Perspectives of Motor Functional Upper Extremity Recovery with the Use of Immersive Virtual Reality in Stroke Patients
Abstract
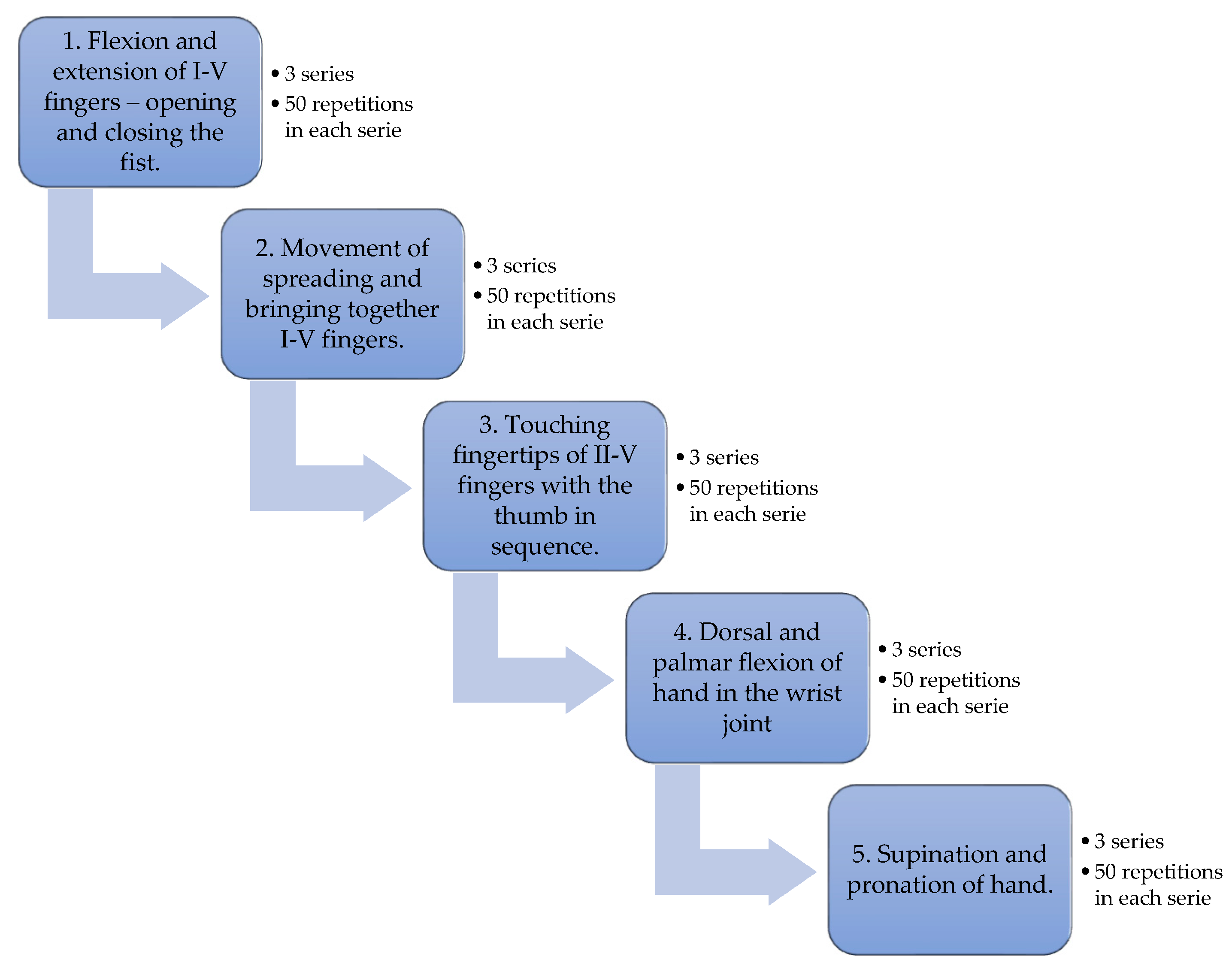
1. Introduction
2. Materials and Methods
2.1. Participants
- -
- diagnosis of first-episode stroke,
- -
- age range 40–64,
- -
- acquired motor impairment of hemiplegic upper limb,
- -
- maximum of 12 months period since diagnosis,
- -
- functional brain damage specified with Rankin scale 1–4 at the last hospital discharge.
