A.S.; Cheporov, R.Y. Increase the information content of control of thermodynamic activity of oxygen in heavy liquid metal coolants. Probl. At. Sci. Technol. Nucl. React. Constants 2018, 5, 5–12. [Google Scholar]
- Salaev, S.V.; Askhadullin, R.S.; Ivanov, K.D.; Legkikh, A.Y.; Niyazov, S.-A.S. The results of studies on the extension of methodological capabilities of determining physico-chemical conditions of lead-based liquid-metal coolants. Izv. Vuzov. Yadenaya Energetika. 2020, 98–106. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Aich, S.; Kailasa, S.; Parangusan, H.; Ibrahim, M.; Eldeib, S.; Shehata, O.; Ismail, M.; et al. Sensors in advancing the capabilities of corrosion detection: A review. Sens. Actuators A Phys. 2021, 332, 113086. [Google Scholar] [CrossRef]
- Wright, R.F.; Lu, P.; Devkota, J.; Lu, F.; Ziomek-Moroz, M.; Ohodnicki, P.R., Jr. Corrosion Sensors for Structural Health Monitoring of Oil and Natural Gas Infrastructure: A Review. Sensors 2019, 19, 3964. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Wang, Z.; Li, G.; Wang, X.; Huang, Y. Probing and separating corrosion and erosion of pipeline steel using electrical resistance method in conjunction with electrochemical measurements. Measurement 2021, 183, 109797. [Google Scholar] [CrossRef]
- Shevtsov, D.; Cao, N.L.; Nguyen, V.C.; Nong, Q.Q.; Le, H.Q.; Nguyen, D.A.; Zartsyn, I.; Kozaderov, O. Progress in Sensors for Monitoring Reinforcement Corrosion in Reinforced Concrete Structures—A Review. Sensors 2022, 22, 3421. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. Electrochemical behavior and compressive strength of Al-Cu/xCu composites in NaCl solution. J. Solid State Electrochem. 2021, 25, 1303–1317. [Google Scholar] [CrossRef]
- Mishra, P.; Yavas, D.; Bastawros, A.F.; Hebert, K.R. Electrochemical impedance spectroscopy analysis of corrosion product layer formation on pipeline steel. Electrochim. Acta 2020, 346, 136232. [Google Scholar] [CrossRef]
- Duarte, T.; Meyer, Y.A.; Osório, W.R. The Holes of Zn Phosphate and Hot Dip Galvanizing on Electrochemical Behaviors of Multi-Coatings on Steel Substrates. Metals 2022, 12, 863. [Google Scholar] [CrossRef]
- Hafez, B.; Mokhtari, M.; Elmsellem, H.; Steli, H. Environmentally friendly inhibitor of the corrosion of mild steel: Commercial oil of Eucalyptus. Int. J. Corros. Scale Inhib. 2019, 8, 573–585. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Stoynov, Z.B.; Grafov, B.M.; Savova-Stoynova, B.; Elkin, V.V. Electrochemical Impedance; Nauka Publishing House: Moscow, Russia, 1991; p. 336. [Google Scholar]
- Lillard, R.S.; Valot, C.; Hanrahan, R.J. Relationships between the Impedance of Oxide Scales on Martensitic and Austenitic Steels and Corrosion Rate in Liquid Lead-Bismuth Eutectic. Corrosion 2004, 60, 1134–1143. [Google Scholar] [CrossRef]
- Stubbins, J.F.; Bolind, A.M.; Chen, X. The electrical impedance response of steel surface oxide layers in molten lead–bismuth eutectic. J. Nucl. Mater. 2008, 377, 243–252. [Google Scholar] [CrossRef]
- Stubbins, J.F.; Bolind, A.M.; Hosseman, P.; Chen, X. Analysis of corrosion scale structure of pre-oxidized stainless steel 316 in molten lead bismuth eutectic and the relation to impedance spectroscopy response. J. Nucl. Mater. 2010, 398, 172–179. [Google Scholar] [CrossRef]
- Stubbins, J.F.; Bolind, A.M.; Chen, X. Real Time Corrosion Monitoring in Lead and Lead-Bismuth Systems; Final Report; University of Illinois: Champaign, IL, USA, 2010. [Google Scholar]
- Chen, X.; Haasch, R.; Stubbins, J.F. Impedance spectroscopy and microstructural characterization of the corrosion behavior of FeCrAl alloy in lead–bismuth eutectic. J. Nucl. Mater. 2012, 431, 125–132. [Google Scholar] [CrossRef]
- Kondo, M.; Suzuki, N.; Nakajima, Y.; Tanaka, T.; Muroga, T. Electrochemical impedance spectroscopy on in-situ analysis of oxide layer formation in liquid metal. Fusion Eng. Des. 2014, 89, 1201–1208. [Google Scholar] [CrossRef]
- Kawarai, A.; Kondo, M. Self-Healing Behavior of Oxide Layer in Liquid Metal. Plasma Fusion Res. 2022, 17, 2405059. [Google Scholar] [CrossRef]
- Gongi, W.; Rube, M.; Ouada, H.B.; Tamarin, O.; Dejois, C. Elaboration and Characterization of a New Heavy Metal Sensor Functionalized by Extracellular Polymeric Substances Isolated from a Tunisian Thermophilic Microalga Strain Graesiella sp. Sensors 2023, 23, 803. [Google Scholar] [CrossRef]
- Qiu, J.; Macdonald, D.D.; Li, N.; Schoell, R.; Kaoumi, D.; Hosemann, P. An Electrochemical Impedance Spectroscopic Study of Oxide Films in Liquid Metal. JOM 2020, 72, 2082–2088. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlov, S.N.; Bogachev, N.A.; Mereshchenko, A.S.; Zmitrodan, A.A.; Skripkin, M.Y. Electrochemical Sensors for Controlling Oxygen Content and Corrosion Processes in Lead-Bismuth Eutectic Coolant—State of the Art. Sensors 2023, 23, 812. https://doi.org/10.3390/s23020812
Orlov SN, Bogachev NA, Mereshchenko AS, Zmitrodan AA, Skripkin MY. Electrochemical Sensors for Controlling Oxygen Content and Corrosion Processes in Lead-Bismuth Eutectic Coolant—State of the Art. Sensors. 2023; 23(2):812. https://doi.org/10.3390/s23020812
Chicago/Turabian StyleOrlov, Sergey N., Nikita A. Bogachev, Andrey S. Mereshchenko, Alexandr A. Zmitrodan, and Mikhail Yu. Skripkin. 2023. "Electrochemical Sensors for Controlling Oxygen Content and Corrosion Processes in Lead-Bismuth Eutectic Coolant—State of the Art" Sensors 23, no. 2: 812. https://doi.org/10.3390/s23020812
APA StyleOrlov, S. N., Bogachev, N. A., Mereshchenko, A. S., Zmitrodan, A. A., & Skripkin, M. Y. (2023). Electrochemical Sensors for Controlling Oxygen Content and Corrosion Processes in Lead-Bismuth Eutectic Coolant—State of the Art. Sensors, 23(2), 812. https://doi.org/10.3390/s23020812







