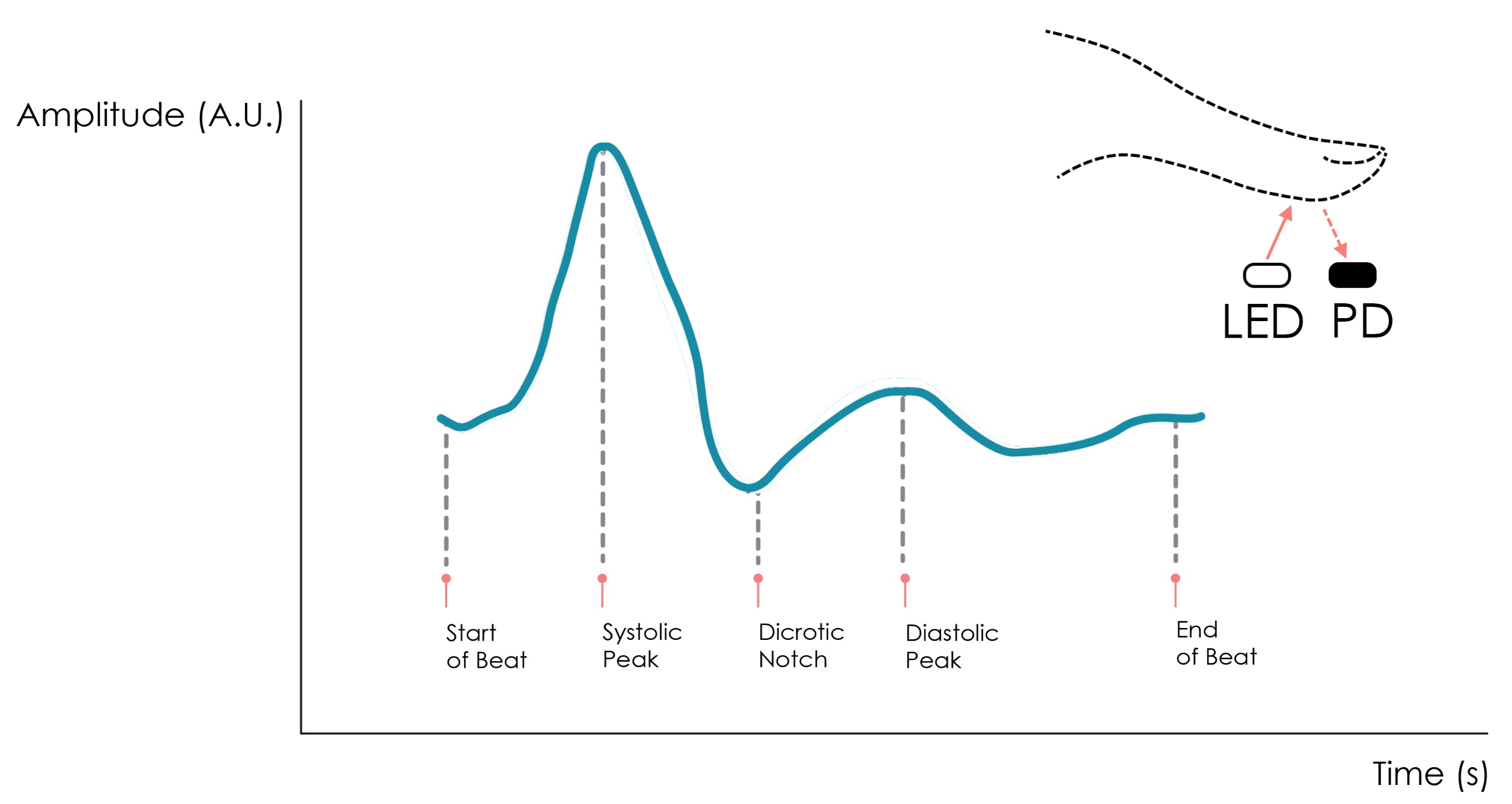
5.3. EDA Data Analysis
EDA signals were successfully recorded at the hand in all of the 19 subjects; however, in 8 of these subjects, no EDA signal could be measured at the foot (i.e., constant null signal for the full duration), resulting in 11 subjects with measurable foot-EDA signals. Furthermore, a full non-zero EDA signal was only observed in 3 out of these 11 subjects, whereas in the remaining 8, the EDA signals were only non-zero during part of the acquisition.
To understand if there were anthropometric or physiological factors associated with cases of non-measurable EDA, the following variables were first considered: continuous numerical candidate (independent) variables and the ratio of measurable EDA,
, given by Equation (
3) (where
S is the signal). The candidate variables consisted of height, weight, and foot size (from the questionnaire in
Figure 7a), and statistical features extracted from ACC and temperature signals (for temperature features, we considered only 15 subjects). The statistical features consisted of the mean, median, variance, standard deviation, skewness, entropy, min, max, range (i.e., max–min), interquartile range, and mean absolute deviation. We computed the Pearson correlation between these candidate variables and
. None resulted in a statistically significant correlation (i.e.,
p-value <
).