- -
- requirement of constant, intensive medical surveillance,
- -
- active comorbidities significantly influencing rehabilitation process (ex. bone fractures occurred during medical treatment, pressure ulcers, etc.),
- -
- circulatory insufficiency, kidney, liver failure, condition after myocardial infarction with ejection fraction less than 30%,
- -
- vascular disease (active thromboembolism),
- -
- heart aneurysm, aortic aneurysm, malformation of cerebral vessels,
- -
- active inflammation,
- -
- uncompensated endocrine disruption,
- -
- cancer (palliative care or need of urgent treatment),
- -
- severe arterial or pulmonary hypertension,
- -
- uncontrolled diabetes,
- -
- epilepsy.
2.2. VR Application
2.3. Research Procedures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palumbo, A. Microsoft HoloLens 2 in Medical and Healthcare Context: State of the Art and Future Prospects. Sensors 2022, 22, 7709. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.F.; Prahm, C.; Kolbenschlag, J.; Oliveira, E.; Rodrigues, N.F. A Virtual Reality Serious Game for Hand Rehabilitation Therapy. In Proceedings of the 2020 IEEE 8th International Conference on Serious Games and Applications for Health (SeGAH), Vancouver, BC, Canada, 12–14 August 2020; pp. 1–7. [Google Scholar]
- Pereira, M.F.; Oliveira, E.; Rodrigues, N.F.; Bressler, M.; Kolbenschlag, J.; Prahm, C. Hand Rehabilitation with Virtual Reality: Preliminary learning results. In Proceedings of the 2021 IEEE 9th International Conference on Serious Games and Applications for Health (SeGAH), Dubai, United Arab Emirates, 4–6 August 2021; pp. 1–8. [Google Scholar]
- Rojo, A.; Santos-Paz, J.Á.; Sánchez-Picot, Á.; Raya, R.; García-Carmona, R. FarmDay: A Gamified Virtual Reality Neurorehabilitation Application for Upper Limb Based on Activities of Daily Living. Appl. Sci. 2022, 12, 7068. [Google Scholar] [CrossRef]
- Zong, Y.; Shin, H.H.; Wang, Y.C.; Li, S.; Zhou, P.; Li, X. Assessing Hand Muscle Structural Modifications in Chronic Stroke. Front. Neurol. 2018, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Cauraugh, J.; Light, K.; Sangbum, K.; Thigpen, M.; Behrman, A. Chronic Motor Dysfunction After Stroke. Stroke 2000, 31, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Israely, S.; Leisman, G.; Carmeli, E. Improvement in arm and hand function after a stroke with task-oriented training. BMJ Case Rep. 2017, 2017, bcr2017219250. [Google Scholar] [CrossRef]
- Jiang, L.; Dou, Z.L.; Wang, Q.; Wang, Q.Y.; Dai, M.; Wang, Z.; Wei, X.M.; Chen, Y.B. Evaluation of clinical outcomes of patients with post-stroke wrist and finger spasticity after ultrasonography-guided BTX-A injection and rehabilitation training. Front. Hum. Neurosci. 2015, 9, 485. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, C.Y.; Kim, H.D. Short-Term Effects of Whole-Body Vibration Combined with Task-Related Training on Upper Extremity Function, Spasticity, and Grip Strength in Subjects with Poststroke Hemiplegia: A Pilot Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2016, 95, 608–617. [Google Scholar] [CrossRef]
- Noma, T.; Matsumoto, S.; Etoh, S.; Shimodozono, M.; Kawahira, K. Anti-spastic effects of the direct application of vibratory stimuli to the spastic muscles of hemiplegic limbs in post-stroke patients. Brain Inj. 2009, 23, 623–631. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, P.C.; Tso, H.H.; Yang, Y.C.; Ho, T.L.; Leong, C.P. Effects of kinesio taping on hemiplegic hand in patients with upper limb post-stroke spasticity: A randomized controlled pilot study. Eur. J. Phys. Rehabil. Med. 2019, 55, 551–557. [Google Scholar] [CrossRef]
- Nakipoğlu Yuzer, G.F.; Köse Dönmez, B.; Özgirgin, N.A. Randomized Controlled Study: Effectiveness of Functional Electrical Stimulation on Wrist and Finger Flexor Spasticity in Hemiplegia. J. Stroke Cerebrovasc. Dis. 2017, 26, 1467–1471. [Google Scholar] [CrossRef]
- Yu-Shen, Y.; Emzain, Z.F.; Shyh-Chour, H. Biomechanical Evaluation of Dynamic Splint Based on Pulley Rotation Design for Management of Hand Spasticity. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 683–689. [Google Scholar]
- Kagawa, S.; Koyama, T.; Hosomi, M.; Takebayashi, T.; Hanada, K.; Hashimoto, F.; Domen, K. Effects of constraint-induced movement therapy on spasticity in patients with hemiparesis after stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, 364–370. [Google Scholar] [CrossRef]
- Germanotta, M.; Gower, V.; Papadopoulou, D.; Cruciani, A.; Pecchioli, C.; Mosca, R.; Speranza, G.; Falsini, C.; Cecchi, F.; Vannetti, F.; et al. Reliability, validity and discriminant ability of a robotic device for finger training in patients with subacute stroke. J. Neuroeng. Rehabil. 2020, 17, 1. [Google Scholar] [CrossRef]
- Smorenburg, A.R.; Ledebt, A.; Deconinck, F.J.; Savelsbergh, G.J. Practicing a matching movement with a mirror in individuals with spastic hemiplegia. Res. Dev. Disabil. 2013, 34, 2507–2513. [Google Scholar] [CrossRef]
- Saha, S.; Sur, M.; Chaudhuri, G.R.; Agarwal, S. Effects of mirror therapy on oedema, pain and functional activities in patients with poststroke shoulder-hand syndrome: A randomized controlled trial. Physiother. Res. Int. 2021, 26, e1902. [Google Scholar] [CrossRef]
- Yavuzer, G.; Selles, R.; Sezer, N.; Sütbeyaz, S.; Bussmann, J.B.; Köseoğlu, F.; Atay, M.B.; Stam, H.J. Mirror therapy improves hand function in subacute stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2008, 89, 393–398. [Google Scholar] [CrossRef]
- Lin, K.C.; Chen, Y.T.; Huang, P.C.; Wu, C.Y.; Huang, W.L.; Yang, H.W.; Lai, H.T.; Lu, H.J. Effect of mirror therapy combined with somatosensory stimulation on motor recovery and daily function in stroke patients: A pilot study. J. Formos. Med. Assoc. 2014, 113, 422–428. [Google Scholar] [CrossRef]
- Lee, D.; Lee, M.; Lee, K.; Song, C. Asymmetric training using virtual reality reflection equipment and the enhancement of upper limb function in stroke patients: A randomized controlled trial. J. Stroke Cerebrovasc. Dis. 2014, 23, 1319–1326. [Google Scholar] [CrossRef]
- Gagnon, C.; Lavoie, C.; Lessard, I.; Mathieu, J.; Brais, B.; Bouchard, J.P.; Fluet, M.C.; Gassert, R.; Lambercy, O. The Virtual Peg Insertion Test as an assessment of upper limb coordination in ARSACS patients: A pilot study. J. Neurol. Sci. 2014, 347, 341–344. [Google Scholar] [CrossRef]
- Oh, Y.B.; Kim, G.W.; Han, K.S.; Won, Y.H.; Park, S.H.; Seo, J.H.; Ko, M.H. Efficacy of Virtual Reality Combined with Real Instrument Training for Patients With Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2019, 100, 1400–1408. [Google Scholar] [CrossRef]
- Johnson, L.; Bird, M.L.; Muthalib, M.; Teo, W.P. Innovative STRoke Interactive Virtual thErapy (STRIVE) online platform for community-dwelling stroke survivors: A randomised controlled trial protocol. BMJ Open 2018, 8, e018388. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 49, e160–e161. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.H.; Zeng, M.; Shi, M.F.; Gu, X.D.; Shen, F.; Zheng, Y.P.; Jia, Y.P. Visual feedback therapy for restoration of upper limb function of stroke patients. Int. J. Nurs. Sci. 2020, 7, 170–178. [Google Scholar] [PubMed]