To test the correlation between age range and
, we used the Spearman correlation, which also did not result in a statistically significant correlation. In addition, we tested whether
differed between gender groups or groups answering differently (binary answers) in each of the 5 questions regarding sweating tendency (from the questionnaire in
Figure 7b). For this, we used ANOVA and Mann–Whitney U tests when the
was normally and non-normally distributed in the groups, respectively. Again, for these variables, no significant differences were observed.
From the extracted full hand and foot EDA signals, we computed the Pearson correlations between the two body locations.
Figure 9 shows the correlation values for each subject and type of extracted signals (
and
). Considering all subjects, the Pearson correlation for
was
(mean and standard deviation) within a range of
. In the same order, for
, we obtained
within
. The obtained correlation values are dispersed as a result of a high inter-subject variability regarding the similarity of the signals from the two body locations. For example, for
, a strong correlation was observed (i.e., >0.75) in 3 of the subjects, whereas in 3 other subjects, a negative value was registered. Moreover, if only the SCR component is considered (
), generally higher correlations are obtained.
Figure 10 shows the
values for the signals obtained from each subject, along with the corresponding durations of the signals in which they were not saturated. For all subjects, the measurable portions of EDA signals had approximately at least 150 s in total. Considering both
Figure 9 and
Figure 10, some signals yielding the highest Pearson correlation values preserved approximately the full acquisition time. For instance, for subjects with IDs 2, 20, and 21, in which strong Pearson correlation values were observed for
, their
was
(i.e., summed measurable portions had a duration
s), thus, confirming the significance of such cases.
It is known that not every SCR event is expressed in all body locations. For instance, one SCR peak arising from a given stimulus can be expressed at the hand and be absent at the foot or vice-versa. Moreover, events elicited by the same stimulus can be expressed with latency in different body locations. For this reason, we studied the similarity of the EDA signals in regions with co-occurring SCR events and minimal lag. For this, we first extracted SCR onsets and peaks from the hand and foot
signals, using the algorithm described in [
47]. On average, considering all 11 recordings, the foot EDA resulted in
extra onsets and peaks that were undetected in the hand signals.
Then, similarly as in [
11], we defined a time window for co-occurring peaks of
s such that, for each peak expressed in the hand signal (i.e., reference), the co-occurring peak in the foot signal would be assigned as the closest one within
to
s with respect to the reference peak. Thus, if no foot peak was within the defined window, the respective hand peak would be neglected. It is worth mentioning that we considered the hand as the reference body location (i.e., by searching nearby foot peaks) because, on average, there were fewer peaks detected at the hand.
Once the co-occurring peaks were found, segments of hand and foot
were extracted from a larger time window around each reference peak. We considered a window of
s, which, based on testing and visualization of different windows, seemed to include the full waveform of the peaks.
Figure 11 shows two of the windows used in the correlation analysis.
Figure 12a shows the statistical distributions of the Pearson correlation values for the obtained
segments extracted from the peak windows. The obtained correlation was
(mean and standard deviation) within a range of
, which is unexpectedly more dispersed compared to the full
signals.
Using the same method in [
11] (and the same window of 4 s), we computed the consensus of peaks, considering the peaks from the hand signals as the reference events. For all subjects, the consensus of peaks was
(mean and standard deviation) within a range of
.
We also analyzed the lag between events, by computing the time difference between each peak at the hand and the closest peak at the foot, within a maximum window of 4 s (i.e.,
s).
Figure 12b shows the distributions of these time lags. Considering all subjects, the time lags were
s. The mean value is positive, meaning the peaks measured at the hand occurred, on average, before the closest peak at the foot, which is in accordance with the expected slower response at the foot.