- Zhan, T.; Yin, K.; Xiong, J.; He, Z.; Wu, S.T. Augmented Reality and Virtual Reality Displays: Perspectives and Challenges. iScience 2020, 23, 101397. [Google Scholar] [CrossRef]
- Holcomb, P.S. Adding New Dimension to Neuroscience. J. Neurosci. Res. 2018, 96, 1123–1124. [Google Scholar] [CrossRef]
- Gallos, P.; Georgiadis, C.; Liaskos, J.; Mantas, J. Augmented Reality Glasses and Head-Mounted Display Devices in Healthcare. Stud. Health Technol. Inform. 2018, 251, 82–85. [Google Scholar]
- Weber, L.M.; Nilsen, D.M.; Gillen, G.; Yoon, J.; Stein, J. Immersive virtual reality mirror therapy for upper limb recovery following stroke: A pilot study. Am. J. Phys. Med. Rehabil. 2019, 98, 783–788. [Google Scholar] [CrossRef]
- Kassutto, S.M.; Baston, C.; Clancy, C. Virtual, Augmented, and Alternate Reality in Medical Education: Socially Distanced but Fully Immersed. ATS Sch. 2021, 2, 651–664. [Google Scholar] [CrossRef]
- Mutai, H.; Furukawa, T.; Nakanishi, K.; Hanihara, T. Longitudinal functional changes, depression, and health-related quality of life among stroke survivors living at home after inpatient rehabilitation. Psychogeriatrics 2016, 16, 185–190. [Google Scholar] [CrossRef]
- Grefkes, C.; Fink, G.R. Recovery from stroke: Current concepts and future perspectives. Neurol. Res. Pract. 2020, 2, 17. [Google Scholar] [CrossRef]
- Wade, D.T.; Wood, V.A.; Hewer, R.L. Recovery after stroke--the first 3 months. J. Neurol. Neurosurg. Psychiatry 1985, 48, 7–13. [Google Scholar] [CrossRef]
- Zupanic, E.; von Euler, M.; Kåreholt, I.; Escamez, B.V.; Fastbom, J.; Norrving, B.; Religa, D.; Kramberger, M.G.; Winblad, B.; Johnell, K.; et al. Thrombolysis in acute ischemic stroke in patients with dementia: A Swedish registry study. Neurology 2017, 89, 1860–1868. [Google Scholar] [CrossRef]
- Zondervan, D.K.; Friedman, N.; Chang, E.; Zhao, X.; Augsburger, R.; Reinkensmeyer, D.J.; Cramer, S.C. Home-based hand rehabilitation after chronic stroke: Randomized, controlled single-blind trial comparing the MusicGlove with a conventional exercise program. J. Rehabil. Res. Dev. 2016, 53, 457–472. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, X.; Yang, F.; Sui, G.; Sui, Y.; Xu, B.; Qu, B. Increased quality of life in patients with stroke during the COVID-19 pandemic: A matched-pair study. Sci. Rep. 2021, 11, 10277. [Google Scholar] [CrossRef]
- Peng, Q.C.; Yin, L.; Cao, Y. Effectiveness of Virtual Reality in the Rehabilitation of Motor Function of Patients with Subacute Stroke: A Meta-Analysis. Front. Neurol. 2021, 12, 639535. [Google Scholar] [CrossRef]
- Kil-Byung, L.; Hong-Jae, L.; Jeehyun, Y.; Hyun-Ju, Y. Efficacy of Mirror Therapy Containing Functional Tasks in Poststroke Patients. Ann. Rehabil. Med. 2016, 40, 629–636. [Google Scholar]
- Wen, Z.; Yonghong, G.; Guofeng, W.; Xueyan, L.; Qian, F. Mirror therapy for motor function of the upper extremity in patients with stroke: A meta-analysis. J. Rehabil. Med. 2018, 50, 8–15. [Google Scholar]
- Rajendran, V.; Jeevanantham, D.; Larivière, C.; Singh, R.J.; Zeman, L.; Papuri, P. Effectiveness of self-administered mirror therapy on upper extremity impairments and function of acute stroke patients: Study protocol. Trials 2021, 22, 439. [Google Scholar] [CrossRef]
- Thieme, H.; Morkisch, N.; Mehrholz, J.; Pohl, M.; Behrens, J.; Borgetto, B.; Dohle, C.; Cochrane Stroke Group. Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 2018, 2018, CD008449. [Google Scholar] [CrossRef]
- Aramaki, A.L.; Sampaio, R.F.; Reis, A.C.S.; Cavalcanti, A.; Silva E Dutra, F.C.M. Virtual reality in the rehabilitation of patients with stroke: An integrative review. Arq. Neuropsiquiatr. 2019, 77, 268–278. [Google Scholar] [CrossRef]
- Spiegel, B.; Fuller, G.; Lopez, M.; Dupuy, T.; Noah, B.; Howard, A.; Albert, M.; Tashjian, V.; Lam, R.; Ahn, J.; et al. Virtual reality for management of pain in hospitalized patients: A randomized comparative effectiveness trial. PLoS ONE 2019, 14, e0219115. [Google Scholar] [CrossRef] [PubMed]
- Nickel, R.; Lange, M.; Stoffel, D.P.; Navarro, E.J.; Zetola, V.F. Upper limb function and functional independence in patients with shoulder pain after stroke. Arq. Neuropsiquiatr. 2017, 75, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Sungbae, J.; Hyunjin, K.; Changho, S. A Novel Approach to Increase Attention during Mirror Therapy among Stroke Patients: A Video-Based Behavioral Analysis. Brain Sci. 2022, 12, 297. [Google Scholar]







| Experimental Group | Control Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | M1 | M2 | Mean ± SD | Median | p | Test | M1 | M2 | Mean ± SD | Median | p |
| FMA | 67.8 ± 18.2 | 75.3 ± 22.1 | 7.5 ± 4.7 | 7.0 | 0.009 | FMA | 49.0 ± 13.3 | 55.0 ± 11.6 | 6.0 ± 4.4 | 4.0 | 0.019 |
| SF-36 | 119.1 ± 10.2 | 100.0 ± 24.1 | 19.1 ± 21.4 | 15.5 | 0.001 | SF-36 | 131.5 ± 16.5 | 126.2 ± 19.9 | 5.3 ± 6.1 | 6.0 | 0.022 |
| pain | 19.4 ± 4.9 | 21.0 ± 3.7 | 1.6 ± 2.5 | 1.0 | 0.035 | pain | 16.5 ± 6.8 | 18.2 ± 5.7 | 1.7 ± 2.5 | 0.5 | 0.056 * |
| Experimental Group | Control Group | ||||
|---|---|---|---|---|---|
| Symptom | M1 | M2 | Symptom | M1 | M2 |
| fingers tingling | 0 | 3 | fingers tingling | 0 | 0 |
| fingertips numbness | 0 | 9 | fingertips numbness | 0 | 2 |
| heat sensation | 0 | 5 | heat sensation | 0 | 0 |
| fingertip sensation | 0 | 6 | fingertip sensation | 0 | 0 |
| impression of movement | 0 | 3 | impression of movement | 0 | 0 |
| movement | 0 | 3 | movement | 0 | 0 |
| Experimental Group | Control Group | ||
|---|---|---|---|
| Question | Answer | Question | Answer |
| previous contact with VR technology | 0 | previous knowledge of mirror therapy treatment | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sip, P.; Kozłowska, M.; Czysz, D.; Daroszewski, P.; Lisiński, P. Perspectives of Motor Functional Upper Extremity Recovery with the Use of Immersive Virtual Reality in Stroke Patients. Sensors 2023, 23, 712. https://doi.org/10.3390/s23020712
Sip P, Kozłowska M, Czysz D, Daroszewski P, Lisiński P. Perspectives of Motor Functional Upper Extremity Recovery with the Use of Immersive Virtual Reality in Stroke Patients. Sensors. 2023; 23(2):712. https://doi.org/10.3390/s23020712
Chicago/Turabian StyleSip, Paweł, Marta Kozłowska, Dariusz Czysz, Przemysław Daroszewski, and Przemysław Lisiński. 2023. "Perspectives of Motor Functional Upper Extremity Recovery with the Use of Immersive Virtual Reality in Stroke Patients" Sensors 23, no. 2: 712. https://doi.org/10.3390/s23020712
APA StyleSip, P., Kozłowska, M., Czysz, D., Daroszewski, P., & Lisiński, P. (2023). Perspectives of Motor Functional Upper Extremity Recovery with the Use of Immersive Virtual Reality in Stroke Patients. Sensors, 23(2), 712. https://doi.org/10.3390/s23020712