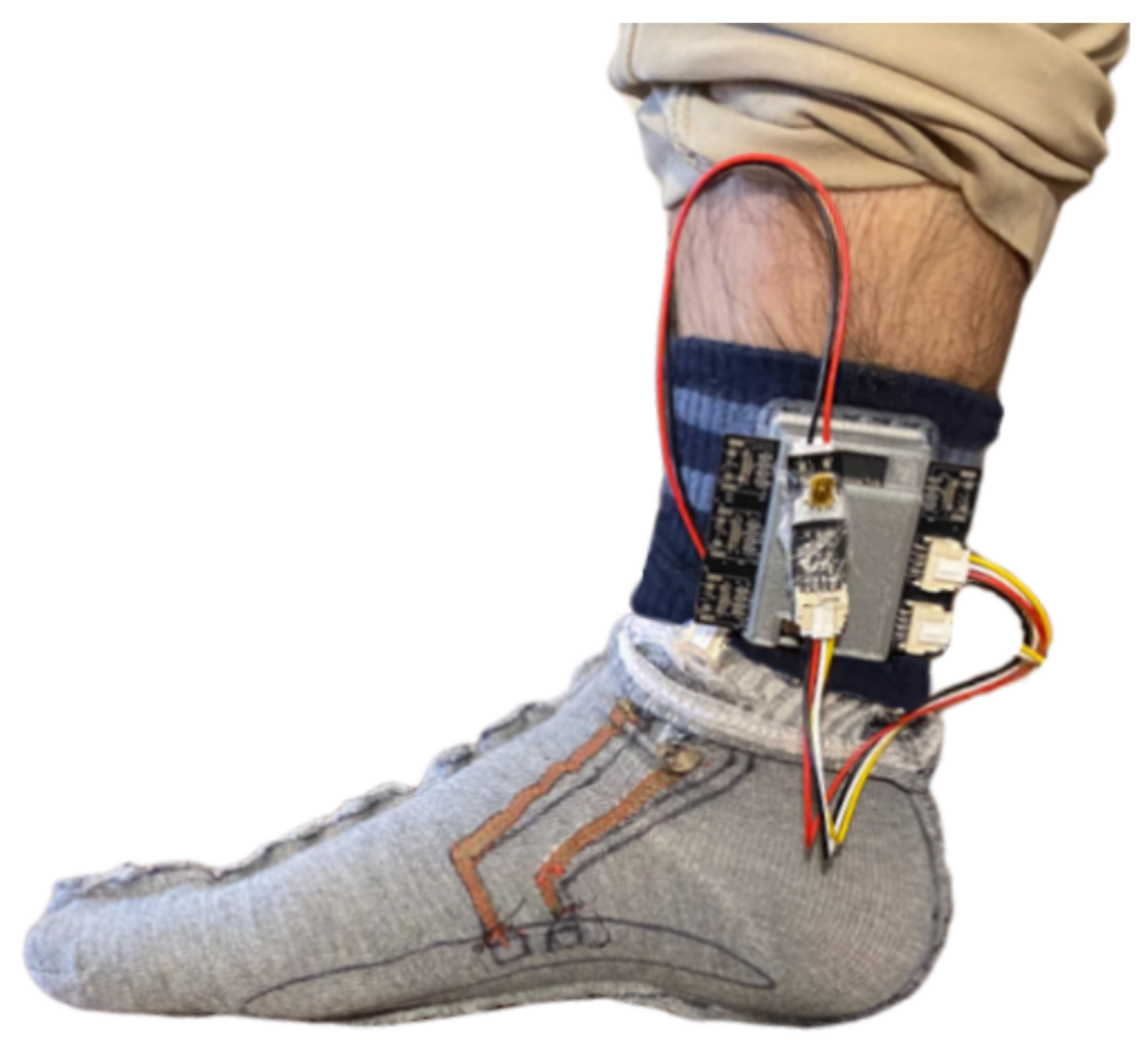
5.4. PPG Data Analysis
Initially, using the filtering step described in
Section 4.5 resulted in a poor peak detection performance for the ankle PPG (i.e., a considerable number of false positives), which motivated us to fine-tune this pre-processing step. Thus, we adjusted the frequency band of the filtering step. This required the systolic peaks to be manually labeled for finger and ankle PPG. Afterward, two grid searches were performed to obtain the highest peak detection accuracy for the ankle PPG, considering the
and
cut-off frequencies of the filtering step as hyper-parameters.
In the first grid search, the following linearly spaced values were considered: 5 values in Hz for and 20 values in Hz for (i.e., a total of 100 combinations). To increase granularity, the second grid search was performed in sub-intervals, which resulted in higher accuracy than the first one. The second one was performed as follows: 10 values in Hz for and 30 values in Hz for .
A band-pass frequency range of
Hz was empirically found to yield the highest accuracy for ankle PPG, without compromising the performance for the finger PPG. In fact, this same frequency range also resulted in the maximum average accuracies for both body locations. The results are shown in
Table 5.
Moreover, for each segment, the HR was estimated based on the predicted PPG peaks. Considering all segments, for the index finger, the difference between the true HR (i.e., computed from manually labeled peaks) and the predicted one was BPM (mean ± standard deviation). For the ankle, this difference was BPM, meaning that the predicted peaks resulted in a significant overestimation of the HR. Additionally, we computed the root mean squared error of the HR in terms of the true and predicted HR estimations at the foot. For this purpose, we employed linear interpolation on the HR values because the algorithm we used computed HR values only at the instances of the PPG peaks. The obtained mean squared error of the HR among all segments was BPM.
To study the association between anthropometric data and the performance of the peak detection for ankle PPG signals, we considered candidate (independent) and target (possibly dependent) variables. The candidate variables consisted of age, gender, height, weight, and foot size, obtained from the questionnaire in
Figure 7a. For the target variables, we considered the accuracy, sensitivity, and positive predictive value of the peak detection from the ankle signals, as well as the mean difference between the true HR and the predicted one also for the ankle. For age, Spearman correlations were computed, and for gender, the Mann–Whitney U test was performed (none of the target variables was normally distributed). Moreover, for the remaining continuous candidate variables, Pearson correlations were computed. When testing the null hypotheses, candidate variables age, gender, weight, and BMI resulted in
p-values
against sensitivity. These results are shown in
Table 6, suggesting the peak detection and HR performance tends to increase with age, weight, and BMI and is higher in the male group.